Journal of Biology and Medicine
Evaluation of Fucoidan for Anti-microbial Potency- An In-vitro Study
Prakruthi P, Rudrakshi C*, Prabhuji MLV and Arul Selvan
Department of Periodontology, Krishnadevaraya College of Dental Sciences and Hospital, India
Cite this as
Prakruthi P, Rudrakshi C, Prabhuji MLV, Selvan A. Evaluation of Fucoidan for Anti-microbial Potency- An In-vitro Study. J Biol Med. 2025;9(1):010-013. Available from: 10.17352/jbm.000047Copyright License
© 2025 Prakruthi P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Seaweed extracts are key ingredients in many modern bio-stimulant products, widely recognized for their richness in polysaccharides, minerals, and certain vitamins. While the benefits of seaweed have been known for biocompatibility, it is only in recent times that their bioactive compounds have gained scientific attention. These extracts contain phytochemicals such as polysaccharides, lipids, and polyphenols, which possess antibacterial, antifungal, and antiviral properties. Because of these properties, fucoidan extracts are of growing interest in nutraceutical, pharmaceutical, and medical applications (e.g., nutritional supplements, wound healing, skincare, periodontal therapy, etc.).
Introduction
Oral health is critical for the general health and well-being of the individual. Globally, periodontal disease, which includes gingivitis and periodontitis, is a prevalent oral infection that impacts the tissues surrounding and supporting the teeth [1]. The Global Burden of Disease Study (2025) highlights systemic implications of periodontal disease beyond oral health [2]. Comprehensive treatment of periodontal disease includes mechanical therapy, although considered a global standard is not without any limitations such as accessing certain areas, root surface irregularities, and tissue invasive micro-organisms. This has paved way for adjunctive therapies along with Phase I therapy. Sites specificity of periodontal disease necessities a personalised approach for treatment planning [3]. Adverse effect and high dose of systemic medication have promoted towards disease site specific delivery of drug, shifting from synthetic preparations towards herbal extracts as native /natural remedies [4].
Ethno-pharmacology represents a multifaceted strategy for drug discovery, encompassing the observation, documentation, and experimental analysis of native drugs and their biological effects [5]. Marine algae represent some of the most significant biomass producers within the marine ecosystem. They generate a diverse array of chemically active metabolites in their environment. Marine macro algae (Seaweed), particularly brown algae (Phaeophyta), have gained much research interest [6]. Seaweeds are a great source of a broad spectrum of biological activities. Brown algae have been utilized by coastal communities for their nutritional, functional, and technological purposes. Ascophyllum, Laminaria, Saccharina, Macrocystis, Nereocystis, and Sargassum represent the most common genera. Sargassum, a genus of brown seaweed (Phaeophyceae) belonging to the Sargassaceae family, comprises around 400 species [6].
Brown algae are known to produce different polysaccharides like Fucoidan, alginic acid, and laminarin [7]. Fucoidan has phytochemical benefits, playing a significant role in the medicinal and pharmacological arena. Fucoidan is a compound, specifically a sulphated polysaccharides possess biological activities which are highly varied, encompassing anticancer [8]. antioxidant, anticoagulant [9], antithrombotic [10], immunomodulatory, antiviral [11] and anti-inflammatory properties. Fucoidan is also recognized for its ability to mitigate metabolic syndrome. Additionally, it possesses angiogenic and osteogenic potential [12]. Fucoidan was procured from marine environments found on southeastern coast of India, particularly in the Gulf of Mannar region near Rameswaram, Tamil Nadu. It was extracted by the traditional method using heat and water [13]. In our study, Fucoidan was obtained from the Sigma Aldrich® company.
Fucoidan with the above-mentioned properties, as an herbal product, would be advantageous to patients with periodontal disease. In an in vitro study on the antibacterial efficacy of the extract of Sargassum wightii against oral pathogens, demonstrated highest zone of inhibition was demonstrated at a concentration of 1000µg, and it is an effective biologically active compound against oral pathogens [14]. Recently, in 2023, a study on the evaluation of the osteogenic potential of Fucoidan-Chitosan hydrogel in the treatment of intra-bony defects has highlighted the presence of Fucoidan contributing to bone regeneration [15]. We intended to investigate in the present study - “Evaluation of Fucoidan for anti-microbial potency- An In-vitro study.” This study was done in Department of Microbiology, Krishnadevaraya College of Dental Sciences and Hospital, Bangalore, Karnataka, India.
Materials and methods
This in-vitro, analytical study was conducted in Krishnadevaraya Dental College and Hospital, Bangalore, Karnataka. Patients presenting with Stage II/III and Grade B periodontitis were enrolled for collection of Gingival Crevicular Fluid (GCF) using Paper points (Dia Dent), ® from Out Outpatient Department (OPD) of Periodontology. The following selection criteria will be included.
Inclusion criteria for this study include patients with at least 18 years of age, patients presenting with Stage II/III and Grade B periodontitis, and patients who will comply with oral health care instructions and necessary visits. Exclusion criteria include patients with uncontrolled diabetes and severe cardiovascular or infectious diseases, smokers, lactating and pregnant women, patients who are psychologically unable to participate and patients with poor oral hygiene are not included in this study.
Site selection: For the collection of GCF samples, paper points (Dia Dent). ®Patients presenting 3-5 mm of loss of attachment were included. The deepest probing depth in the selected quadrant, preferably premolar/molar area, was included for GCF sample collection through the extra crevicular method.
Ethical approval: This is an ex vivo study where a total of 5 patients with Stage II/III and Grade B periodontitis satisfying the inclusion criteria and exclusion criteria were included for GCF sample collection at the pre-selected sites (deepest probing depth with ≥5 mm). The study went through the institutional review board of Krishnadevaraya College of Dental Sciences and Hospital in the pre-selection category, and considered Fucoidan compound was approved for biocompatible use. No screened patients were excluded.
GCF sample collection
GCF samples were obtained from the buccal aspect of the selected site using paper points. For a distinct standardization of the sampling site, the GCF sample was collected from the interproximal sites, among which the site showing the greatest probing depth. An unstimulated GCF sample was collected from the patients for which a sterile paper point was gently inserted into the entrance of the selected site (extracrevicular). The paper point was held in place for 30 seconds. Attempts were made to avoid the insertion of points to the depth of the pocket. To minimise the contamination of GCF with blood, paper points contaminated with blood were discarded and an alternate site was sampled [16].
Procedure for MIC estimation
Antimicrobial activity was assessed through an agar well diffusion test. A sterile paper point (Dia Dent) ® of size 0.06 mm was placed in the deepest periodontal pocket under aseptic conditions and kept for 30 seconds. The paper point was transferred to 1ml of sterile thioglycolate broth containing hemin and vitamin K and incubated at 37 ◦C for 1 hour.
The specimen was vortexed for 30 seconds in a vortex mixer to elute the bacteria from the paper point. 10µl of the specimen was transferred onto a dry anaerobic blood agar (Himedia Laboratories Pvt. Ltd) and spread uniformly using a sterile L spreader. Wells of 6 mm diameter were punched on the agar plate using sterile templates.
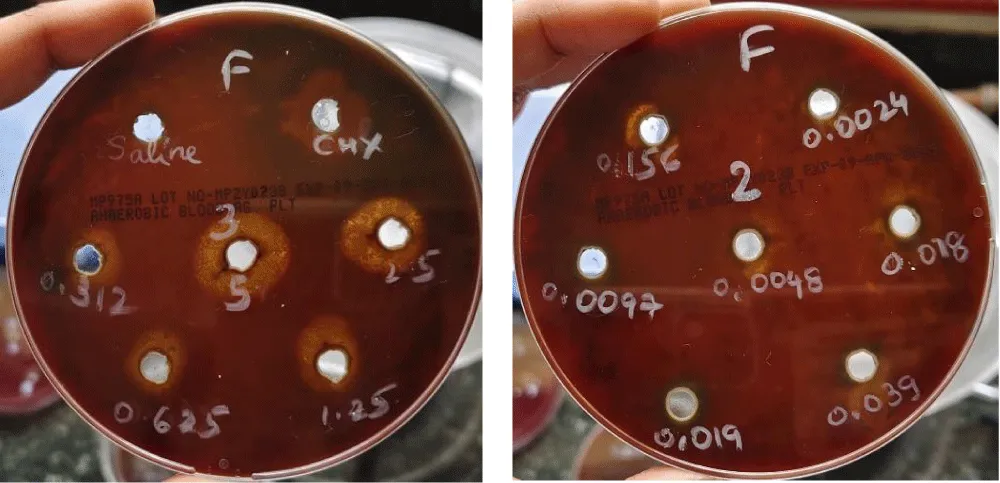
Fucoidan was diluted in sterile distilled water by serial doubling dilution to obtain dilution of 50 mg/ml,25 mg/ml,12.5 mg/ml,6.25 mg/ml,3.12 mg/ml,1.56 mg/ml,0.78 mg/ml,0.39 mg/ml,0.19 mg/ml,0.09 mg/ml,0.04 mg/ml,0.02 mg/ml and taking chlorhexidine as positive control and saline as negative control. Chlorhexidine is considered as gold standard, which can be used as an adjunct to mechanical therapy in periodontitis (Figure 1) [17].
50 µl of the Fucoidan dilution was inoculated onto the designated wells under aseptic condition. The plates were incubated at 37 ◦C in an anaerobic jar with gas pack for 48 hours.
Result
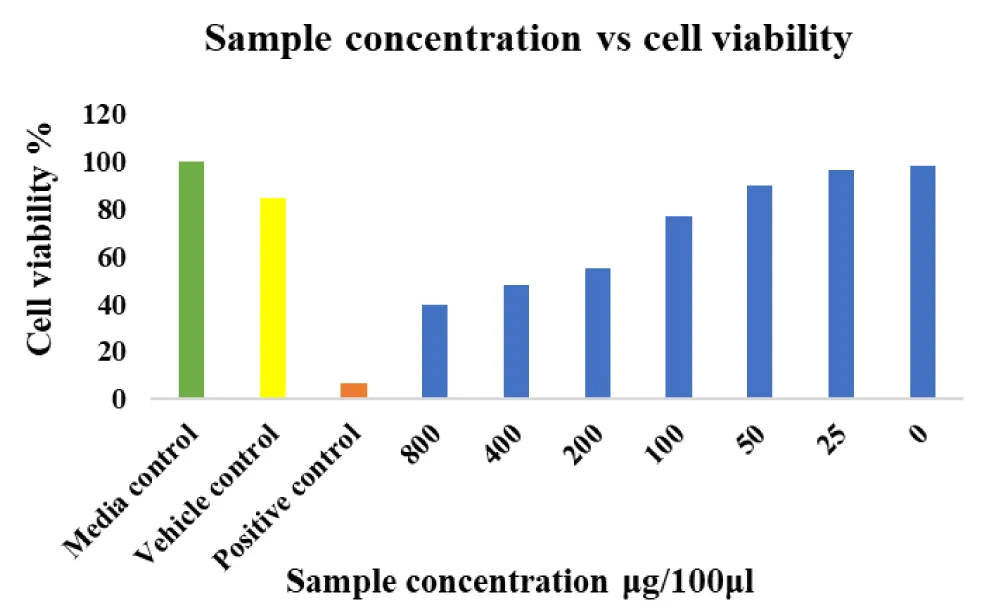
MIC value: The minimal dilution of Fucoidan that gave a visible zone of inhibition was considered as its MIC at a dilution of 156 mg/ml according to doubling dilution. The effective concentration was followed by the determination of cell viability-MTT Assay. (Stroma Lifesciences Pvt Ltd). The test sample induced 50% cell death in Human fibroblast cells at a concentration of 204.5 μg
Cell viability was determined using the MTT Assay
(3- [4, 5-dimethylthiazol-2-yl]-2,5diphenyltetrazolium bromide). Cells were seeded in a fibronectin-coated 24 x 12 well plate for cultivation at 37 °C. After 48 h, 20 mL of MTT 5g/L (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) was added to each well at a final concentration of 0.5mg/ml after 4 hours of shock wave treatment. Cells were cultured in a 24 x 12 well plate to 70% confluency and treated with shock wave pulses as described above. Cells were incubated in MTT medium for 3 hours at 37 °C in the dark, and formazan crystals were solubilized using 50% Dimethyl Sulfoxide (DMSO). Absorbance was read at 570 nm using Eppendorf Bio-spectrometer with DMSO as reference. The test sample induced 50% cell death in Human fibroblast cells at a concentration 204.5 μg (Graph 1).
Discussion
Treatment of chronic inflammatory diseases, associated with dysbiosis and immune-inflammatory reactions is a challenging field in contemporary medicine. Adverse effects of drugs and Anti-microbial Resistance (AMR) have shifted the focus towards natural remedies through herbal medication. Herbal preventive approaches are gaining popularity using effective and safe doses of Homeopathy. Fucoidan, a sulphated polysaccharides obtained from Sargassum wightii has shown to have immunomodulatory and anti-inflammatory effects [18].
Rani V, et al. (2017) in their study on ‘Extraction of Fucoidan yield, influences species, and geographical location of Gulf of Mannar’ concluded that more Fucoidan was obtained from S.wightii and T. conoides during their reproductive stages. Their study demonstrated that differences in chemical structure play a role in the yield of Fucoidan from brown seaweed, which varies with season, species, and method of extraction. In our study, we have obtained Fucoidan through Ethanolic extract of Sargassum wightii. S.wightii is the best source of phytochemicals with anti-oxidant properties, which improves anti-bacterial activity too. Rajivgandhi, et al. 2021 have demonstrated that S.wightii possesses potential antioxidant and anti-cholinesterase activity along with a wide range of biological activities [18].
Eshwar S, et al. (2023) evaluated the ‘Osteogenic potential of Fucoidan with chitosan as hydrogel in treatment of periodontal intra-bony defects [15]. We have evaluated the concentration of Fucoidan through the detection of Minimum inhibitory concentration (MIC) and investigated its Cytotoxic effect through this in-vitro study. Fang CY, et al. (2022) in their in-vitro study confirmed Fucoidan-mediated inhibition of fibrotic properties via MEG-3/miR-181a/Egr1 axis in oral submucous fibrosis. They investigated through Cell culture, Cell viability, Cell migration assay, RNA Isolation, Sequencing, and RT-qPCR [19]. We have investigated the concentration of Fucoidan through Cell viability assay.
In the present study, ethanolic extracts of different dilutions of Fucoidan were evaluated against anaerobic microorganisms to determine the antibacterial activity. Previously, same study design has been applied for detection of periodontal pathogens [20]. Our study showed insignificant values for dilution of Fucoidan at concentrations of 0.78 mg/ml, 0.39 mg/ml, 0.19 mg/ml, 0.09 mg/ml, 0.04 mg/ml, and 0.02 mg/ml.
Our study is also not without any limitations. We had procured fewer GCF samples for Minimum Inhibitory Concentration (MIC) of Fucoidan. As we had to estimate the inhibitory concentration of Fucoidan in anaerobic culture, we used GCF samples with Fucoidan dilutions to check the MIC. No ethical clearance was obtained for this study. We have used Gingival Crevicular Fluid (GCF) samples at periodontal disease sites instead of plaque samples to provide MIC. As Fucoidan has multi-faceted properties [15,21,22], it holds promise for the treatment of multi-factorial periodontal disease.
Conclusion
Sulphated polysaccharides like Fucoidan possess antibacterial and cytotoxic properties. The biological effects depend heavily upon the source species, extraction method, and purity. Comprehensive studies and animal research are required to validate the in-vivo effectiveness of Sargassum wightii extract as herbal antibacterial agent for periodontal therapy. The current study aims to use Fucoidan extract, which has antimicrobial properties by inhibiting anaerobic microorganisms. Hence, Fucoidan can be used as herbal extract as adjunct to periodontal therapy.
- Newman MG, Takei H, Klokkevold PR, Carranza FA. Carranza's clinical periodontology. Elsevier Health Sciences; 2011. Available from: https://books.google.co.in/books/about/Carranza_s_Clinical_Periodontology.html?id=BspTzxVK6-kC&redir_esc=y
- Hashim NT, Babiker R, Padmanabhan V, Ahmed AT, Chaitanya NC, Mohammed R, et al. The Global Burden of Periodontal Disease: A Narrative Review on Unveiling Socioeconomic and Health Challenges. Int J Environ Res Public Health. 2025;22(4):624. Available from: https://doi.org/10.3390/ijerph22040624
- Salvi GE, Roccuzzo A, Imber JC, Stähli A, Klinge B, Lang NP. Clinical periodontal diagnosis. Periodontol 2000. 2023. Available from: https://doi.org/10.1111/prd.12487
- Hashim NT, Babiker R, Rahman MM, Mohamed R, Priya SP, Chaitanya NC, et al. Natural bioactive compounds in the management of periodontal diseases: a comprehensive review. Molecules. 2024;29(13):3044. Available from: https://doi.org/10.3390/molecules29133044
- Khamkar AD, Motghare VM, Deshpande R. Ethnopharmacology—a novel approach for drug discovery. Indian J Pharm Pharmacol. 2015;2(Suppl 4):222–225. Available from: http://dx.doi.org/10.5958/2393-9087.2015.00007.2
- Gupta S, Abu-Ghannam N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci Technol. 2011;22(6):315–326. Available from: http://dx.doi.org/10.1016/j.tifs.2011.03.011
- El-Beltagi HS, Mohamed AA, Mohamed HI, Ramadan KM, Barqawi AA, Mansour AT. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar Drugs. 2022;20(6):342. Available from: https://doi.org/10.3390/md20060342
- Phull AR, Ali A, Dhong KR, Zia M, Mahajan PG, Park HJ. Synthesis, characterization, anticancer activity assessment and apoptosis signaling of fucoidan mediated copper oxide nanoparticles. Arab J Chem. 2021;14(8):103250. Available from: https://doi.org/10.1016/j.arabjc.2021.103250
- Qi Y, Wang L, You Y, Sun X, Wen C, Fu Y, et al. Preparation of low-molecular-weight fucoidan with anticoagulant activity by photocatalytic degradation method. Foods. 2022;11(6):822. Available from: https://doi.org/10.3390/foods11060822
- Zhao X, Guo F, Hu J, Zhang L, Xue C, Zhang Z, et al. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb Res. 2016;144:46–52. Available from: https://doi.org/10.1016/j.thromres.2016.03.008
- Balasubramanian A, Muniyappan S, Ramalingam K. In-silico antiviral screening of native seaweed fucoidan and its derivatives against dengue virus-2 RNA-dependent RNA polymerase (RdRp). Mater Today Proc. 2023. Available from: http://dx.doi.org/10.1016/j.matpr.2023.07.014
- Luthuli S, Wu S, Cheng Y, Zheng X, Wu M, Tong H. Therapeutic effects of fucoidan: A review on recent studies. Mar Drugs. 2019;17(9):487. Available from: https://doi.org/10.3390/md17090487
- Eluvakkal T, Murugan M, Arunkumar K. Extraction of antibacterial substances, galactofucoidan and alginate successively from the Gulf of Mannar brown seaweed Sargassum wightii Greville ex J. Agardh. Indian J Nat Prod Resour. 2014;5(3):249–257. Available from: http://op.niscair.res.in/index.php/IJNPR/article/view/3634
- Magesh KT, Aravindhan R, Kumar MS, Sivachandran A. Antibacterial efficacy of the extract of Sargassum wightii against oral pathogen− an in vitro study. J Orofac Sci. 2020;12(2):96–100. Available from: https://journals.lww.com/joro/fulltext/2020/12020/antibacterial_efficacy_of_the_extract_of_sargassum.6.aspx
- Eshwar S, Konuganti K, Manvi S, Bharadwaj AN, Sajjan S, Boregowda SS, et al. Evaluation of osteogenic potential of fucoidan containing chitosan hydrogel in the treatment of periodontal intra-bony defects—a randomized clinical trial. Gels. 2023;9(7):573. Available from: https://doi.org/10.3390/gels9070573
- Bibi T, Khurshid Z, Rehman A, Imran E, Srivastava KC, Shrivastava D. Gingival crevicular fluid (GCF): a diagnostic tool for the detection of periodontal health and diseases. Molecules. 2021;26(5):1208. Available from: https://doi.org/10.3390/molecules26051208
- da Costa LF, Amaral CD, da Silva Barbirato D, Leão AT, Fogacci MF. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: A meta-analysis. J Am Dent Assoc. 2017;148(5):308–318. Available from: https://doi.org/10.1016/j.adaj.2017.01.021
- Afreen AB, Rasool F, Fatima M. Bioactive properties of brown seaweed, Sargassum wightii and its nutritional, therapeutic potential and health benefits: A review. J Environ Biol. 2023;44(2):146–158. Available from: http://dx.doi.org/10.22438/jeb/44/2/MRN-5081
- Fang CY, Chen SH, Huang CC, Liao YW, Chao SC, Yu CC. Fucoidan-mediated inhibition of fibrotic properties in oral submucous fibrosis via the MEG3/miR-181a/Egr1 axis. Pharmaceuticals. 2022;15(7):833. Available from: https://doi.org/10.3390/ph15070833
- Nagraj BK, Koregol AC, Sulakod K, Patil K, Gore S. Antibacterial efficacies of brown seaweed Sargassum wightii on periodontal pathogens: An in vitro microbiological analysis. J Ayurveda Integr Med Sci. 2022;7(11):52–8. Available from: https://doi.org/10.21760/jaims.7.11.9
- Pal A, Kamthania MC, Kumar A. Bioactive compounds and properties of seaweeds—a review. Open Access Libr J. 2014;1(4):1–7. Available from: http://dx.doi.org/10.4236/oalib.1100752
- Sarithakumari CH, Renju GL, Kurup GM. Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology. 2013;21:261–8. Available from: https://doi.org/10.1007/s10787-012-0159-z
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
