Journal of Biology and Medicine
Integrative Review: Hemoglobin Biosynthesis and the Nutritional Roles of Vitamins, Enzymes, and Minerals (2000–2025)
Marco Santana Institute, Brazil
Author and article information
Cite this as
Vinícios de Oliveira Santana M, Marchiori CH, Paula Malheiros KD, de Melo ÈM. Integrative Review: Hemoglobin Biosynthesis and the Nutritional Roles of Vitamins, Enzymes, and Minerals (2000–2025). J Biol Med . 2025; 9(1): 014-020. Available from: 10.17352/jbm.000048
Copyright License
© 2025 Vinícios de Oliveira Santana M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Hemoglobin plays a central role in oxygen transport, carbon dioxide removal, acid-base balance maintenance, of cellular homeostasis in humans. Its structural characteristics and biochemical properties are essential for physiological adaptation in both health and disease. Alterations in hemoglobin concentration, molecular structure, or biological function are associated with a wide range of clinical conditions, including anemia, thalassemia, hemoglobinopathies, inflammatory disorders, kidney disease, and chronic degenerative conditions. This review provides an updated overview of the biological functions, molecular mechanisms, regulatory pathways, diagnostic significance, and clinical applications of hemoglobin. A comprehensive review of the literature was conducted, covering scientific publications from 2000 to 2025, including research from the fields of molecular biology, clinical hematology, pathophysiology, and translational medicine. The purpose of this review is to support a better understanding of hemoglobin as a clinical biomarker, with relevance for early diagnosis, disease monitoring, and personalized medicine.
RBCs: Red Blood Cells; CO₂: Carbon Dioxide; RBCs: Red Blood Cells; α₂β₂: Hemoglobin tetramer; ALAS: 5-aminolevulinate synthase; ALA: 5-aminolevulinic acid; Fe²⁺: Ferrous iron; DNA: deoxyribonucleic acid; ALAD: Aminolevulinate Dehydratase; MnSOD: Manganese Superoxide Dismutase
Introduction
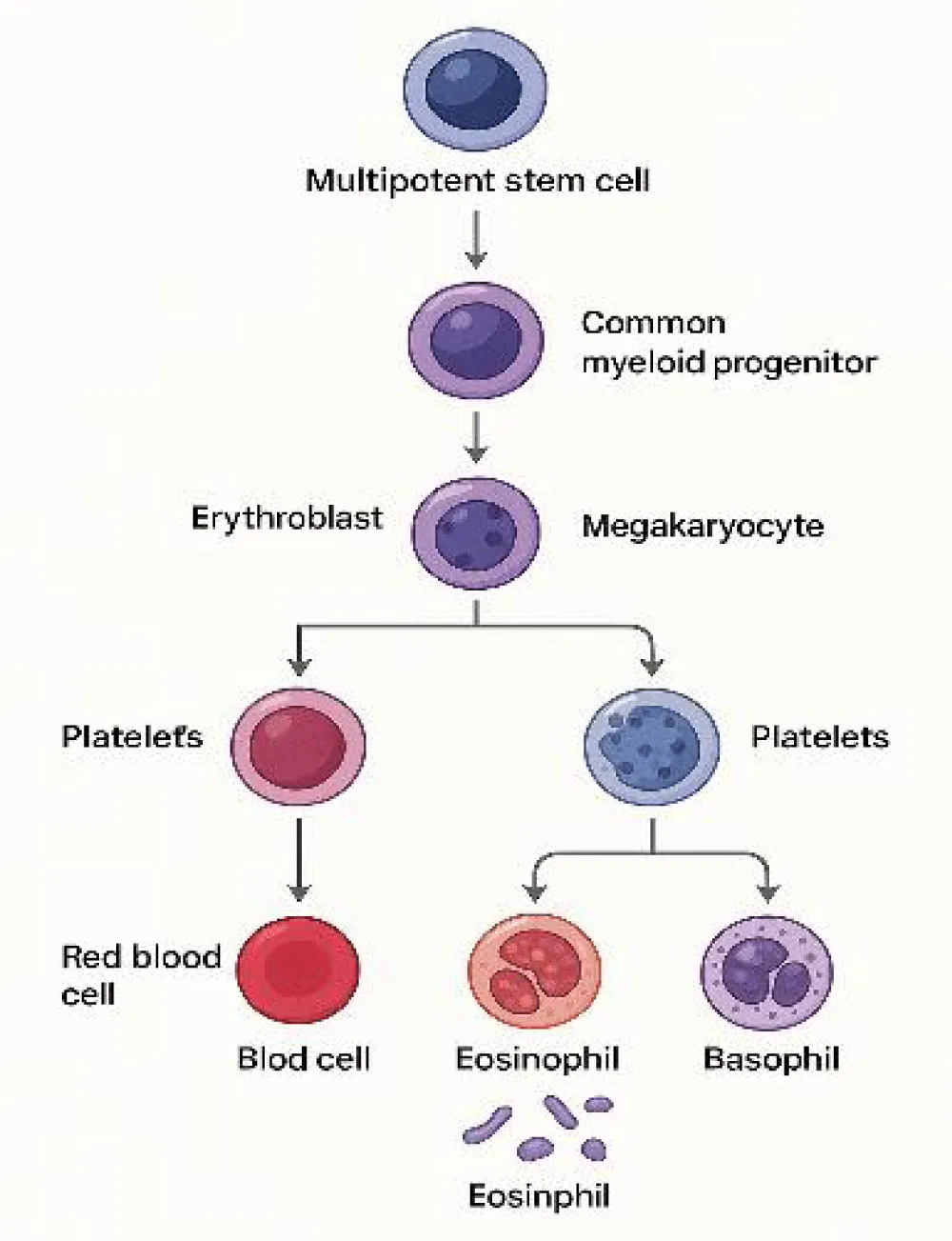
Red Blood Cells (RBCs) are anucleate, biconcave disc-shaped cells that contain large amounts of hemoglobin, a protein responsible for the transport of oxygen and carbon dioxide (CO₂). Their primary function is to carry oxygen from the lungs to peripheral tissues, supporting cellular respiration, ATP production, and physiological homeostasis. Due to their structural conformation, RBCs facilitate efficient gas exchange, favoring both oxygen release and carbon dioxide clearance. Erythropoiesis and hemoglobin structure are illustrated in Figures 1,2 [1-3].
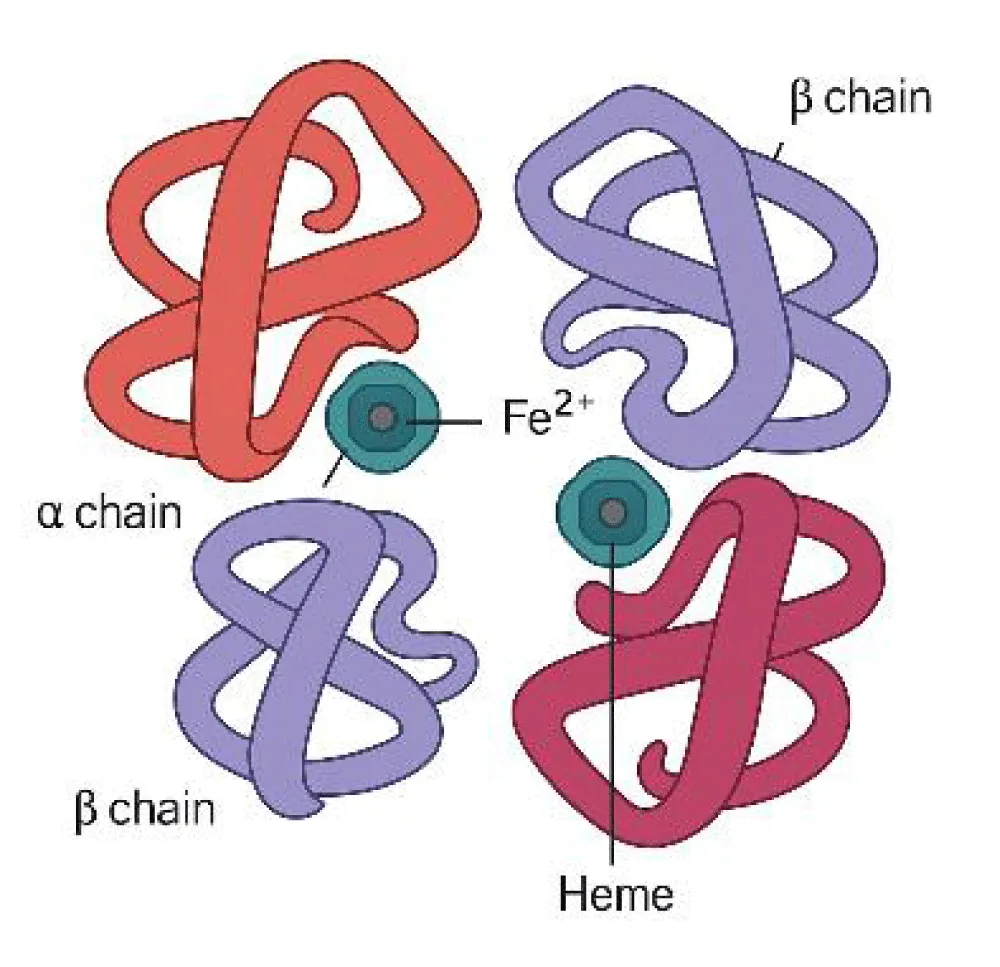
Figure 1 illustrates erythropoiesis as a multistage process regulated by essential growth factors. Efficient erythropoiesis ensures adequate hemoglobin production and systemic oxygen transport. Figure 2, Structure of hemoglobin showing an iron atom (Fe²⁺) at the center of the porphyrin ring coordinated by nitrogen atoms and anchored to the globin chain via a histidine residue. Each Fe²⁺ reversibly binds one O₂ or CO₂ molecule, allowing efficient gas transport throughout the body.
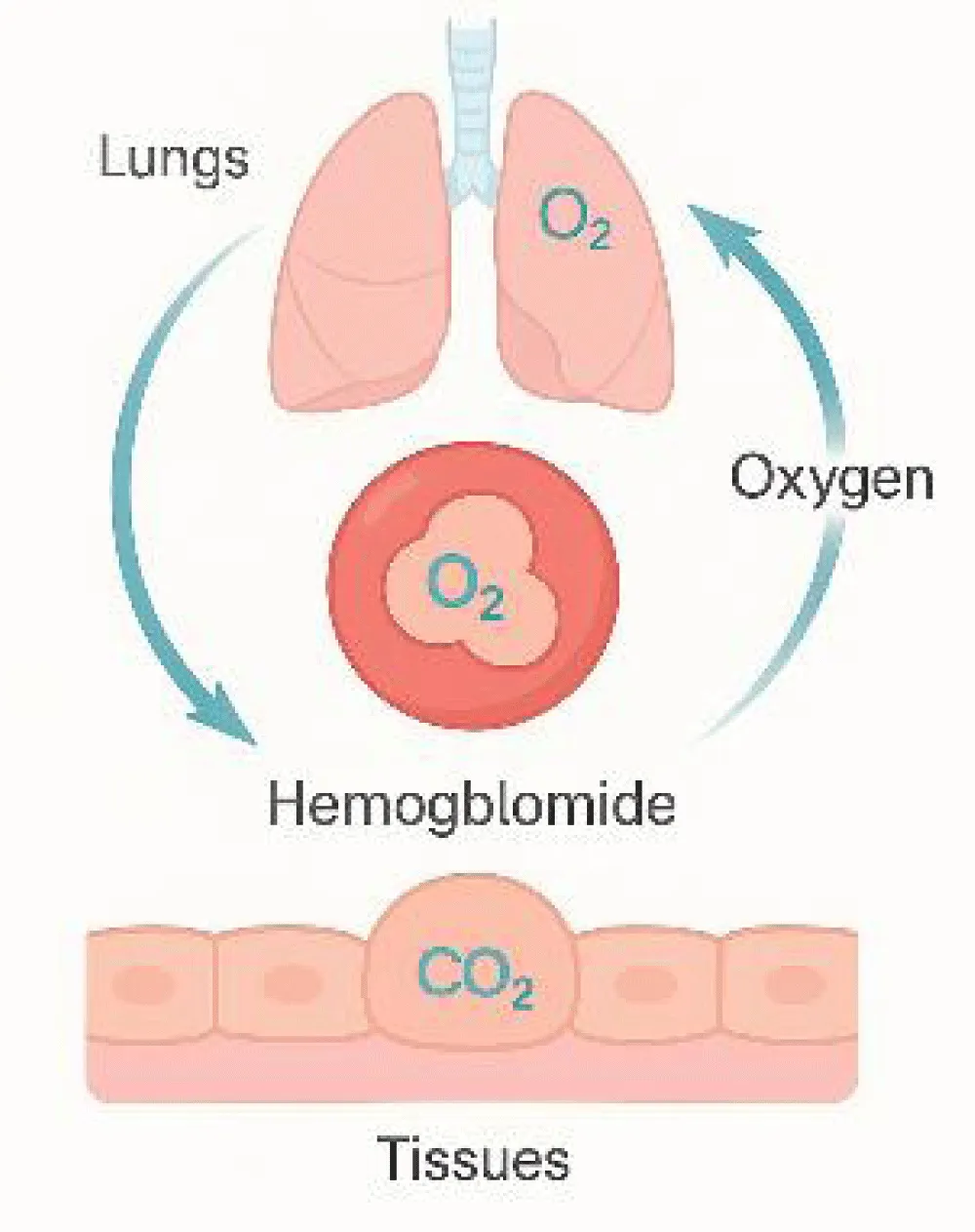
Hemoglobin comprises approximately 95% of the total protein content in RBCs, facilitating gas exchange and contributing indirectly to immune processes through the transport of inflammatory mediators and antibodies [4,5]. Hematopoiesis encompasses the formation, proliferation, and maturation of precursor cells into functional blood elements, ensuring oxygen delivery through hemoglobin (Figure 3) [6,7].
(Figure 3) Mechanism of oxygen transport and gas exchange through hemoglobin. Hemoglobin binds oxygen in the lungs and releases it in tissues while carrying carbon dioxide back to the lungs. This reversible exchange maintains efficient gas transport and blood pH balance. Over the past two decades, research has revealed that hemoglobin synthesis depends not only on iron availability but also on a complex network of vitamins, minerals, and enzymes that regulate iron absorption, heme formation, and erythrocyte maturation.
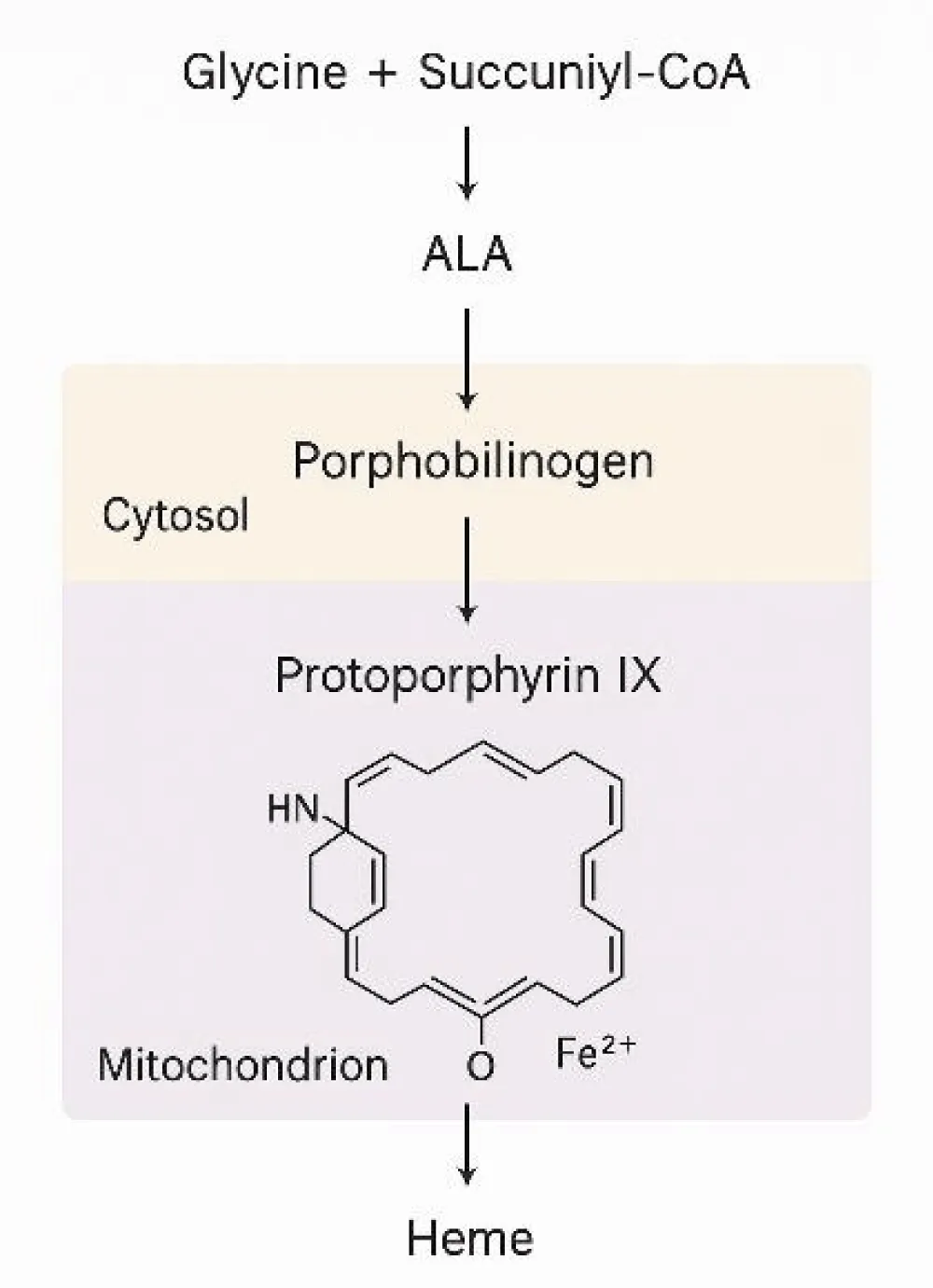
The discovery of the hepcidin–ferroportin axis clarified how inflammation, chronic disease, and nutritional deficiencies impact iron metabolism and erythropoiesis. This process is illustrated in the hemoglobin tetramer structure (α₂β₂) with four heme groups bound to globin chains (Figure 4) [8-10].
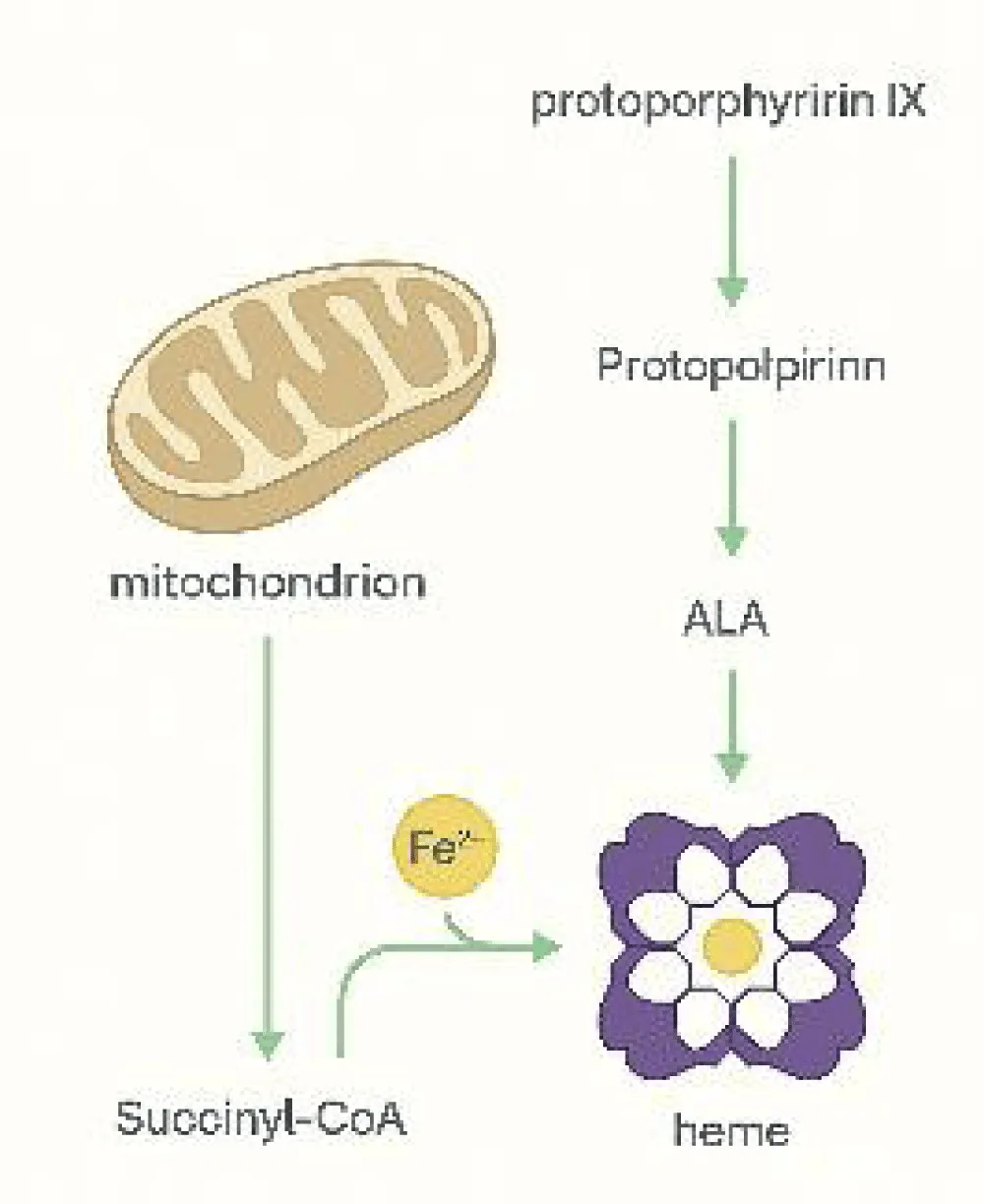
Figure 4, Heme biosynthesis begins in the mitochondria, where glycine reacts with succinyl-CoA via ALA synthase, producing 5-aminolevulinic acid (ALA). This reaction is the initial and rate-limiting step in heme formation, which later contributes to hemoglobin production.
Heme synthesis progresses through sequential enzymatic reactions involving zinc-dependent dehydratases and ferrochelatase, which incorporates ferrous iron into protoporphyrin IX. Genetic defects or deficiencies in vitamin B₆, zinc, or iron can impair hemoglobinization, leading to specific anemias [8-11].
Hepcidin, produced by the liver, regulates systemic iron balance by inducing internalization of ferroportin, the only known iron-exporting protein. In inflammatory states, increased hepcidin causes iron sequestration, contributing to anemia of chronic disease and reducing the effectiveness of oral iron supplementation [5,6,12].
In addition to iron, vitamins such as B₁₂, B₆, folate, and vitamin C play essential roles in hemoglobin synthesis and erythropoiesis. Their deficiencies lead to macrocytic or sideroblastic anemia and impaired iron absorption [13-15]. Trace minerals, including copper, zinc, and manganese, also support enzymatic activity and protect erythrocytes from oxidative stress [16-18].
Hemoglobin’s functions extend beyond oxygen and carbon dioxide transport; it also contributes to acid–base regulation, blood buffering, nitric oxide signaling, and redox balance. Through the Bohr effect, hemoglobin binds protons during oxygen release, stabilizing deoxyhemoglobin and preserving pH homeostasis [19,20]. These multifaceted functions justify its importance across nutritional, hematological, and clinical research.
Accordingly, this review integrates biochemical, nutritional, and clinical perspectives on hemoglobin biosynthesis and function, emphasizing the regulatory roles of vitamins, enzymes, and trace minerals in maintaining physiological balance and preventing anemia.
Methods
This integrative review sought to gather and interpret current biochemical, nutritional, and clinical evidence regarding hemoglobin synthesis and the micronutrients involved in its regulation. A structured methodological approach was applied, including the definition of the research problem, systematic literature search, application of predefined eligibility criteria, critical evaluation of selected studies, and thematic integration of key concepts.
The literature search was conducted between January and October 2025 in major scientific databases, including PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar. Additionally, authoritative hematology textbooks, clinical laboratory protocols, and biochemical manuals were consulted to complement the data and contextualize mechanisms related to erythropoiesis, iron metabolism, and micronutrient regulation.
Search strategies used Boolean operators (AND, OR) to combine terms, including: hemoglobin biosynthesis, heme pathway, iron regulation, hepcidin ferroportin axis, vitamin cofactors AND erythropoiesis, trace minerals AND red blood cells, nutritional anemia mechanisms. Publications from 2000 to 2025 were included to integrate classical mechanisms with recent molecular discoveries, nutritional insights, technological advancements, and emerging therapeutic perspectives.
Studies were considered eligible if they met at least one of the following criteria: addressed biochemical or molecular pathways involved in hemoglobin or heme formation; evaluated vitamins, minerals, or enzymatic cofactors involved in erythropoiesis; examined regulatory mechanisms of iron metabolism; provided clinical or nutritional insights into anemia; or included experimental, clinical, or review data with methodological clarity.
Excluded materials were non-scientific publications, opinion essays, studies lacking methodological description, reports unrelated to hemoglobin or micronutrient metabolism, duplicated content, and publications with insufficient scientific rigor. Relevant information from eligible sources was manually extracted and categorized into the following analytical themes: biochemical mechanisms of heme formation; enzymatic regulation and cofactor dependency; systemic iron homeostasis and hepcidin–ferroportin dynamics; vitamin roles in erythroblast maturation; and trace mineral functions in heme stabilization.
The selected studies were evaluated according to methodological approach, biochemical accuracy, consistency of findings, and clinical applicability. Conflicting results were examined to identify differences in methodology, sample characteristics, inflammatory status, comorbidities, or nutritional deficiencies. The synthesis emphasized mechanistic pathways integrating iron flux, vitamin-dependent enzymatic activity, and trace mineral interactions, aligning molecular biology with clinical nutrition.
Results
In erythroid cells, heme biosynthesis proceeds through a coordinated series of enzymatic reactions distributed between the cytosol and mitochondria. Porphobilinogen is converted into hydroxymethylbilane, which is cyclized by uroporphyrinogen III synthase to form uroporphyrinogen III. This compound is then decreasingly produced by uroporphyrinogen decarboxylase, producing coproporphyrinogen III, which subsequently enters the mitochondria.
Within the mitochondrial matrix, coproporphyrinogen oxidase catalyzes its conversion to protoporphyrinogen IX, which is further oxidized by protoporphyrinogen oxidase to generate protoporphyrin IX. The final step is mediated by ferrochelatase, which inserts ferrous iron (Fe²⁺) into protoporphyrin IX to produce heme, the essential prosthetic group of hemoglobin and other hemoproteins (Figure 5).
Figure 5, schematic representation of the heme biosynthetic pathway illustrating the sequential cytosolic and mitochondrial enzymatic reactions that transform metabolic precursors into porphyrin intermediates, ultimately forming protoporphyrin IX and culminating in the insertion of Fe²⁺ by ferrochelatase to produce heme.
Cytosolic reactions convert 5-aminolevulinic acid (ALA) into porphobilinogen, hydroxymethylbilane, uroporphyrinogen III, and coproporphyrinogen III before the intermediates re-enter the mitochondria. The final mitochondrial stages involve oxidation to protoporphyrin IX and insertion of Fe²⁺, completing heme formation [4-6]. This pathway highlights the dependence of each enzyme on specific vitamins and minerals, which serve as cofactors essential for catalytic efficiency and erythrocyte maturation.
(Table 1) This table shows that each enzyme in heme biosynthesis depends on specific vitamins or trace elements for catalytic activity. 5-aminolevulinate synthase (ALAS) requires vitamin B₆, 5-aminolevulinic acid dehydratase depends on zinc, and ferrochelatase uses ferrous iron. Deficiencies or inhibition by heavy metals, particularly lead, cause porphyrin accumulation and microcytic anemia. ALAS: 5-aminolevulinate synthase; ALA: 5-aminolevulinic acid.
Deficiencies of these micronutrients or inhibition by heavy metals such as lead impair heme synthesis, resulting in porphyrin accumulation and microcytic or sideroblastic anemia [7-10]. The balance of these elements is crucial for maintaining hemoglobin production and adequate erythropoiesis. Systemic iron regulation and nutritional interactions.
Iron regulation in the human body is primarily governed by the hepcidin–ferroportin axis. Hepcidin, synthesized in the liver, binds to ferroportin on enterocytes, macrophages, and hepatocytes, inducing its internalization and degradation. This mechanism restricts iron efflux into circulation and regulates systemic iron homeostasis [11-13].
During periods of high erythropoietic demands, such as growth, pregnancy, or recovery from anemia, hepcidin expression decreases, enhancing iron absorption and mobilization to support hemoglobin synthesis and red blood cell production [11-13].
In inflammatory states, however, interleukin-6 and other cytokines stimulate hepatic hepcidin synthesis, leading to sequestration of iron within reticuloendothelial cells. Consequently, serum iron levels decline despite adequate or elevated body iron stores, contributing to anemia of chronic disease. Table 2 summarizes the major regulators involved in systemic iron metabolism and their role in hemoglobin synthesis [11-13].
Table 2, systemic iron metabolism is primarily regulated by the hepcidin–ferroportin axis, which controls iron absorption, release, and availability for erythropoiesis. Supporting nutrients and proteins, such as vitamin C, copper/ceruloplasmin, transferrin, and ferritin, facilitate iron absorption, transport, and storage. Together, these elements ensure adequate iron supply for hemoglobin synthesis while preventing deficiency or overload. Fe³⁺ and Fe²⁺; Ferrous.
Vitamin C enhances iron absorption by reducing ferric (Fe³⁺) to ferrous (Fe²⁺) form. Copper supports ceruloplasmin-mediated oxidation of Fe²⁺ to Fe³⁺, allowing transferrin binding. Zinc participates indirectly in erythropoiesis by supporting DNA synthesis and cell division [14-16].
Folate and vitamin B₁₂ are required for DNA synthesis and erythroblast proliferation, and deficiency results in megaloblastic anemia. Vitamin B₆, in the form of pyridoxal phosphate, is essential for ALAS, the rate-limiting enzyme in heme synthesis. Its deficiency is associated with sideroblastic anemia, which often responds to pyridoxine supplementation [17-20].
(Table 3) Mechanism of biochemical roles of vitamins in hemoglobin production. Folate and vitamin B₁₂ are essential for DNA synthesis and erythroblast division; deficiencies cause megaloblastic anemia. Vitamin B₆, as pyridoxal phosphate, is required for ALAS, the first step of heme synthesis. Its deficiency causes sideroblastic anemia, which is reversible with pyridoxine therapy. ALAS: 5-aminolevulinate synthase. Adequate intake of these vitamins is necessary to maintain efficient erythropoiesis and prevent both microcytic and macrocytic anemia.
Trace minerals such as copper, zinc, and manganese are fundamental for hemoglobin metabolism, enzyme stabilization, and protection against oxidative stress. Copper enables ceruloplasmin and hephaestin to oxidize Fe²⁺ to Fe³⁺ for transferrin binding. Zinc stabilizes Aminolevulinate Dehydratase (ALAD), while manganese is essential for Manganese Superoxide Dismutase (MnSOD), an enzyme responsible for defending erythrocyte membranes against oxidative damage [21-23].
(Table 4) Describes the minerals such as copper, zinc, and manganese, which are vital for hemoglobin metabolism. Copper is required for ceruloplasmin and hephaestin, which oxidize Fe²⁺ to Fe³⁺ for transferrin.
Loading. Zinc stabilizes aminolevulinate dehydratase activity, while manganese supports antioxidant defense systems. Deficiencies lead to impaired iron utilization and oxidative stress. ALA: 5-aminolevulinic acid. Optimal levels of these trace elements are required to maintain hemoglobin integrity, iron mobilization, enzymatic activity, and redox balance during erythropoiesis [24-60].
Despite substantial progress in understanding hemoglobin metabolism, several gaps persist. There is still incomplete knowledge regarding how inflammation, genetic variability, and micronutrient status interact to determine erythropoietic efficiency. Although secondary biomarkers such as hepcidin and erythroferrone have improved diagnostic accuracy, their use remains limited due to cost and methodological inconsistencies [61].
Clinical studies show considerable variability due to differences in disease status, age, supplementation dosage, and control of inflammatory conditions. This heterogeneity complicates interpretation and limits translational applicability [62]. Socioeconomic disparities, including limited access to diagnosis, fortified foods, and supplementation, continue to hinder effective anemia prevention strategies in vulnerable populations [63]. Environmental exposure to heavy metals disrupts enzymatic reactions in heme synthesis and worsens nutritional deficiencies [64-70].
There is a need to integrate molecular, nutritional, and clinical data into public health frameworks to create targeted and equitable strategies for anemia prevention and management.
Conclusion
The metabolism and oxygen physiology. Its synthesis relies not only on iron availability but also on a coordinated regulatory system involving enzymes, vitamins, and trace minerals that sustain erythrocyte maturation and heme production. Deficiencies or imbalances in these nutrients impair hemoglobinization, disrupt iron homeostasis, and contribute to different types of anemia, many of which remain underdiagnosed.
Advances in the understanding of regulatory pathways, such as the hepcidin–ferroportin axis and erythroferrone signaling, have improved diagnostic and therapeutic perspectives. Furthermore, technologies such as multi-omics, nutrigenomics, and artificial intelligence offer promising tools for early detection and personalized interventions. Integrating these molecular insights into clinical and public health strategies is essential to optimize prevention, improve hemoglobin-related outcomes, and reduce the global burden of anemia.
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper. All authors have participated in the work and agree with the contents of the manuscript. There has been no financial support, personal relationship, or professional affiliation that could have influenced the results or interpretation of this study.
Acknowledgment
The author expresses sincere gratitude to colleagues and collaborators who contributed to the development of this work through scientific discussion and constructive suggestions. Special thanks are extended to the research teams and institutions involved in studies on hemoglobin biosynthesis, nutrition, and trace element metabolism, whose work provided essential references for this review. The authors also acknowledge the valuable editorial support and guidance during the preparation of this manuscript.
- Benz EJ Jr, Forget BG. Regulation of hemoglobin synthesis. N Engl J Med. 2018;378(17):1643–1655.
- Bain BJ. Blood Cells: A Practical Guide. 6th ed. Wiley-Blackwell; 2020. https://www.scribd.com/document/889727507/Blood-Cells-a-Practical-Guide-6th-Edition-by-Barbara-J-Bain
- Clegg JB. The structure and function of hemoglobin. Clin Haematol. 2018;31(4):245–259.
- Hoffbrand AV, Moss PAH. Essential Haematology. 8th ed. Wiley-Blackwell; 2022.
- Ganz T, Nemeth E. Iron homeostasis and its disorders. N Engl J Med. 2019;381(20):1986–1995.
- Weatherall DJ, Clegg JB. The Thalassemia Syndromes. 5th ed. Wiley-Blackwell; 2018.
- Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Blood Rev. 2004;18(3):211–230. Available from: https://doi.org/10.1146/annurev.nutr.24.012003.132306
- Stojanovski D. Mitochondrial regulation of heme biosynthesis. Biochim Biophys Acta. 2019;1866(4):694–706.
- Brown FG. Enzymatic steps in heme formation and clinical implications. Br J Haematol. 2018;183(2):232–245.
- Rosove MH. Disorders of heme metabolism. Blood. 2023;141(15):1782–1790.
- Nemeth E. Hepcidin and iron regulation. Blood. 2006;107(10):4103–4110.
- Ganz T. Hepcidin — a key regulator of iron metabolism. N Engl J Med. 2011;366(19):1773–1783.
- Donovan A. The hepcidin–ferroportin axis controls systemic iron. Cell Metab. 2005;1(3):191–200.
- Cook JD, Dassenko SA, Whittaker P. Effects of ascorbic acid on iron absorption. Am J Clin Nutr. 2001;73(1):93–98. Available from: https://doi.org/10.1093/ajcn/73.1.93
- Gulec S, Anderson GJ, Collins JF. Mechanistic and regulatory aspects of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2014;307(4):G397–G409. Available from: https://doi.org/10.1152/ajpgi.00348.2013
- Myint ZW, Oo TH, Thein KZ. Role of micronutrients in red blood cell production. Nutr Rev. 2018;76(4):263–279.
- Cappellini MD, Graziadei G. Pathophysiology and management of sideroblastic anemia. Haematologica. 2021;106(7):1791–1802.
- Cappellini MD. Iron metabolism and its disorders. Br J Haematol. 2021;193(4):720–733.
- Steinberg MH, Forget BG, Higgs DR. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge University Press; 2017.
- Bain BJ. Haematology Illustrated. 5th ed. Wiley-Blackwell; 2021.
- Andrews NC. Disorders of iron metabolism. N Engl J Med. 2021;384(8):776–789.
- Azzini E, Raguzzini A, Polito A. Micronutrients and anemia: an updated review. Nutrients. 2021;13(6):1958.
- Selhub J. Folate and vitamin B12 in red blood cell formation. Am J Clin Nutr. 2022;115(3):725–735.
- O’Leary F, Samman S. Vitamin B12 in health and disease. Nutrients. 2010;2(3):299–316. Available from: https://doi.org/10.3390/nu2030299
- Okazaki Y, Ikeda K. Recent advances in iron deficiency and supplementation. Nutrients. 2023;15(4):923.
- Pagani A, Nai A, Silvestri L. Hepcidin regulation and anemia of chronic disease. Biochim Biophys Acta. 2019;1865(3):539–547.
- Xu L, Mikhael J. Management of anemia in chronic inflammation. Blood Rev. 2020;42:100712.
- Piskin O. Dietary vitamin C and iron absorption. Eur J Nutr. 2022;61(5):2443–2454.
- Moustarah F, Mohiuddin SS. Physiology, iron metabolism. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
- Donovan A. Copper-dependent ferroxidases in iron transport. J Biol Chem. 2005;280(13):11735–11742.
- Higgs DR, Weatherall DJ. The alpha thalassemias. Cell Mol Life Sci. 2019;76(7):1291–1303.
- Mayasari N. Micronutrient deficiencies in anemia: global perspectives. Nutrients. 2023;15(8):1736.
- Li H. Advances in gene therapy for hemoglobinopathies. Nat Med. 2024;30(2):245–259.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2017;390(10091):311–323. Available from: https://professionaleducation.blood.ca/sites/default/files/2021-11/Reading%20ware2017%20ADD.pdf
- Ware RE, de Montalembert M, Tshilolo L. Sickle cell disease. Lancet. 2020;395(10221):1648–1660.
- Dahal P. Hemoglobinopathies: genetic basis and therapeutic outlook. Front Med. 2025;12:1587.
- PNH News. Oral complement inhibitors in paroxysmal nocturnal hemoglobinuria. PNH News. 2025;3(1):15–18
- Novartis. Clinical data on iptacopan (Fabhalta) for PNH treatment. Novartis Press Release; 2025.
- Shao T. Nutritional management of anemia: digital health strategies. Clin Nutr. 2024;43(5):1981–1992.
- Kosugi T. Software-assisted anemia management in dialysis. Am J Kidney Dis. 2025;86(1):45–59.
- Ferrari P. Hepcidin modulators and iron metabolism disorders. Haematologica. 2024;109(2):412–426.
- Sandnes M. Inflammatory regulation of hepcidin and new therapeutic targets. J Transl Med. 2024;22(1):455.
- Quintana-Castanedo L. Novel anti-hepcidin strategies in anemia of inflammation. Front Pharmacol. 2025;16:1520.
- Fuertinger DH. Decision-support systems for anemia management in dialysis patients. Kidney Int Rep. 2024;9(3):398–411.
- Fresenius Medical Care. Clinical update: individualized anemia management software trials. Fresenius Medical Care; 2024.
- Warner J. Post-ICU anemia bundles and hemoglobin recovery. Crit Care Med. 2025;53(2):147–160.
- Zulfikar R. Digital adherence tools for iron–folic acid supplementation. Public Health Nutr. 2025;28(5):801–812.
- Shao L. Nutrition-guided hemoglobin targets in elderly dialysis patients. Clin Nutr. 2024;43(1):225–236.
- Kosugi T. Machine learning for personalized anemia treatment in CKD. Nephrol Dial Transplant. 2025;40(2):310–322.
- Novartis AG. Complement inhibition in paroxysmal nocturnal hemoglobinuria: 2024–2025 clinical updates. Novartis; 2025.
- PNH News. Oral iptacopan shows hemoglobin increase in the phase IIIB study. PNH News. 2025; March 14.
- Li C. Gene therapy and editing approaches for hemoglobinopathies. Mol Ther. 2024;32(7):1215–1230.
- Ribeil JA. Gene therapy in β-thalassemia and sickle cell disease. N Engl J Med. 2017;376(9):848–855.
- Cappellini MD, Porter JB, Viprakasit V, Taher AT. Emerging therapies for β-thalassemia. Blood Rev. 2020;40:100635.
- Weiss G. Hepcidin–ferroportin axis and anemia of chronic disease. Nat Rev Nephrol. 2021;17(6):386–401.
- Ganz T, Nemeth E. Hepcidin and iron homeostasis in health and disease. Biochim Biophys Acta. 2019;1863(7):157–168.
- The Guardian. Gene therapy rollout for sickle cell disease begins in NHS England. The Guardian. 2025; February 22.
- Wired. Inside the manufacturing challenges of CRISPR gene therapy. Wired Magazine. 2025; January 9.
- Mayasari NR. Manganese and selenium in erythropoiesis: an integrative review. Nutrients. 2023;15(3):520–534.
- Andrews NC. Trace elements and the molecular basis of anemia. N Engl J Med. 2021;384(2):185–197.
- De Franceschi L. Hepcidin and erythroferrone: Current insights into iron homeostasis and their diagnostic potential. Blood Rev. 2021;48:100806.
- Zlotkin S. Challenges and controversies in iron and micronutrient supplementation studies: methodological limitations and global implications. Am J Clin Nutr. 2020;111(1):144–150.
- Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low and middle-income countries. Lancet. 2011;378(9809):2123–2135. Available from: https://doi.org/10.1016/s0140-6736(10)62304-5
- Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5(2):47–58. Available from: https://doi.org/10.2478/v10102-012-0009-2
- McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2021. Public Health Nutr. 2022;25(6):1473–1488.
- McMahon LP. Integration of artificial intelligence into clinical hematology: future directions and ethical implications. Hematology Am Soc Hematol Educ Program. 2023;2023(1):85–93.
- Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK, et al. CRISPR–Cas9 gene editing for sickle-cell and β-thalassemia: long-term outcomes. N Engl J Med. 2021;384(3):252–260. Available from: https://doi.org/10.1056/nejmoa2031054
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. Available from: https://doi.org/10.1056/nejmra041809
- Nemeth E, Ganz T. Anemia of chronic disease and iron regulation. Semin Hematol. 2019;56(3):190–198.
- Weiss G, Goodnough LT. Anemia of inflammation. N Engl J Med. 2019;381(12):1148–1157. Available from: https://doi.org/10.1056/NEJMra1804281
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
