Journal of Biology and Medicine
A New Horizon in Biocompatible Membrane: The Role of Collagen and Silk Fibroin in Tissue Regeneration-Case series
Jyoti T Naik1, Rudrakshi C2* and M L V Prabhuji3
2Reader, Department of Periodontology, Krishnadevaraya Dental College Bangaluru, 562157, India
3Prof and Head, Department of Periodontology, Krishnadevaraya Dental College Bangaluru, 562157, India
Cite this as
Naik JT, Rudrakshi C, Prabhuji MLV. A New Horizon in Biocompatible Membrane: The Role of Collagen and Silk Fibroin in Tissue Regeneration-Case series. J Biol Med. 2024;8(1):018-024. Available from: 10.17352/jbm.000043Copyright License
© 2024 Naik JT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Silk extracted from the silkworm is prized for its exceptional biological and mechanical qualities for its versatility in multiple fields as a biomaterial, most prominently showcased in bone tissue engineering scaffolding. In recent times, silk protein-derived biomaterials have been utilized as scaffolds for guiding bone regeneration. Bovine collagen is selected to enhance the biological function of silk fibroin, as a composite membrane. Thus, considering all these characteristics, we have fabricated silk fibroin into a collagen membrane for evaluation. Therefore, we have a cross-linked collagen membrane with silk fibroin for soft tissue augmentation.
Materials and methods: We have fabricated a silk fibroin-collagen membrane, a composite membrane using lyphophilization to form a fibroin/collagen scaffold for the evaluation of gingival tissue thickness. A total of 10 patients with Stage II/III, Grade B periodontitis received this composite membrane. Patients were re-evaluated at 6 and 12 weeks for clinical parameters of soft tissue gingival thickness, keratinized tissue width, and wound healing. Values obtained were statistically analyzed with p - value ≤ 0.05.
Results: The addition of silk fibroin to collagen membrane for augmentation of ridge defects, infrabony defects, socket augmentation, and in immediate implant placement for soft tissue augmentation resulted in statistically significant improvement of clinical parameters.
Conclusion: Soft tissue augmentation using a composite membrane with silk fibroin demonstrated marginal but statistically significant changes in gingival phenotype. In various oral soft tissue defects. This composite membrane shows a promising alternative to connective tissue graft.
Introduction
Periodontal disease is an inflammatory and infectious disease affecting the tooth-supporting structures leading to tooth loss when left untreated [1].The detrimental effect of periodontitis results in soft tissue and hard tissue loss. Periodontal regeneration deals with the reconstruction of injured and lost tooth-supporting tissues i.e. cementum, periodontal ligament, and alveolar bone or tissues in such a way that the architecture and function are restored to normal. Collagen is a biocompatible natural polymer that has a good function to promote new tissue formation [2]. When it comes to naturally occurring polymers, silk stands out as the strongest and most durable of them all. Silk produced by silkworms and orb-weaving spiders exhibits remarkable mechanical characteristics, along with environmental resilience, biocompatibility, controlled proteolytic biodegradability, morphological flexibility, and the capacity for modifying amino acid side chains to immobilize growth factors [3]. Bovine collagen is selected to enhance the biological function of silk fibroin, as a composite membrane [4]. Thus, considering all these characteristics, this green material [5] is incorporated into the collagen membrane for evaluation. As there is no literature assessing this composite membrane, in this study we evaluated of Cross-linked collagen membrane with Silk fibroin for soft tissue augmentation.
Materials and methods
Patients from the outpatient department of periodontology, Krishnadevaraya College of Dental Science and Hospital, satisfying the inclusion and exclusion criteria were included in this study.
This case report of clinical trials has been done in Krishnadevaraya College of Dental Sciences and Hospital, Hunsmaranahalli, Bangalore. The study was conducted in accordance with the declaration of ethical principles of the World Medical Association Declaration of Helsinki, version VI, 2002. The present study was approved by the institutional review board and ethical committee and has been registered in “Clinical Trials.gov” with identity number NCT06552065.
Preparation
Bovine Collagen membrane Type-1*1 and Silk Fibroin*2 are commercially available. The composite membrane was prepared by incorporating silk fibroin in a cross-linked collagen membrane at Fibroheal Woundcare Pvt Limited. Newtown Yelahanka Bengaluru 560064.
----------------------------
1FIX GUIDE- Fibroheal woundcare Pvt Limited. Newtown Yelahanka Bengaluru 560064
2FIBROHEAL PRODUCTS- - Fibroheal woundcare Pvt Limited. Newtown Yelahanka Bengaluru 560064.
Composite membrane incorporating collagen and silk fibroin
Preparation of hydrogel collagen gel was added to the fibroin solution to create fibroin/collagen blends in water. In this study, the weight percentage of collagen in the dried blend hydrogel was fixed at 20%. In fibroin solution, the collagen gel dissolved when heated to 40 0C with gentle stirring.
At 60 0C, all of the collagen gel denatured and dissolved in the fibroin solution, forming a translucent fibroin/collagen solution in the process. The collagen and fibroin aqueous solution was concentrated at 50 0C – 60 0C while being gently stirred until the fibroin concentration reached 3.4% - 5%. After the mix solution had cooled to room temperature, different components of Ethylene Diamine Chloride (EDC) were added and the pH value was adjusted to 4 - 5 by adding 1 mol/L sodium hydroxide. The fibroin/collagen hydrogel was immediately freeze-dried using a lyophilizer. The freeze-dried scaffolds were cleaned with 1 M NaCl and 0.1 M Na2HPO4 (pH 9.1), in that order. The matrices were then freeze-dried once more after being cleaned with distilled water. To create fibroin/collagen scaffolds devoid of EDC, the fibroin/collagen solution was immediately freeze-dried using a lyophilizer once the pH value was brought to 4-5 [6].
Sterilization and storage
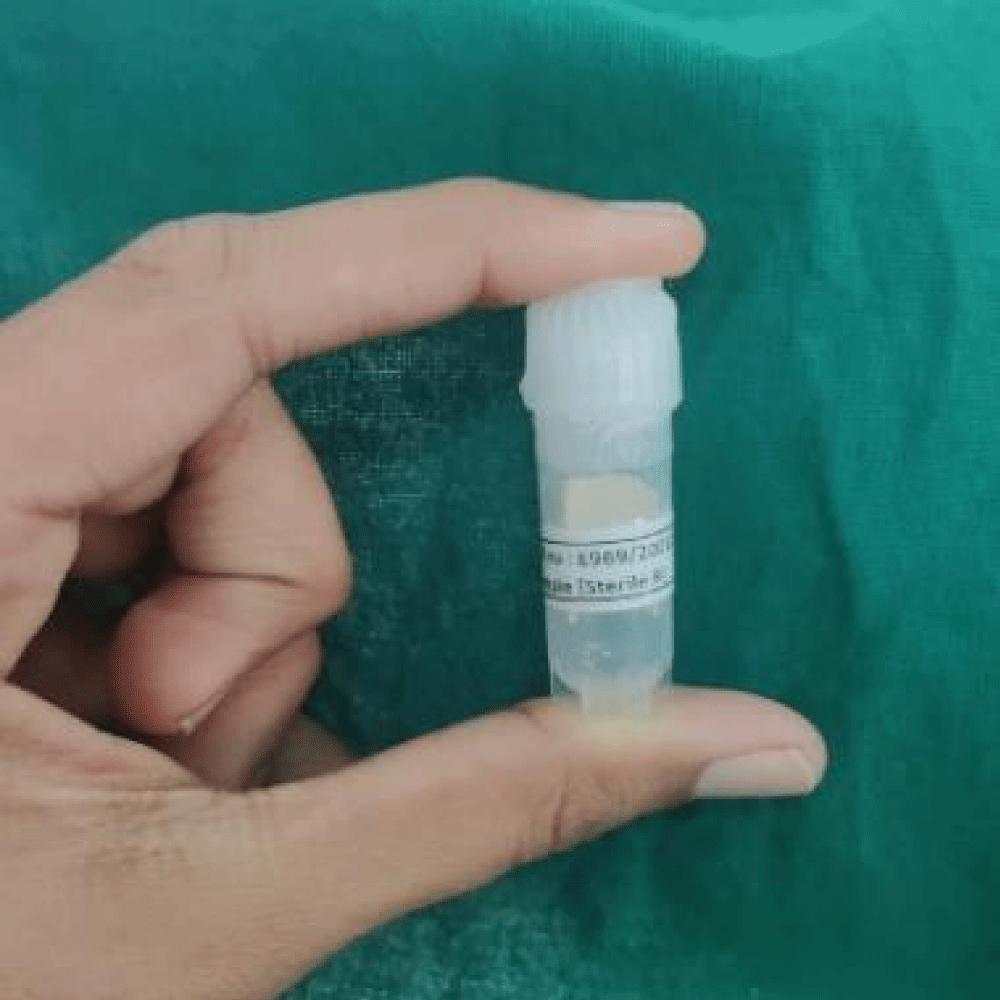
The membrane was prepared and was in 15 x 30 mm. Following it was sterilized using UV radiation and provided in sealed Ependorff packages (Figures 1,2). Packages were opened during the intervention.
Case definition
According to the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions, patients with Stage II /III and Grade B Periodontitis with various periodontal defects (infrabony defect, horizontal/vertical bone loss, fresh extraction socket, etc.) were included for the study.
A total of 10 patients received Cross-linked Collagen Membrane with Silk Fibroin for soft tissue augmentation of the defects.
Inclusion criteria
- Systemically healthy patients in the age group of 18 to 55 years.
- Stage II /Stage III and Grade B Periodontitis patients.
- Patients undergoing horizontal augmentation, treatment of infrabony defects, and immediate dental implant placement.
- Radiographic assessment using Radiovisiography (RVG) with grid.
Exclusion criteria
- Medical conditions contraindicating surgical interventions.
- Patients who are known smokers and alcoholics.
- Acute infections at the site of extraction. Ex Abscess.
- Patients who have undergone radiotherapy or chemotherapy.
Clinical parameters
- Gingival thickness was assessed using a digital caliper.
- Keratinized tissue width was measured from the mucogingival junction to the free gingival margin using a UNC 15 probe.
Sample size estimation
The effect size of this study was determined as previously described. Since the study was to be conducted with 2 groups, it was determined that the number of in-groups should be at least n = 10 and a total of n = 20 individuals were required. The effect size level of the study was 1.35, a = 0.05 for significance, and calculated by 81% power analysis.
Study design
- Informed consent was obtained from all selected patients.
- After the screening examination, patients were enrolled for the trial. Patients who underwent routine scaling, root planning, and oral hygiene instructions were emphasized and recalled for flap surgery.
- Detailed medical, and dental history and blood investigations (complete blood count) were taken.
- Gingival thickness and keratinized tissue width were recorded before the procedure and this was considered as baseline.
Pre-surgical procedure
- All patients were evaluated by prior clinical history and clinical and radiographic examination.
- Phase 1 therapy was carried out for all patients.
- Gingival thickness was measured using a digital calliper and Keratinized width using a UNC15 probe at the pre-determined defect site.
These measurements were recorded at baseline, 6 weeks, and at completion of 12 weeks.
Surgical procedure
- Pre-procedural chlorhexidine mouth rinse was used prior to the procedure.
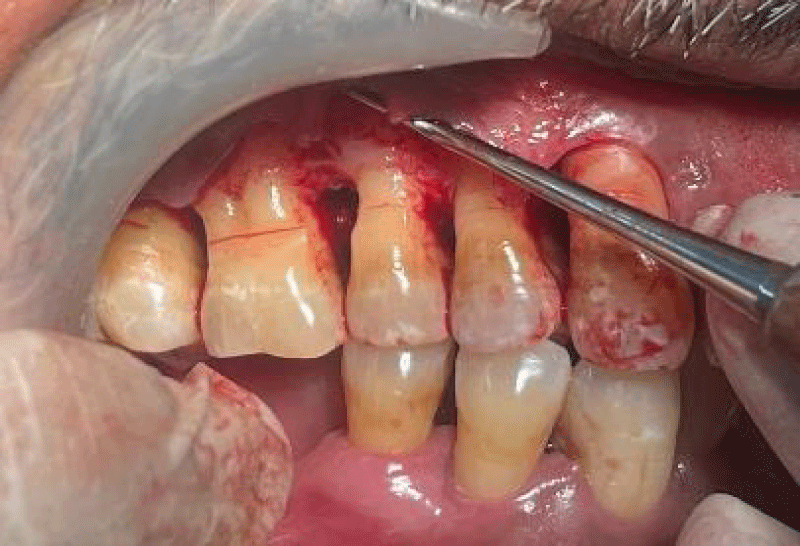
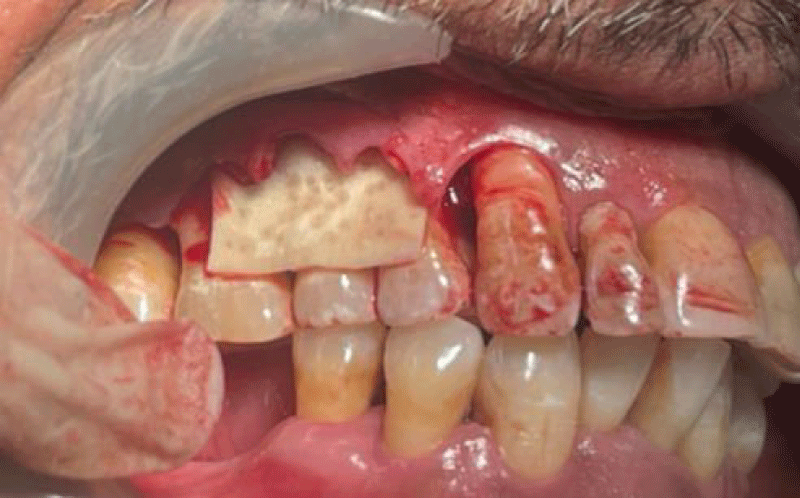
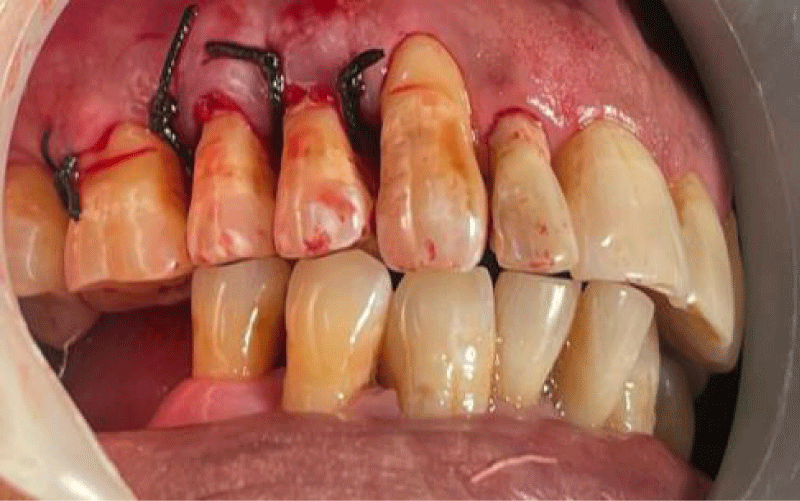
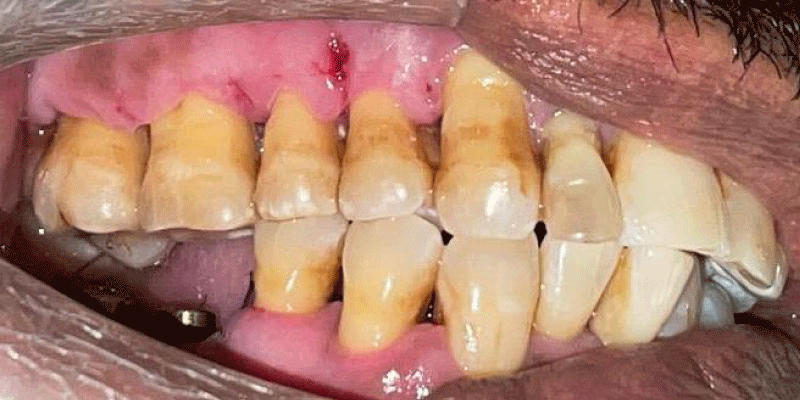
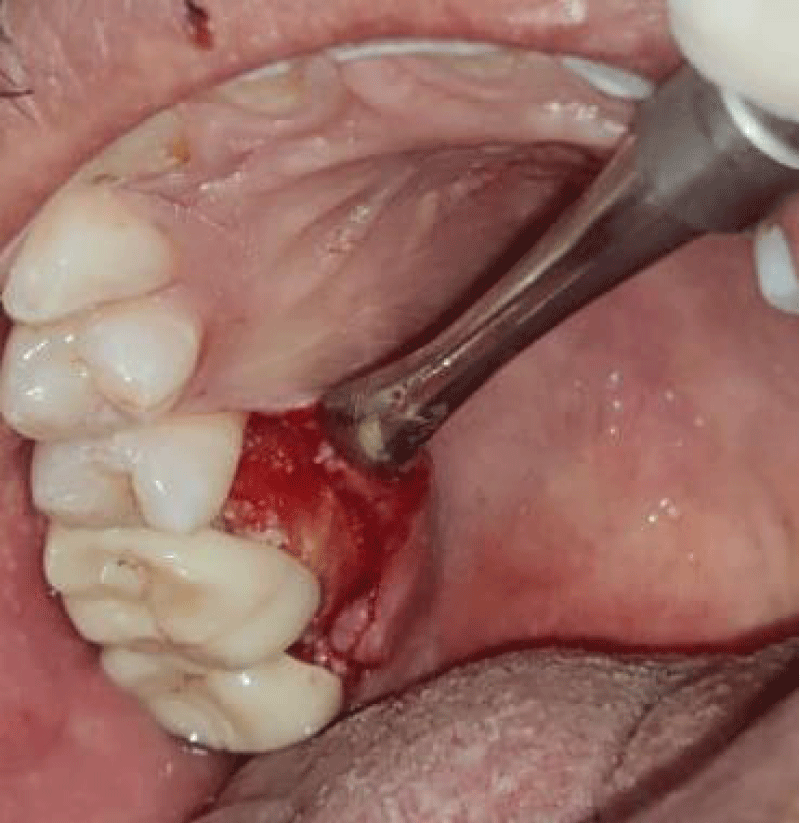
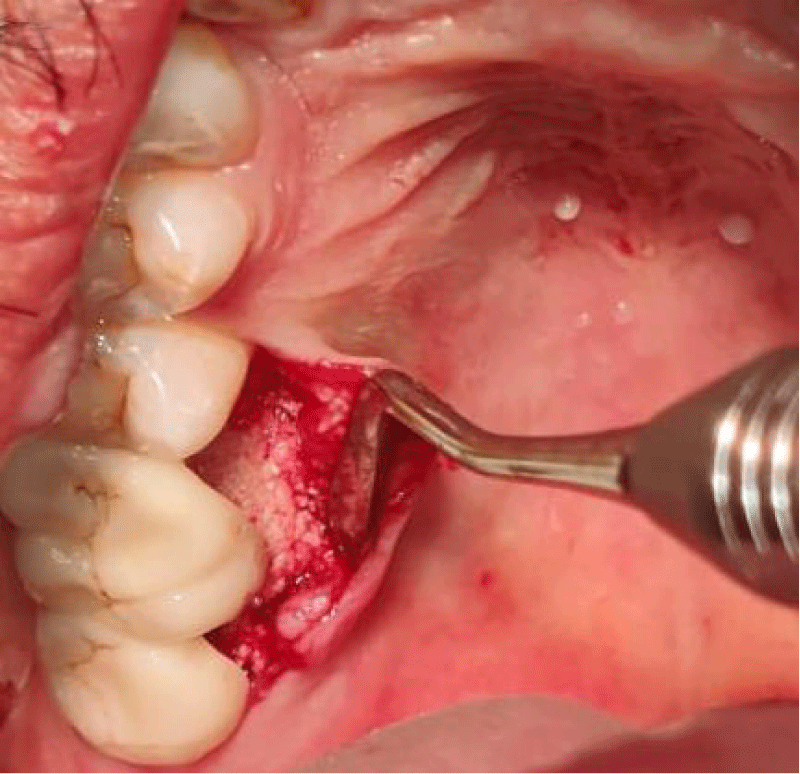
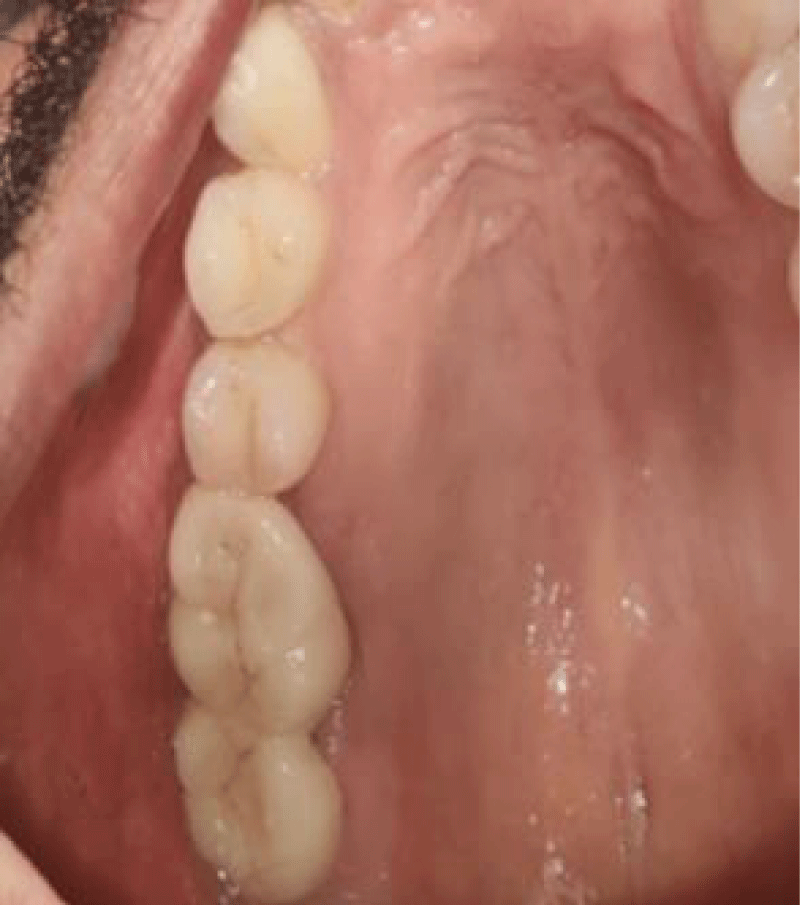
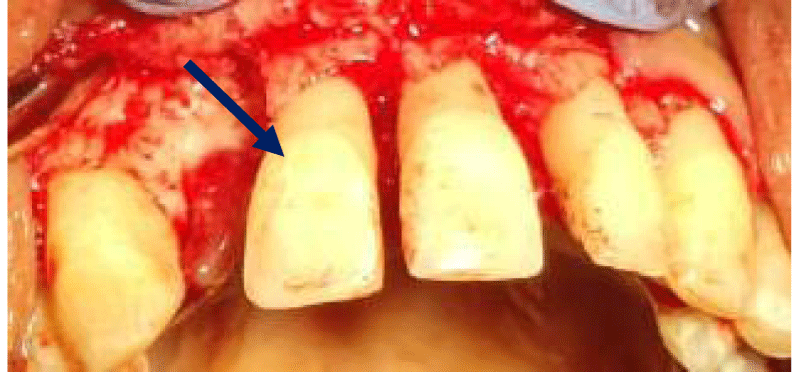
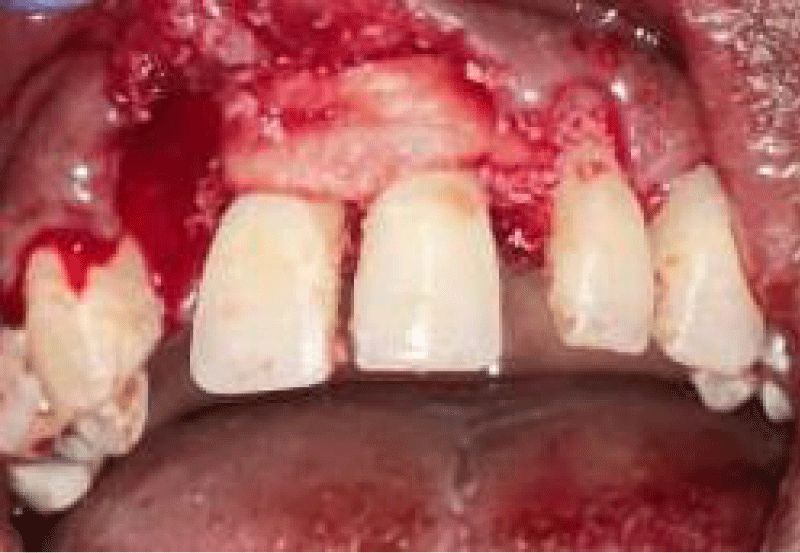
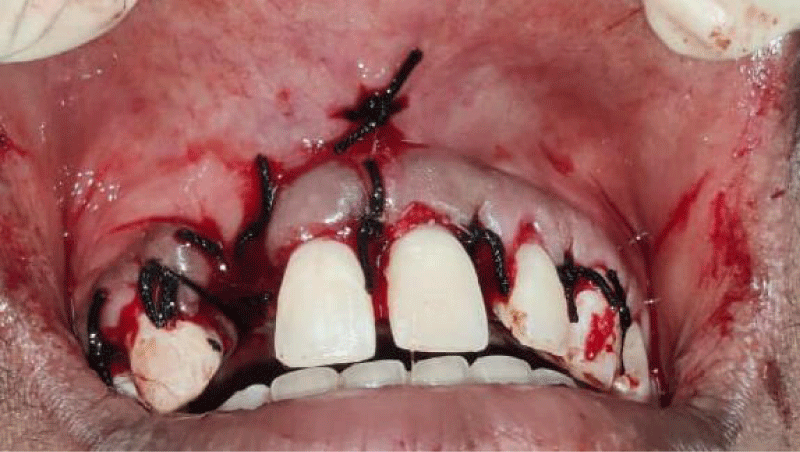
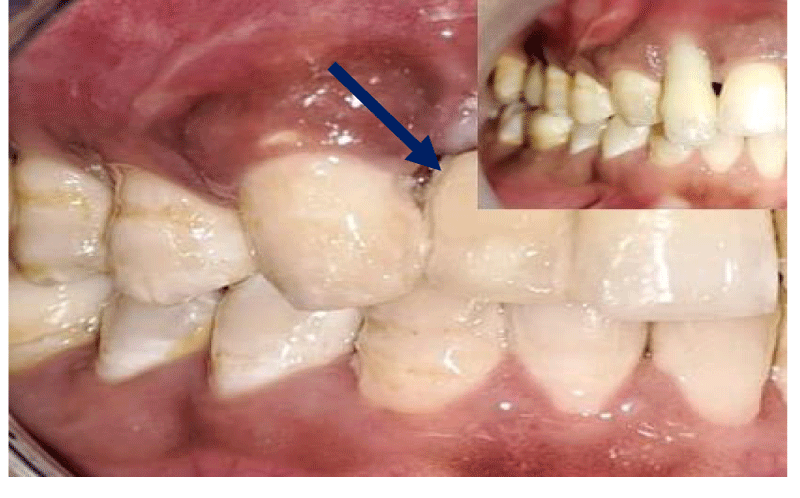
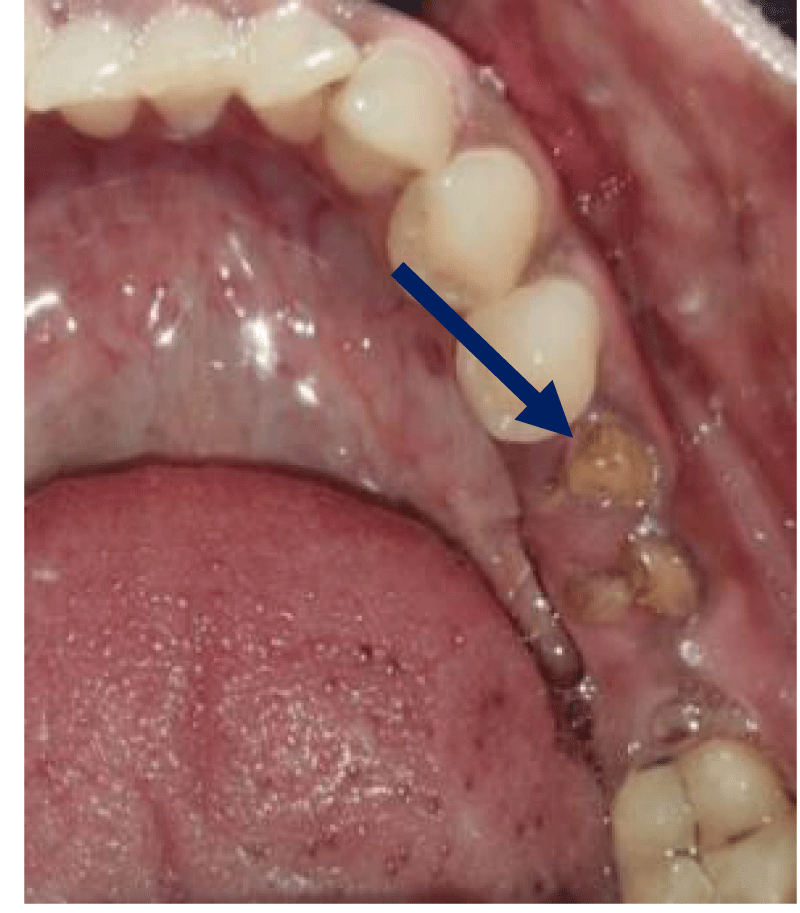
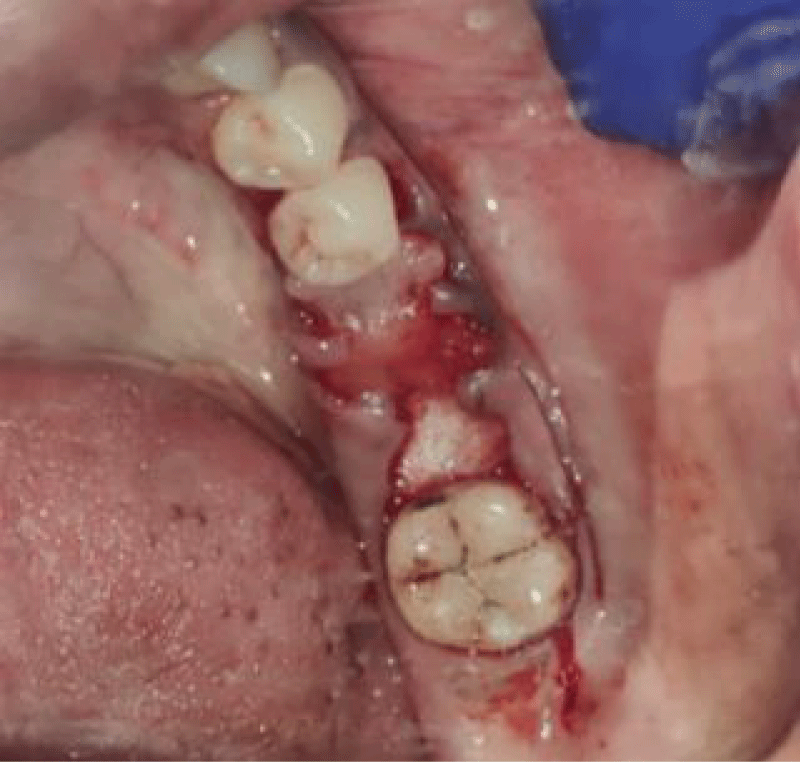
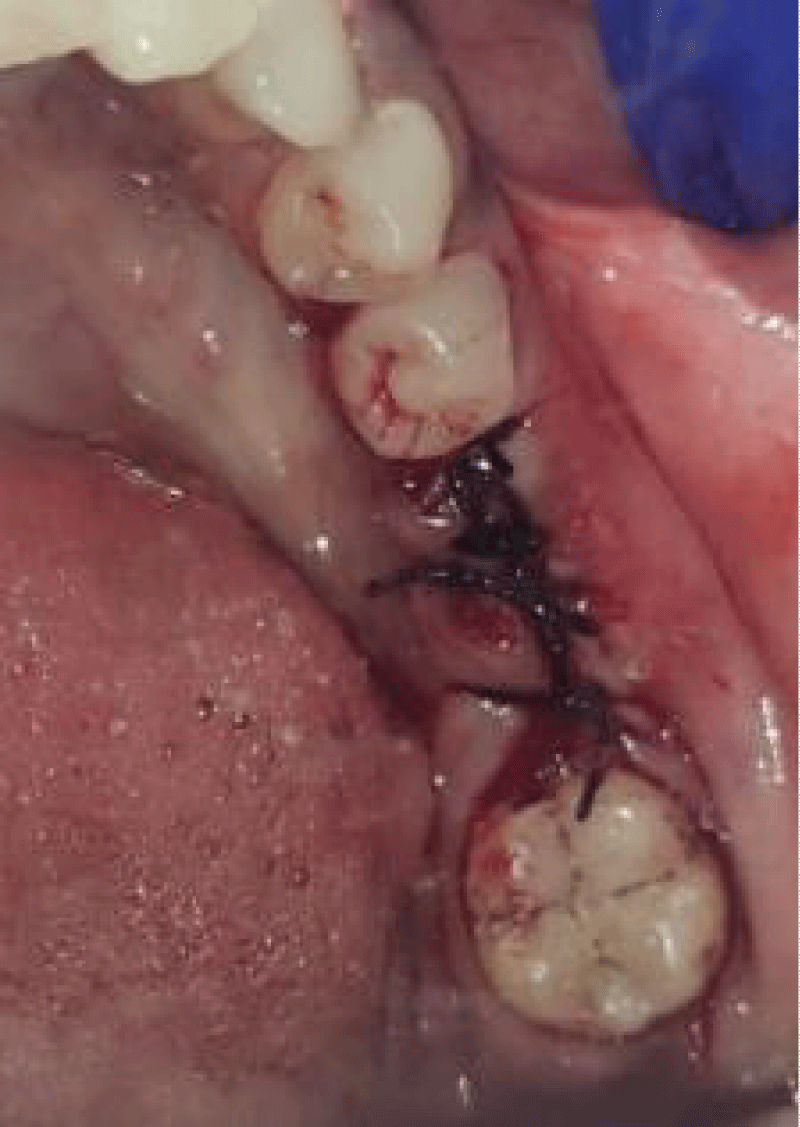
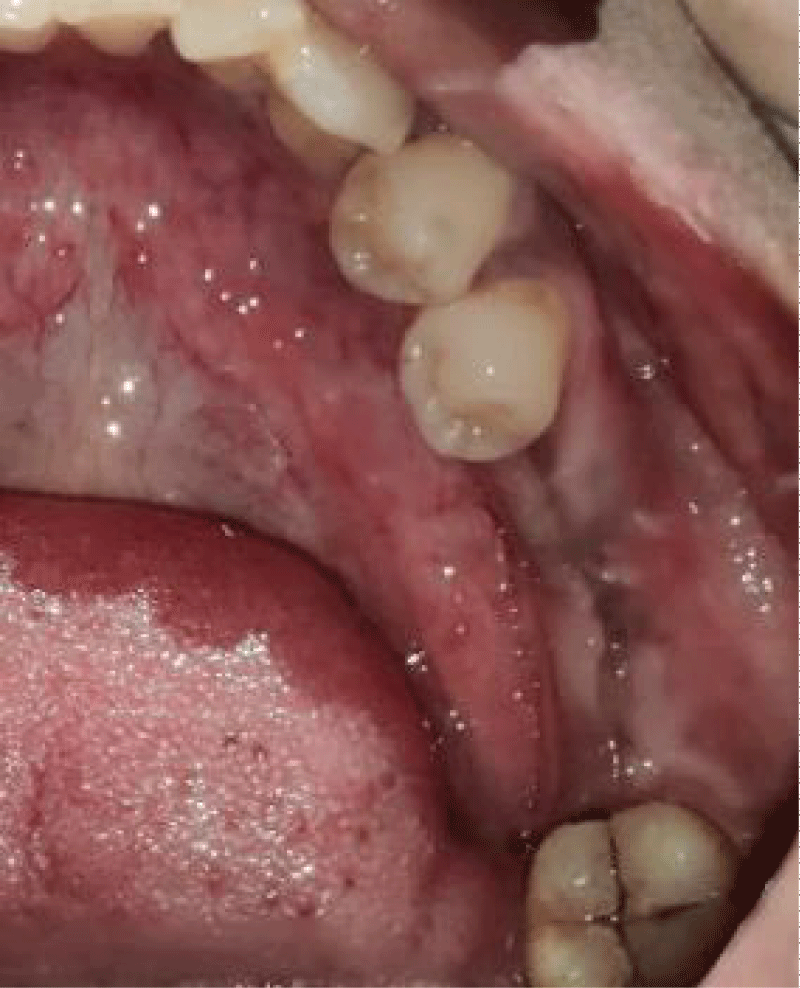
- Local anesthesia was injected, an incision was made with a 15 mm blade, and a mucoperiosteal flap was elevated with a periosteal elevator for cases with horizontal/vertical (2 cases) Figures 3-6 and Figures 7-10, with infrabony defects (2 cases) Figures 11-14 and during immediate implant placement. (4 cases) Figures 15-18.
- Thorough debridement of inflamed granulation tissue was done.
- A cross-linked collagen membrane with silk fibroin was randomly allocated to the area with gingival thickness ≤ 1 mm.
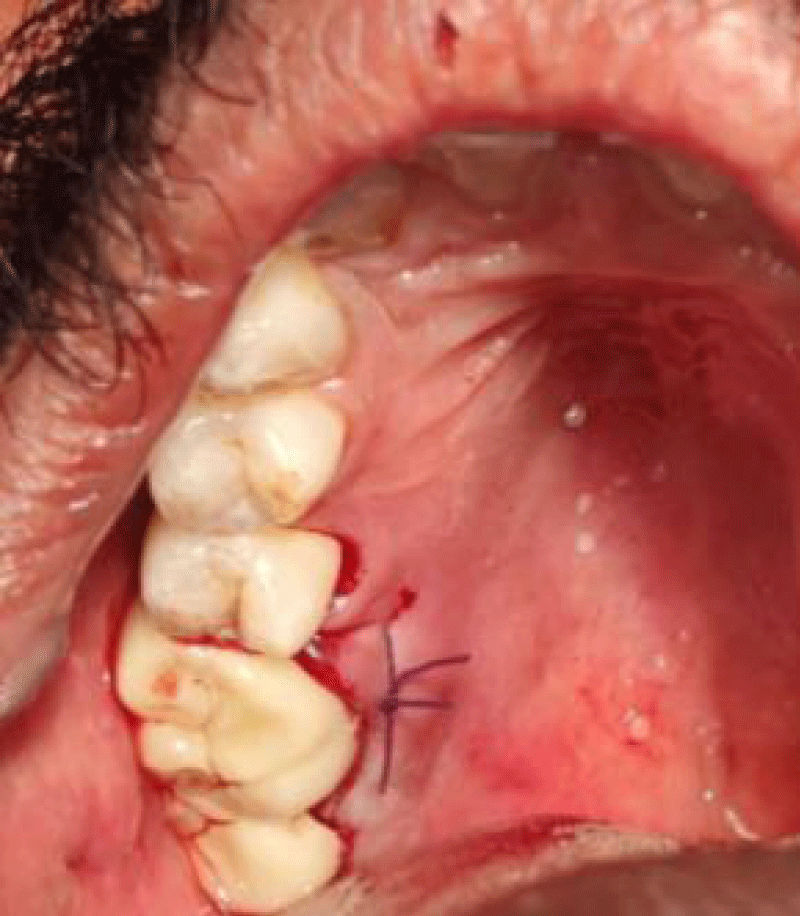
- Suture was placed by approximating flaps, followed by periodontal dressing.
Case series
Horizontal augmentation (Figures 3-6)
Vertical augmentation (Figures 7-10)
Immediate implant placement (Figures 11-14)
Socket augmentation (Figures 15-18)
Postoperative care
- Post-operative instructions consist of gargling with saline and betadine mouth rinse 2 times daily for 1 minute and avoidance of brushing at the surgical site for 2 weeks.
- Postoperative pain and edema were controlled with non-steroidal anti-inflammatory drugs and antibiotics.
- Patients were advised to consume only soft and warm food during the first week.
- After 10 days, periodontal dressing and sutures were removed and the surgical site was evaluated with Landry Turnbull and Howley Healing Index and was considered as the baseline value.
- Patients were later recalled for reinforcement of oral hygiene instructions and supra-gingival debridement as maintenance care. Clinical parameters were recorded for wound healing at the end of the first week, 6th week, and 12th week after the surgical procedure.
- Patients were assessed for gingival tissue thickness and keratinized tissue width in the 6th week and in the 12th week following membrane placement.
- Patients were assessed at baseline and at the 12th week for radiographic changes using RVG with the grid using a grid with marking of 1mm2.
Results
In the present study, a collagen membrane with silk fibroin was placed in periodontal defects. Clinical parameters like gingival tissue thickness using an endodontic reamer with a stopper measured through a digital caliper, keratinized tissue width measured using a UNC 15 probe, and the Landry Turnbull and Howley Healing Index were used for wound healing assessment. Pre-operative measurements were recorded at baseline before intervention. Following surgical protocol for placement of composite membrane with post-operative care, patients were recalled at 6 weeks and 12 weeks for re-evaluation and measurement of clinical parameters.
Values recorded were subjected to statistical analysis with p-value ≤ 0.05. Friedman test and Wilcoxon signed-ranked test, both non-parametric test was used to compare repeated measurements.
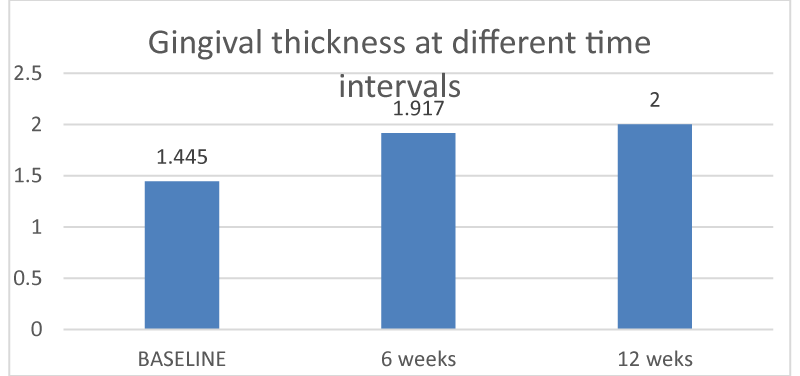
Cases treated with composite membranes demonstrated statistically significant improvement. This was evident through significant improvement in gingival tissue thickness, (Graph I). There was statistically very significant improvement in augmented gingival tissue at 12 weeks (p value - 0.006) when compared to baseline and 6 weeks (p value - 0.005).
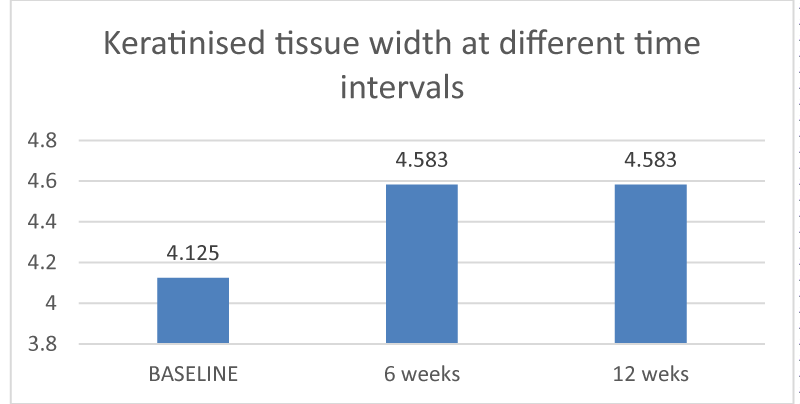
Keratinized tissue width was measured from marginal gingiva to mucogingival junction using a UNC 15 probe at baseline, 6 weeks, and 12 weeks. There was an increase in the keratinized tissue width from baseline to 6 weeks (p -value of 0.005) and 12 weeks. (p - value of 0.005) The difference in the mean values observed was statistically significant. Between the three time intervals, the difference observed between the baseline and 6 weeks and between baseline and 12 weeks was statistically significant (Graph II).
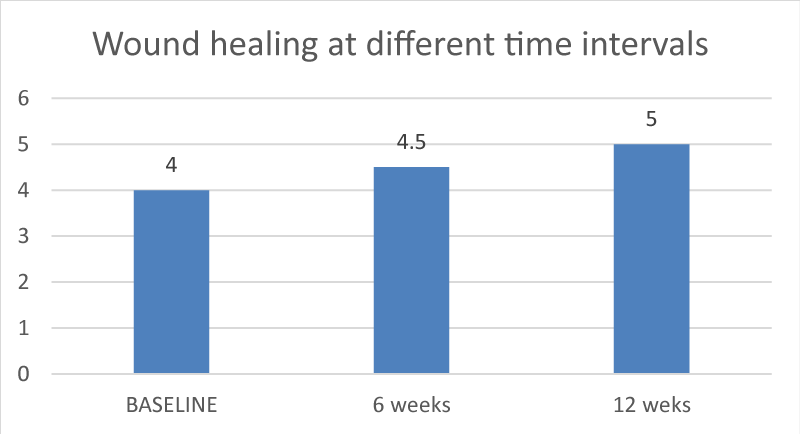
Assessment of wound healing on the 10th day following intervention was considered as baseline. Re-evaluation of healing was assessed at 6 weeks and 12 weeks. There was an increase in wound healing index scores from baseline to 12 weeks (p value of 0.001). The difference in the mean values observed was statistically highly significant (Graph III). Comparison between 6 weeks (p - value of 0.004) and 12 weeks (p - value of 0.046) were statistically significant.
Discussion
Silk Fibroin has been used because of its unique set of properties which include biocompatibility, low immunogenicity, biodegradability, high water and oxygen uptake, robust mechanical properties, and cost-effectiveness. Silk Fibroin is used in various forms like films, sponges, hydrogels, electrospun mats, and tubes [7]. With recent knowledge about the green material, silk fibroin, this study is the first of its kind to investigate the efficacy of silk fibroin with collagen membrane for soft tissue augmentation.
Evaluating the preparation of the membrane, Zheng, et al. in their study showed that silk fibroin with tannic acid mediated modification to prepare silk fibroin-based Guided Tissue Regeneration (GTR) membrane with better mechanical performance and osteogenic function [8]. Compared to that, in our study, we used silk fibroin membrane prepared using ethylene Diamine Chloride showed significant improvement in soft tissue augmentation.
Silk Fibroin (SF) has been widely used as wound dressings due to its good biocompatibility. In the Shao et al study, Silver particles were added to Silk fibroin to enhance the antibacterial properties of the dressings [9] which is in contrast to our study where type I collagen is added to enhance the biological properties of Silk fibroin.
Silk Fibroin (SF), which is a protein-based bio-macromolecule, proved to be an important biomaterial in biomedical applications and tissue engineering. In their study mainly they focused on investigating the effectiveness of antibiotics with silk fibroin against the common pathogens found at the site of burn wounds [10]. Contrary to their study, we have used type I collagen to silk fibroin to improve healing efficacy. Hasatsri, et al. reported reduced healing time in wounds treated with Silk Fibroin dressings (11 ± 6 days) when compared to wounds treated with Paraffin Gauze (14 ± 6 days) [11]. Similar results were reported by Zhang et al. in their study, with a mean healing time of 9.86 ± 1.79 days for partial thickness wounds treated with Silk Fibroin films. Both studies reported faster rates of wound healing when compared to control groups, which is comparable to the findings of our study.
Zhang, et al. [12] reported that the Silk Fibroin membrane provided coverage of the sensitive nerve endings present in the wound surface area, thus contributing to the pain relief reported by patients. The lack of an inflammatory response to silk fibroin has also been cited as a factor influencing pain relief. These findings are comparable to our study demonstrating better wound healing in the silk fibroin membrane group. Contrary to our study, Schulz, et al. [13] found no significant difference in the levels of pain reported by patients treated with Silk Fibroin dressings and other dressings during their study period.
In the clinical trial by Shulz, et al [13] only minor inflammatory reactions to silk dressings were reported, while other studies described no adverse reactions. In this study, no local inflammatory or allergic reactions to silk fibroin were noted. This finding reinforces the fact that Silk Fibroin is a clinically safe biomaterial with minimal antigenic potential.
Wessing B, Urban I, et al. [14] in their study compared the clinical performance of a resorbable non-cross-linked collagen membrane with a bioguide for the treatment of dehisced implant sites using Guided Bone Regeneration (GBR). This study evaluates bone augmentation results, soft tissue healing, and implant survival rate. Their results show bone gain to support implant placement in expected dehiscence defects. In our study, we used cross-linked collagen with silk-fibroin membrane during vertical soft tissue augmentation around the immediate implant. We have evaluated the wound healing pattern, soft tissue thickness, and keratinized tissue width unlike their study evaluating bone regeneration.
Researchers have investigated silk fibroin as a natural biomaterial to be utilized in bone tissue engineering scaffolding. Silk Fibroin (SF) is a natural polymer that can cause inflammation when implanted in the body. To avoid this, low molecular SF powder is used as a bone graft scaffold [15]. In studies, low molecular SF powder with platelet-rich fibrin has shown promising osteogenic properties, increasing the expression of osteogenic genes and promoting bone regeneration in rabbit models. We have restricted the advantages of silk fibroin for soft tissue augmentation. Longitudinal studies evaluating the effect of silk fibroin on bone regeneration may pave the way for more cost-effective outcomes.
Conclusion
Silk fibroin expands the family of regenerative barrier membranes with an enhanced performance for soft tissue regeneration in the field of dentistry. By considering all the characteristics of cross-linked collagen membrane with silk fibroin it can be considered as a good substitute for connective tissue graft. It can thus be considered a better material for periodontal regeneration with further longitudinal clinical trials for tissue augmentation.
- Sankar AR, Gujjari SK. The treatment of intrabony defect using silk fibroin as GTR xenograft–a case report: Silk Fibroin as GTR in Intrabony Defect. Indian J Clin Res Dent. 2020;16(1):5-9. Available from: https://ijcrd.in/index.php/clinicaldentistry/article/view/8
- Neto AM, Sartoretto SC, Duarte IM, Resende RF, Neves Novellino Alves AT, Mourão CF, et al. In vivo comparative evaluation of biocompatibility and biodegradation of bovine and porcine collagen membranes. Membranes. 2020;10(12):423. Available from: https://doi.org/10.3390/membranes10120423
- Pripatnanont P, Chankum C, Meesane J, Thonglam J. Physical and biological performances of a semi-resorbable barrier membrane based on silk fibroin-glycerol-fish collagen material for guided bone regeneration. J Biomater Appl. 2021;36(5):930-42. Available from: https://doi.org/10.1177/08853282211025781
- Sbricoli L, Guazzo R, Annunziata M, Gobbato L, Bressan E, Nastri L. Selection of collagen membranes for bone regeneration: A literature review. Materials. 2020;13(3):786. Available from: https://doi.org/10.3390/ma13030786
- Lujerdean C, Baci GM, Cucu AA, Dezmirean DS. The contribution of silk fibroin in biomedical engineering. Insects. 2022;13(3):286. Available from: https://doi.org/10.3390/insects13030286
- Lin XL, Gao LL, Li RX, Cheng W, Zhang CQ, Zhang XZ. Mechanical property and biocompatibility of silk fibroin–collagen type II composite membrane. Mater Sci Eng. 2019;105:110018. Available from: https://doi.org/10.1016/j.msec.2019.110018
- Aditya NK, Lakshmi S. The use of a silk fibroin-based dressing on full thickness facial skin wounds: A randomized controlled clinical trial. RGUHS J Dent Sci. 2023;15(4). Available from: https://doi.org/10.26463/rjds.15_4_14
- Zheng X, Ke X, Yu P, Wang D, Pan S, Yang J, et al. A facile strategy to construct silk fibroin based GTR membranes with appropriate mechanical performance and enhanced osteogenic capacity. J Mater Chem B. 2020;8(45):10407-10415. Available from: https://pubs.rsc.org/en/content/articlelanding/2020/tb/d0tb01962c
- Shao J, Cui Y, Liang Y, Liu H, Ma B, Ge S. Unilateral silver-loaded silk fibroin difunctional membranes as antibacterial wound dressings. ACS Omega. 2021;6(27):17555–17565. Available from: https://doi.org/10.1021/acsomega.1c02035
- Yerra A, Mamatha DM. Antibiotic-based silk fibroin films for burn wound healing. Polym Adv Technol. 2021;32(2):861–871. Available from: https://doi.org/10.1002/pat.5137
- Hasatsri S, Angspatt A, Aramwit P. Randomized clinical trial of the innovative bilayered wound dressing made of silk and gelatin: Safety and efficacy tests using a split-thickness skin graft model. Evid Based Complement Alternat Med. 2015:1-8. Available from: https://doi.org/10.1155/2015/206871
- Zhang W, Chen L, Chen J, Wang L, Gui X, Ran J, et al. Wound healing: Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv Healthc Mater. 2017;6(10):PMID: 28337854. Available from: https://doi.org/10.1002/adhm.201700121
- Schulz A, Depner C, Lefering R, Kricheldorff J, Kästner S, Fuchs PC, et al. A prospective clinical trial comparing Biobrane® Dressilk® and PolyMem® dressings on partial-thickness skin graft donor sites. Burns. 2016;42(2):345-55. Available from: https://doi.org/10.1016/j.burns.2014.12.016
- Wessing B, Urban I, Montero E, Zechner W, Hof M, Alandez Chamorro J, et al. A multicenter randomized controlled clinical trial using a new resorbable non‐cross‐linked collagen membrane for guided bone regeneration at dehisced single implant sites: Interim results of a bone augmentation procedure. Clin Oral Implants Res. 2017;28(11):218-226. Available from: https://doi.org/10.1111/clr.12995
- Kim JY, Yang BE, Ahn JH, Park SO, Shim HW. Comparable efficacy of silk fibroin with the collagen membranes for guided bone regeneration in rat calvarial defects. J Adv Prosthodont. 2014;6(6):539-46. Available from: https://doi.org/10.4047/jap.2014.6.6.539
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
