Journal of Biology and Medicine
Reviewing the Bio-Applications of SrAl2O4:Eu2+, Dy3+ Phosphor
Maryam Mollazadeh-Bajestani1, AmirHossein Bahmanpour2, Maryam Ghaffari3, Fathollah Moztarzadeh3 Azadeh Sepahvandi4* and Korebami Adebajo5
2Biomaterial Group, Biomedical Engineering Department, University of North Carolina at Charlott, Charlott, NC, USA
3Biomaterial Group, Faculty of Biomedical Engineering (Center of Excellence), Amirkabir University of Technology, Tehran, Iran
4Biomaterials and Tissue Engineering Laboratory, Department of Mechanical Engineering, University of South Carolina, Columbia, SC 29208, USA
5Mechanical Engineering, College of Engineering and Computing, University of South Carolina
Cite this as
Mollazadeh-Bajestani M, Bahmanpour A, Ghaffari M, Moztarzadeh F, Sepahvandi A, et al. (2023) Reviewing the Bio-Applications of SrAl2O4:Eu2+, Dy3+ Phosphor. J Biol Med 7(1): 044-052. DOI: 10.17352/jbm.000040Copyright License
© 2023 Mollazadeh-Bajestani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Strontium aluminate (SrAl2O4) phosphor nanoparticles with Eu2+, and Dy3+ co-doping exhibit high brightness and long afterglow properties, storing light energy and glowing slowly under different conditions. It has been widely studied that SrAl2O4:Eu2+, Dy3+ (SAO) phosphor nanoparticles with a green visible emission can penetrate deep into the tissue, show low self-fluorescence, cause minimal light damage, and are biocompatible. SAO phosphor nanoparticle synthesis and modification mechanisms are outlined in this review. Biological therapies, in addition to the detection of substances in organisms, are provided by these excellent priorities. Despite the existing research, it has been demonstrated that nanostructures of SAO luminescence particles have great potential to be applied to tissue engineering and drug delivery systems. The current scientific achievements can provide a reference for research in the areas mentioned above, as well as stimulate biomedical disciplines to pay attention to SAO luminescence nanoparticles.
Introduction
Phosphorescent substances with Eu2+ activated alkaline earth aluminates are extensively identified for his or her quantum efficiency in the seen area [1]. Primarily based on the photoluminescence emission spectrum of SrAl2O4:Eu2+, a green top exists at 520 nm due to the parity-allowed 5d1-4f7 transition of Eu2+. Dy3+ ion is used on this inexperienced phosphor to lengthen the afterglow period by way of increasing electron traps and trap depths close to Eu2+ [2]. In evaluation to sulfide phosphorescent phosphors, SAO phosphor has a safe, chemically strong, bright, and long-lasting photoluminescence without radiation [3-9], which leads to a vast range of optoelectronic materials and devices utilized in biomedicine.
The development of clinical technology has brought about numerous chemical synthesis techniques to prepare SrAl2O4 and/or its phosphors, including co-precipitation [10], sol-gel [11], and combustion synthesis strategies [12]. Each of these techniques was carried out in liquid levels to ensure correct control and uniform combination of every element. Processing workouts to put together precursor powders for sol-gel or co-precipitation techniques is complicated and time-consuming. Despite the fact that the combustion technique could be very clean and take only some minutes, the precursor powders have been substantially used to put together various nano-scale oxide substances [13].
Rare earth factors have the ability to transform NIR mild into UV and visible light through up-conversion luminescence [14]. because of their unique characteristic of emitting mild at lengthy wavelengths and having a nanoscale shape [15,16], SAO luminescence nanoparticles have been implemented in a ramification of biomedical programs, which include pH, ion, and biomolecule detectors, bioimaging, and photodynamic, photothermal, drug shipping, tissue engineering, and multimode remedies. Research on SAO luminescence nanoparticles and their application in biomedicine has turned out to be a hot topic in recent years [17]. We will discuss the development of SAO luminescence nanoparticles in organic applications in this assessment. First, we talk about the mechanism of SAO luminescence in rare-earth-based total nanoparticles, as well as their synthesis and surface modification. 2nd, we offer an in-depth evaluation of their packages in Photodynamic Remedy (PDT), bioimaging, and biosensors. The implications of SAO luminescence nanoparticles in tissue regeneration and drug transport systems could be mentioned in the final section of the item.
Luminescence mechanism and synthesis of SAO nano particles
Although the exploration of the luminescence mechanisms of Carbon Dots (CDs) has been a research hotspot in recent years, it is still in the stage of ambiguity due to the diversity of carbon sources, the complex structure of CDs, and different preparation methods. At present, four kinds of luminescence mechanisms have been in use: carbon core-controlled luminescence, surface-controlled luminescence, quantum size effect, and crosslink-enhanced emission effect. Synthesis of CDs is the controlled synthesis of NIR fluorescent CDs and has become one of the bottlenecks restricting the applications of CDs in biological imaging and tumor therapy. The reported synthesis methods of NIR fluorescent CDs can be divided into two categories: “top-down” and “bottom-up”. The “top-down” method refers to the decomposition of large-volume carbon sources into nanosized CDs by means of light, electricity, heat, and chemistry, including arc discharge, electrolysis, and acid etching [18].
The luminescent properties of europium-doped alkaline earth aluminates have attracted much attention since Palilla, et al. [19] published their first work. Upon exposure to UV light, this ceramic phosphor exhibits a greenish emission peaking at 520 nm associated with the parity-allowed transition 4f6 5d→4f7 of Eu2+. The auxiliary activator role of Dy3+ in SAO was discovered by Maruyama, et al. [20] Its enhanced brightness and long-lasting properties remain stable at room temperature for hours, even overnight. As a result of this fact, as well as the excellent chemical stability of these materials, as well as their non-toxic, non-radioactive, and biocompatible properties, these phosphors have achieved unprecedented success in luminescent biomaterial applications. Among strontium aluminates and silicates doped with Eu2+ or other rare earth ions, the SAO phosphor exhibits the longest-lasting after-glowing properties [21,22]. A thermally driven solid-state reaction of Al2O3 and SrCO3 is conventionally used to synthesize rare earth-activated strontium aluminates. In this process, a temperature above 1250 C is necessary because Sr3Al2O6-based pigments quench their photoluminescence at this temperature. To control the valence of the activator, a reducing atmosphere is required (in this case Eu2+ is needed instead of Eu3+). Sol-gel, hydrothermal, and co-precipitation methods have been used to synthesize SAO nanoparticles. It is possible to precisely control particle size, morphology, and luminescent properties using these methods [23]. By altering the dopant concentration and synthesis conditions, SAO nanoparticles can be tailored for specific emission wavelengths, intensities, and stability [20,24].
The liquid phases were used for all of these methods so that each component could be controlled and mixed uniformly. Precursor powders must be prepared through complex processing routines for sol-gel or co-precipitation techniques. Precursor powders are, however, easily prepared using the combustion process, which only takes a few minutes and has been extensively applied to nano-scale oxide materials.
In this synthesis technique, metal nitrates are heated at low igniting temperatures by redox exothermic reactions with urea or other fuels to liberate heat energy. Moreover, the process is fast, safe, and energy-efficient. Thus, combustion synthesis may hold promise for preparing nanosized aluminate phosphors. SAO nanoparticles have rarely been reported [25], although ultrafine SrAl4O4 has been prepared by combustion of metal nitrates with aluminum nitrates and urea mixtures. As nano-scale materials have special properties, combustion synthesis is the most effective way to prepare SAO phosphor nanoparticles. In a weak reductive atmosphere of active carbon, SAO phosphor nanoparticles can be synthesized using combustion synthesis processing along with heating combustion ash precursor powder at 1100 JC. Molecular analysis of the nanometer phosphors revealed a pure monoclinic SAO phase, with particle sizes ranging between 15 nm and 45 nm [13,26].
Surface modification of SAO nano particles
SAO nanoparticles can be modified by altering their surface properties or characteristics in order to enhance their performance or enable specific functions. Coating, ligand exchange, doping, surface functionalization, and surface roughening are the most common surface modification techniques for SAO Nano Particles. Nanoparticle composition, size, and targeted modifications should all be considered when selecting appropriate techniques. A surface modification approach was decided according to the properties and capability required with the aid of nanoparticles and their intended biomedical applications.
Eu2+, Dy3+ in the form of long-lasting phosphorescence co-doped strontium aluminates phosphor and it has been explained by Makishawa, et al. [27] and Yamamoto, et al. [28] by the holes thermally released from the trap levels of Dy3+, which have the optimal trap depth in room temperature. Alternatively, Dorenbos [29,30] proposed a model involving conduction band states of Eu2+ and subsequent trapping by Dy3+.
SAO phosphor was synthesized and studied by Peng’s group [31]. It’s miles recognized that doping phosphorescent materials with unique ions can regulate their luminescent homes, especially in biomedical applications where biocompatibility is crucial. A study by Katsumata, et al. [32] characterized the trap levels in long-lasting phosphors of SrAl2O4:Eu2+ doped with Nd, Sm, Gd, Dy, and Y and found that the density and depth of the traps significantly affected the phosphorescence during the long-term [33]. Investigated the long afterglow phosphorescence of SrAl2O4:Eu2+ doped with various rare earth ions as phosphorescent sensor materials and found that the intensity and lifetime of the long afterglow phosphorescence varied depending on the type of doping auxiliary activator used (Y). As a result of trapped carriers, they were dominated by thermal excitation (La, Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu), and they were dominated by thermal excitation. The excitation and emission spectra of Eu3+ and Cu2+ co-doped SrAl2O4 are substantially extraordinary from the ones of SrAl2O4: ecu and SrAl2O4: Cu, according to Yang, et al. [34]. Doping these materials with rare earth ions could strongly influence their phosphorescent properties. As auxiliary activators, Yb3+ ions have been extensively studied. Yb3+ doped SAO phosphors were synthesized by combustion method and their luminescence was measured. Different doping ratios of doping ions can modify the initial intensity of the phosphors and the afterglow time of the phosphors, which is critical to understanding the properties of the phosphors [35,36].
SAO nanoparticle applications
Photodynamic and photothermal therapy: Photodynamic Therapy (PDT) is a non-invasive therapeutic approach that utilizes light-sensitive compounds (photosensitizer) to selectively treat a variety of diseases, including acne, psoriasis, age-related macular degeneration, and several cancers. The primary function of a photosensitizer is to absorb specific wavelengths of light and then transfer that energy to oxygen molecules in the surrounding tissue. This energy transfer generates Reactive Oxygen Species (ROS), primarily singlet oxygen, which can cause localized cell damage and death [37]. Photosensitizers used in PDT offer several advantages and limitations. PDT provides targeted therapy, concentrating on specific tissues like tumors while sparing healthy ones. It’s minimally invasive, with low systemic toxicity, making it suitable for various medical conditions. However, PDT has some limitations. First, most of the photosensitizers for PDT only work with UV–visible light (wavelength < 700 nm), which has poor tissue penetration. Shorter wavelengths, such as ultraviolet and blue light, are absorbed more strongly by biological tissues and have shallower penetration depths. This means that photosensitizers cannot penetrate deep-seated tumors and produce enough ROS to have the desired photodynamic effect. Second, the optimal PDT treatment requires prolonged light exposure, which can damage normal tissues. Third, PDT is less effective in the inherent microenvironmental hypoxia of tumor tissue [38-43]. The advantages and limitations of common photosensitizers in PDT are overviewed in Table 1. To address these limitations, researchers have investigated a variety of strategies like designing carriers for the delivery of photosensitizers, shifting the photosensitizer absorption efficiency towards the infrared, and supplying oxygen for the hypoxic tumor microenvironment [39].
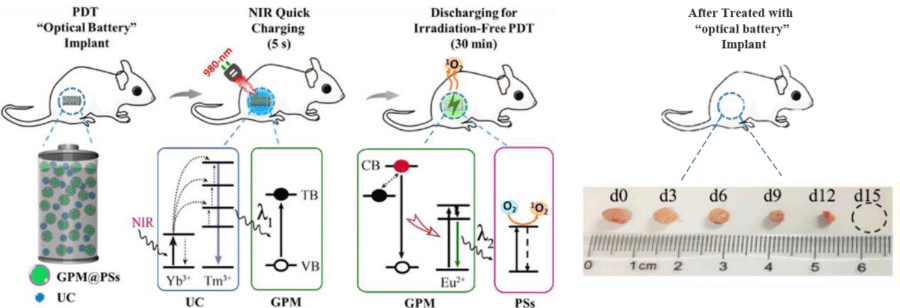
One strategy to enhance the efficacy of photosensitizer is to combine it with Persistent Luminescence Nanoparticles (PLNPs). PLNPs have a special property of emitting light for a long time, from minutes to hours, after being excited. This prolonged luminescence from PLNPs is very beneficial for PDT because it extends the activation time of the photosensitizers without needing more light energy. In addition, this means that PDT can be more localized and controlled, minimizing damage to healthy tissues [40,41]. Lidan Hu, et al. [42] have developed a new type of PDT implant called “optical battery” which is a Near-Infrared (NIR) rechargeable PDT implant for irradiation-free PDT. The implant was fabricated by embedding upconversion materials, green persistent luminescence materials (SrAl2O4: 2%Eu2+, 4%Dy3+), and photosensitizer into Polydimethylsiloxane (PDMS). The implant can be charged quickly with a 980-nm NIR laser for just 5 seconds. After charging, it can produce green persistent luminescence and generate cytotoxic singlet oxygen for continuous irradiation-free PDT for almost 30 minutes without requiring external irradiation. The brightness and fading speed of the persistent luminescence do not change significantly when the charging time is extended from 5 seconds to 30 seconds at a power density of 2 W/cm2. The in vitro results showed the tumor volume greatly decreased after the multiple circulation of NIR irradiation in 15 days (Figure 1). This means that the PLNPs can be fully charged in just 5 seconds of NIR exposure, which minimizes the risk of overheating and cell damage caused by long-term irradiation. X-ray photons have much greater penetration in body tissues. X-ray-activatable PDT could be initiated from virtually any part of a body with high efficiency. The Chen group [44] pioneered in demonstrating in vitro that low energy X-rays can activate a novel integrated nanosystem, which consists of a core made of SrAl2O4: Eu2+ and a silica coating loaded with a photosensitizer, merocyanine 540 for cancer cell destruction. SrAl2O4: Eu2+ is a strong luminescent material that can convert X-ray photons to visible photons. Matching the excitation wavelength of the photosensitizer, the visible photons can activate the photosensitizer, producing cytotoxic O2. SAO also can enhance sensitivity for deep tissue bioimaging in animals through soft X-ray-mediated photon energy charging into it [45]. The long-lasting phosphorescence properties of SAO nanoparticles enable novel PDT modalities with improved therapeutic indices. The exact mechanisms of how SAO nanoparticles store and emit energy are still being investigated. However, their ability to act as an internal light source makes them a promising nanoplatform for advancing PDT.
Photothermal Therapy (PTT) uses photothermal agents and focused NIR light to selectively heat and destroy tumor cells without affecting normal tissues. It provides localized and targeted treatment of cancers. External near-infrared light can only penetrate about 1-2 cm deep into tissues, restricting PTT to superficial tumors. In addition, external light needs to be continuously applied, leading to intermittent PTT. The persistent NIR emission activated by ultrasound provides continuous PTT without on/off external illumination [46]. In a recent study, Zhang, et al. [47] developed ultrasound-chargeable nanomotors that can generate persistent Near-Infrared (NIR) emission for Photothermal Therapy (PTT) and light-triggered Nitric Oxide (NO) release to treat tumors. The nanomotors consist of mesoporous silica nanoparticles decorated with mechanoluminescent SrAl2O4: Eu2+ and persistent luminescent ZnGa2O4: Cr3+ nanodots, and partially coated with NO-loaded polydopamine caps. Ultrasonication activates the mechano-luminescence of SrAl2O4: Eu2+ nanodots, which excites the ZnGa2O4: Cr3+ nanodots to emit persistent NIR. This internal NIR emission heats the polydopamine caps for PTT and triggers NO release. The results showed, that the persistent PTT and NO release synergistically inhibited tumor growth and extended animal survival.
Bioimaging: Bioimaging is a non-invasive technique that allows for real-time visualization of biological functions without the need for invasive tools and minimizing disruption to live processes [48]. Fluorescence imaging is an example of bioimaging which allows real-time imaging and has yielded impressive results. Fluorescence imaging is based on the phenomenon of fluorescence. Certain molecules, known as fluorophores, absorb light at one wavelength (excitation wavelength) and then emit light at a longer wavelength (emission wavelength). This emitted light can be captured and used to create detailed images of biological samples. However, Fluorescence bioimaging presents several challenges that researchers must address to obtain high-quality and reliable results. These challenges include photobleaching, where fluorophores degrade over time, limiting the duration of experiments; phototoxicity, which can harm living cells and tissues with the intense excitation light; autofluorescence, making it difficult to differentiate between specific signals and background fluorescence; and limited imaging depth due to light scattering and absorption in biological samples [49]. Researchers continually work to overcome these challenges through technological advancements and methodological innovations to harness the full potential of fluorescence bioimaging in the field of biology and medicine. Phosphorescent nanoparticles are materials that can store light energy and then release it as light over a long period of time. This property can be used to eliminate short-lived background fluorescence and avoid autofluorescence [50-52]. Meng Sun, et al. [53] use SAO as probes to achieve long-term in vivo imaging. These nanoparticles have persistent luminescence, which means they can emit light for several hours after being excited in vitro. This allows them to be imaged in vivo in real-time after injection, without the need for any external illumination source. In addition, the signal-to-noise ratio is significantly improved because the autofluorescence signals, which can be a source of noise, stop when the excitation is removed. In another study, Calatayud, et al. [54] explored the use of phosphorescent strontium aluminates as potential bioimaging probes. The authors used a commercial Eu2+, Dy3+ -doped Sr4Al14O25 phosphor material as the luminescent core. The phosphor material is a promising candidate for optical imaging because it has size-dependent properties that make it ideal for this application. Additionally, its long-lasting phosphorescence emission allows for imaging well after excitation, which helps to avoid autofluorescence. As mentioned before, the SAO particle is a highly efficient afterglow material. Afterglow materials are more suitable for bioimaging due to their ability to solve the auto-fluorescence issue, which occurs when fluorescent materials within living cells are excited along with the marker [55]. In a recent study, a new approach to synthesizing magnetic and afterglow nanoparticles using a double core-shell strategy was introduced. The method involves combining precipitation and combustion synthesis routes to create bifunctional nanocomposites with unique properties. Specifically, the core of the particles is made up of Fe3O4 nanoparticles, which are coated with a protective SiO2 middle layer. On top of this, an afterglow SAO layer is applied to enhance the nanoparticles’ afterglow properties.
Results showed the bifunctional nanocomposite can potentially be applied for the detection and separation of cells and molecules relevant for diagnosis [56].
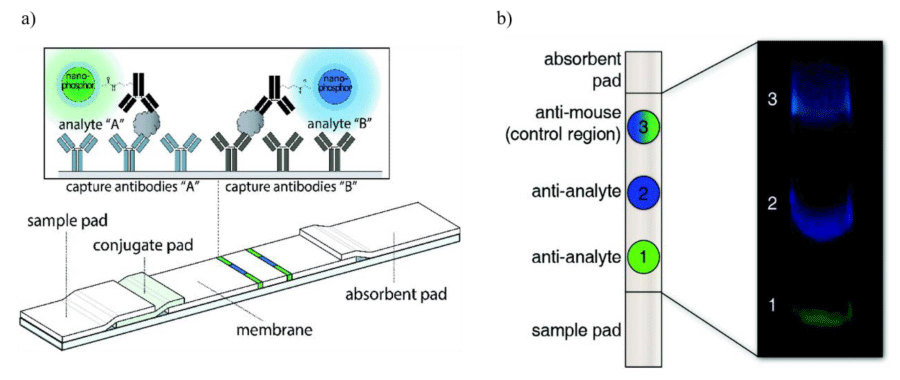
Biosensors: The luminescence of SAO nanoparticles can be harnessed for the development of biosensors. Biosensors are specialized analytical devices that combine a biological component with a physicochemical detector to detect and quantify specific biological molecules or chemical compounds within a sample [57]. The ability of biosensing probes to selectively and sensitively detect target molecules is essential for chemical and biomedical detection. For example, the detection of tumor biomarkers, metabolites, biomolecules, and other signal parameters in living cells is important for disease diagnosis and treatment. PLNPs can be functionalized with specific biomolecules, such as antibodies or DNA probes, to create a highly sensitive and selective sensing platform. These particles can eliminate background noise interferences due to their long-lasting afterglow nature. In particular, NIR-emitting PLNPs have high penetration depth in biological tissues, good photo- and chemical stability, and low toxicity, making them ideal for biosensing applications [58]. Lateral Flow Assays (LFAs), also known as immunochromatographic assays, are a type of diagnostic test commonly used for the rapid and qualitative detection of specific target analytes, such as antigens or antibodies, in a variety of biological samples. To overcome the limitations of conventional fluorescent labels, upconverting phosphors were studied but they showed low quantum yields [59]. Paterson, et al. [60] for the first time suggested using strontium aluminate PLNPs as reporters in LFAs with biotinylated Hen Egg Lysozyme (bHEL) as a model analyte. These PLNPs could emit intense visible light for several minutes after excitation which helps to eliminate the need for optical filters and decrease background autofluorescence. In their recent study [61], they incorporated two persistent PLNPs in a multiplex LFA to simultaneously detect two independent model analytes, Prostate-Specific Antigen (PSA) and human Chorionic Gonadotropin (hCG). The extended emission lifetime of PLNPs means that they don’t require continuous excitation. As a result, they can be imaged using a smartphone-based time-gated imaging system, which allows for the creation of a straightforward, rapid, and cost-effective point-of-need diagnostic method. This method can be used to quickly detect analytes (Figure 2). Jiang, et al. [62] in a novel study, investigated the potential of Mechano-Luminescence (ML) properties of SAO nanoparticles in evaluating the occlusal examination of artificial teeth. The bright and sensitive ML emitted by the artificial tooth models can guide clinicians in adjusting the occlusal surface until a balanced occlusion is established. This can help ensure that the patient has a comfortable and functional bite.
Tissue engineering: Tissue Engineering and Regenerative Medicine (TERM) is a rapidly growing field that merges the biological, material, and engineering sciences to create artificial structures that resemble native tissues or organs. TERM is a promising alternative to traditional organ transplantation, which has several drawbacks, such as limited donor availability, the need for immunosuppression, and the risk of rejection [57,63]. TE is done by combining cells with a scaffold, which is a material that provides a supportive environment for the cells to grow. The cells can then proliferate and differentiate into the desired tissue type. To create scaffolds that are more effective at mimicking the natural Extracellular Matrix (ECM) of tissues, researchers are using a nanoscale approach [64]. The luminescent properties of SAO nanoparticles have garnered attention as potential nanoparticles in cell stimulation and tissue growth, facilitating the development of more efficient tissue engineering strategies. For the first time, our team studied the probability of using SAO in retinal tissue engineering. SAO nanophosphor coated with Polyethylene Glycol (PEG) and then loaded into Chitosan (CS)/Polycaprolactone (PCL) electrospinning scaffold. 30% photoluminescent nanoparticle scaffold showed an optimal combination of emission intensity, wavelength peak at 560 nm, mechanical properties similar to retina tissue, and cell proliferation and differentiation. Differentiation of retinal progenitor cells was enhanced on the scaffolds, especially towards photoreceptor lineages. Gene expression of rhodopsin and MAP2 was upregulated [65]. Later, Cerro [66], et al. used the burn-off technique to fabricate a persistent luminescent amorphous borosilicate scaffold. They added SAO microparticles as a source of luminescence. The results showed Hydroxy Carbonated Apatite (HA) layer precipitates at the surface of the glass particles which is a promising sign of bioactivity.
Drug delivery systems: Nano-delivery systems are a relatively new but rapidly developing science where materials in the nanoscale range are employed to deliver therapeutic agents to specific targeted sites in a controlled manner [67]. Encapsulating bioactive compounds in nanoparticles can help to reduce the frequency of dosing, protect the drug during storage, and control the release of the drug into the body. This can lead to increased bioavailability of the active compound and reduced side effects [68]. PLNPs offer several advantages in drug delivery systems. Firstly, their luminescent properties allow for real-time monitoring of drug release in specific targets, ensuring precise control over dosage and therapeutic efficacy. Additionally, these particles can protect drugs from degradation and provide sustained release, leading to improved treatment outcomes. Researchers loaded PLNPs with anti-tumor drugs to create bioimaging-guided drug delivery systems [69]. Shi, et al. [70] have developed a new type of nanoprobe that can be used for drug delivery and tumor imaging. PLNPs were loaded with drugs and targeted to tumors using folic acid, a molecule that binds to cancer cells.
The potential applicability of the SAO nanoparticles for cancer cell tagging was demonstrated in a study. Breast adenocarcinoma cells have been bio-labeled with FA-SAO nanoparticles and 4′,6-diamidino-2-phenylindole, dihydrochloride for nucleus identification. By measuring the persistent luminescent lifetime process of the internalized SAO nanoparticles without additional staining, it would be possible to apply the proposed combination of light sheet microscopy system and nano phosphor tools to study the cellular dynamics taking advantage of their optical properties [71].
Conclusion
Luminescent materials that exhibit persistent or long-lasting phosphorescence have garnered significant interest in various biomedical applications in recent years. Strontium aluminate co-doped with europium and dysprosium ions (SrAl2O4:Eu2+, Dy3+) is a highly efficient inorganic long persistent phosphor that has shown immense promise for diverse bio-applications. The excellent photostability, low toxicity, and chemical stability of these phosphors make them well-suited for photodynamic therapy, bioimaging, biosensing, tissue engineering, and drug delivery. Surface modification of SAO nanoparticles is often necessary to enhance colloidal stability, allow surface functionalization, and improve biocompatibility and luminescent properties. Photodynamic therapy holds promise for treating diseases with minimal invasiveness. Innovative strategies, such as combining photosensitizers with persistent luminescence nanoparticles, extend PDT activation time, enhancing precision and minimizing damage. The development of a NIR rechargeable implant with the help of SAO demonstrates a significant breakthrough, allowing for nearly 30 minutes of irradiation-free PDT after a brief 5-second charge. Bioimaging, particularly fluorescence imaging, offers a non-invasive means for real-time visualization of biological processes, yet it faces challenges such as photobleaching and autofluorescence. The integration of phosphorescent nanoparticles, with persistent luminescence capabilities, addresses these issues, enabling long-term in vivo imaging without external illumination and improving the signal-to-noise ratio. Recent advancements, including the synthesis of magnetic and afterglow nanoparticles, present promising opportunities for their application in cell detection and molecular separation for diagnostic purposes. The luminescence properties of SAO nanoparticles present a valuable avenue for biosensor development. Functionalized with specific biomolecules, these persistent luminescent nanoparticles offer high sensitivity and selectivity, particularly in near-infrared applications with low toxicity. Their integration into lateral flow assays and innovative uses, such as smartphone-based time-gated imaging and mechano-luminescence for occlusal examination, showcase their potential for creating rapid, cost-effective, and versatile diagnostic methods. Additionally, the integration of SAO nanoparticles into tissue engineering and regenerative medicine presents a novel avenue for enhancing cell stimulation and tissue growth. The use of SAO-coated scaffolds, especially in retinal tissue engineering, demonstrates promising outcomes with optimal luminescent properties, mechanical similarity to native tissue, and improved cell proliferation and differentiation. Furthermore, nano-delivery systems, utilizing persistent luminescent nanoparticles like SAO, present a promising frontier in drug delivery. The luminescent properties of PLNPs enable real-time monitoring of drug release, enhancing precision in dosage and therapeutic efficacy. Moreover, the successful application of SAO nanoparticles in cancer cell tagging demonstrates their potential for advancing both drug delivery systems and cellular dynamics studies in the context of cancer research. While most work has focused on proof-of-concept studies, future research should aim to translate these promising nanomaterials into clinical use. More in-depth investigations on the long-term in vivo behavior and safety are required before human trials can commence. The multi-functionality and versatility of SAO phosphors open doors to creating integrated nanosystems that combine diagnostic imaging, controlled drug release, and light-activated therapies. With additional research and development, these luminescent nanoparticles could make a significant impact in nanomedicine and bio-photonics in the years to come. However, potential toxic effects must always be carefully evaluated when developing any new nanomaterial for human use.
- Blasse G, Brill A. Fluorescence of Eu2+-Activated Alkaline-Earth Aluminates Philips Research Reports. 1968; 23: 201-206. https://pearl-hifi.com/06_Lit_Archive/02_PEARL_Arch/Vol_16/Sec_53/Philips_Rsrch_Reports_1946_thru_1977/Philips%20Research%20Reports-23-1968.pdf
- Kumar A, Kedawat G, Kumar P, Dwivedi J, Gupta BK. Sunlight-activated Eu2+/Dy3+ doped SrAl2O4 water resistant phosphorescent layer for optical displays and defence applications. New J Chem. 2015; 39: 3380-338. https://doi.org/10.1039/C4NJ02333A
- Ito S, Banno S, Suzuki K, Inagaki M. Phase Transition in SrAl2O4. Z Phys Chem Neue Folge.105 (1977): 173-178. https://doi.org/10.1524/zpch.1977.105.3_4.173
- Zhang Y, Chen Z, Zhou Z. Rapid Combustion Synthesis of Light-Storing-Emitting Material SrAl2O4 : Eu2 + , Dy3 + and Its Spectral Characteristics. J Electrochem Soc. 2006;153:H86-H87. https://doi.org/10.1149/1.2170547
- Xu YF, Ma DK, Guan ML, Chen XA, Pan QQ, Huang SM. Controlled synthesis of single-crystal SrAl2O4:Eu2+,Dy3+ nanosheets with long-lasting phosphorescence. J Alloys Compd. 2010; 502: 38-42. http://dx.doi.org/10.1016/j.jallcom.2010.04.186
- Lin Y, Tang Z, Zhang Z. Preparation of long-afterglow Sr Al O -based luminescent 4 14 25 material and its optical properties. Mater Lett. 2001; 51: 14-18. https://doi.org/10.1016/S0167-577X(01)00257-9
- Ntwaeaborwa OM, Nsimama PD, Pitale S, Nagpure IM, Kumar V, Coetsee E, Terblans JJ, Swart HC, Sechogela PT. Photoluminescence properties of SrAl2O4:Eu2+,Dy3+SrAl2O4:Eu2+,Dy3+ thin phosphor films grown by pulsed laser deposition. J Vac Sci Technol A. 2010; 28: 901-905. https://doi.org/10.1116/1.3299255
- Abdul-Allah MH, Chiad SS, Habubi NF. The Effect of Iron Chromate on the Optical Properties of PMMA Films. Diyala Journal for Pure Science. 2010; 6: 161-169. https://www.iasj.net/iasj/download/187123773a726f5f
- Zhang B, Blum FD. Thermogravimetric study of ultrathin PMMA films on silica: effect of tacticity. Thermochim A. 2003; 396: 211-217. https://doi.org/10.1016/S0040-6031(02)00518-X
- Lin Y, Zhang Z, Zhang F, Tang Z, Chen Q. Preparation of the ultrafine SrAl2O4:Eu,Dy needle-like phosphor and its optical properties. Mater Chem Phys. 2000; 65: 103. https://doi.org/10.1016/S0254-0584(00)00222-4
- Kurihara LK, Suib SL. Sol-Gel Synthesis of Ternary Metal Oxides. 1. Synthesis and Characterization of MAI2O4 and Pb2Al2O5. Chem Mater. 1993; 5: 609. https://doi.org/10.1021/cm00029a006
- Kingsley JJ, Suersh K, Patil KC. Combustion synthesis of fine-particle metal aluminates. J Mater Sci. 1990; 25: 1305. https://doi.org/10.1007/BF00585441
- Peng T, Yang H, Pu X, Hu B, Jiang Z, Yan C. Combustion synthesis and photoluminescence of SrAl2O4:Eu,Dy phosphor nanoparticles. Materials Letters. 2004; 58: 352–356. https://doi.org/10.1016/S0167-577X(03)00499-3
- Bai Y, Li Y, Wang R, Li Y. Low Toxicity, High Resolution, and Red Tissue Imaging in the Vivo of Yb/Tm/GZO@SiO2 Core-Shell Upconversion Nanoparticles. ACS Omega. 2020 Mar 2;5(10):5346-5355. doi: 10.1021/acsomega.9b04381. PMID: 32201824; PMCID: PMC7081422.
- Tu L, Xu YL, Ouyang QY. Recent advances on small-molecule fluorophores with emission beyond 1000 nm for better molecular imaging in vivo. Chin Chem Lett. 2019; 30: 1731-1737. https://doi.org/10.1016/j.cclet.2019.05.022
- Zhou H, Xiao YL, Hong XC. New NIR-II dyes without a benzobisthiadiazole core Chin. Chem Lett. 2018; 29: 1425-142. https://doi.org/10.1016/j.cclet.2018.08.009
- Sepahvandi A, Moztarzadeh F. Fabrication and characterization of SrAl2O4: Eu2 + Dy3 +/CS-PCL electrospun nanocomposite scaffold for retinal tissue regeneration. msec.2016.03.028. https://doi.org/10.1016/j.msec.2016.03.028
- Yuqing W, Li X, Zhao S, Wang B, Song X, Xiao J, Lan M. Synthesis strategies, luminescence mechanisms, and biomedical applications of near-infrared fluorescent carbon dots. Coordination Chemistry Reviews. 2022; 470: 214703.
- Palilla FC, Levine AK, Tomkus MR. Fluorescent properties of alkaline earth aluminates of type MAl2O4 activated by divalent europium. J Electrochem Soc 1968; 115: 642–4. https://doi.org/10.1149/1.2411379
- Matsuzawa T, Aoki Y, Takeuchi N, Murayama Y. New long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+,Dy3+. J Electrochem Soc. 1996; 143:2670–3. https://doi.org/10.1149/1.1837067
- Clabau F, Rocquefelte X, Jobic S, Deniard P, Whangbo MH, Garcia A. On the phosphorescence mechanism in SrAl2O4:Eu2+ and its codoped derivatives. Solid State Sci. 2007; 9:608–12. https://doi.org/10.1016/j.solidstatesciences.2007.03.020
- Lakshminarasimhan N, Varadaraju UV. Luminescence and afterglow in Sr2SiO4:Eu2+, RE3+ [RE =Ce, Nd, Sm and Dy] phosphors—role of codopants in search for afterglow. Mater Res Bull 2008; 43: 2946-2953. https://doi.org/10.1016/j.materresbull.2007.12.005
- Song YK, Choi SK, Moon HS, Kim TW, Mho SI, Park HL. Phase studies of SrO–Al2O3 by emission signatures of Eu2+ and Eu3+. Mater Res Bull c. 1997; 32:337-341. https://doi.org/10.1016/S0025-5408(96)00192-4
- Sánchez-Benítez J, de Andrés A, Marchal M, Cordoncillo E, Vallet Regi M, Escribano P. Optical study of SrAl1.7B0.3O4:Eu, R (R= Nd, Dy) pigments with long-lasting phosphorescence for industrial uses. J Solid State Chem. 2003; 171:273–7. https://doi.org/10.1016/S0022-4596(02)00175-5
- Lin Y, Zhang Z, Zhang F, Tang Z, Chen Q. Preparation of the ultrafine SrAl2O4:Eu,Dy needle-like phosphor and its optical properties. Mater. Chem. Phys. 2000; 65: 103. https://doi.org/10.1016/S0254-0584(00)00222-4
- Kingsley JJ, Suersh K, Patil KC. Combustion synthesis of fine-particle metal aluminates. J Mater Sci. 1990; 25: 1305. https://doi.org/10.1007/BF00585441
- Matsuzawa T, Aoki Y, Takeuchi N, Murayama Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2 O 4: Eu2 +, Dy3 +. J Electrochem Soc. 1996; 143: 2670. https://doi.org/10.1149/1.1837067
- Yamamoto H, Matsuzawa T. Mechanism of long phosphorescence of SrAl2O4:Eu2+, Dy3+ and CaAl2O4:Eu2+, Nd3+. J Lumin. 1997; 72-74. 287. https://doi.org/10.1016/S0022-2313(97)00012-4
- Dorenbos P. Mechanism of Persistent Luminescence in Eu2 + and Dy3 + Codoped Aluminate and Silicate Compounds. J Electrochem Soc. 2005; 152: H107. https://doi.org/10.1149/1.1926652
- Dorenbos P. Absolute location of lanthanide energy levels and the performance of phosphors. J Lumin. 2007; 122/123: 315. https://doi.org/10.1016/j.jlumin.2006.01.155
- Peng TY, Yang HP, Pu XL, Hu B, Jiang ZCH, Yan CHH. Combustion synthesis and photoluminescence of SrAl2O4:Eu,Dy phosphor nanoparticles. Mater Lett. 2004; 58(2004): 352. https://doi.org/10.1016/S0167-577X(03)00499-3
- Aizawa H, Katsumata T, Takahashi J, Matsunaga K, Komuro S, Morikawa T. Long afterglow phosphorescent sensor materials for fiber-optic thermometer. Rev Sci Instrum. 2003; 74(3): 1344. https://doi.org/10.1063/1.1540719
- Katsumata T, Toyomane S, Tonegawa A, Kanai Y, Kaneyama U, Shakuno K, Sakai R. Komuro S, Morikawa T. Characterization of trap levels in long-duration phosphor crystals. J Cryst Growth. 2002; 237–239: 361. https://doi.org/10.1016/S0022-0248(01)01942-X
- Yang P, Lu MK, Song CF, Xu D, Yuan DR, Xia GM, Liu SW. Photoluminescence characteristics and mechanism of SrAl2O4 co-doped with Eu3+ and Cu2+. Inorg Chem Commun. 2002; 5: 919. https://doi.org/10.1016/S1387-7003(02)00601-9
- Holsa J, Jungner H, Lastusaari M, Niittykoski J. Persistent luminescence of Eu2+ doped alkaline earth aluminates, MAl2O4:Eu2+. J Alloys Compd. 2001; 323/324: 326. https://doi.org/10.1016/S0925-8388(01)01084-2
- Guo C, Tang Q, Huang D, Zhang C, Su Q. Influence of co-doping different rare earth ions on CaGa2S4: Eu2+, RE3+(RE=Ln) phosphor. J Phys Chem Solids. 2007; 68: 217. https://doi.org/10.1016/j.jpcs.2006.10.013
- Karagianni A, Tsierkezos NG, Prato M, Terrones M, Kordatos KV. Application of carbon-based quantum dots in photodynamic therapy. J Carbon. 2023; 203: 273-310. https://doi.org/10.1016/j.carbon.2022.11.026
- Pivetta TP, Botteon CEA, Ribeiro PA, Marcato PD, Raposo M. Nanoparticle Systems for Cancer Phototherapy. Nano 2021; 11: 3132. https://doi.org/10.3390/nano11113132
- Chen L, Huang J, Li X, Huang M, Zeng S, Zheng J, Peng S, Li S. Progress of Nanomaterials in Photodynamic Therapy Against Tumor. Front Bioeng Biotechnol. 2022; 10: (920162). https://dx.doi.org/10.3389/fbioe.2022.920162
- Escudero A, Carrillo-Carrión C, Castillejos MC, Romero-Ben E, Rosales-Barrios C, Khiar N. Photodynamic therapy: photosensitizers and nanostructures. Mater Chem Front. 2021; 5 (10): (2021) 3788-3812. https://doi.org/10.1039/D0QM00922A
- Chen W, Zhang J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J Nanosci Nanotechnol. 2006 Apr;6(4):1159-66. doi: 10.1166/jnn.2006.327. PMID: 16736782.
- Bessière A, Durand JO, Noûs C. Persistent Luminescence Materials for Deep Photodynamic Therapy. J Nanophotonics. 2021; 10(12):2999-3029. https://dx.doi.org/doi:10.1515/nanoph-2021-0254
- Hu L, Wang P, Zhao M, Liu L, Zhou L, Li B, Albaqami FH, El-Toni AM, Li X, Xie Y, Sun X, Zhang F. Near-infrared rechargeable "optical battery" implant for irradiation-free photodynamic therapy. Biomaterials. 2018 May;163:154-162. doi: 10.1016/j.biomaterials.2018.02.029. Epub 2018 Feb 12. PMID: 29459324.
- Chen H, Wang GD, Chuang YJ, Zhen Z, Chen X, Biddinger P, Hao Z, Liu F, Shen B, Pan Z, Xie J. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett. 2015 Apr 8;15(4):2249-56. doi: 10.1021/nl504044p. Epub 2015 Mar 12. PMID: 25756781; PMCID: PMC5233724.
- Song L, Lin XH, Song XR, Chen S, Chen XF, Li J, Yang HH. Repeatable deep-tissue activation of persistent luminescent nanoparticles by soft X-ray for high sensitivity long-term in vivo bioimaging. Nanoscale. 2017 Feb 23;9(8):2718-2722. doi: 10.1039/c6nr09553d. PMID: 28198899.
- Zhao L, Zhang X, Wang X, Guan X, Zhang W, Ma J. Recent advances in selective photothermal therapy of tumor. J Nanobiotechnology. 2021 Oct 24;19(1):335. doi: 10.1186/s12951-021-01080-3. PMID: 34689765; PMCID: PMC8543909.
- Zhang Z, Yan H, Cao W, Xie S, Ran P, Wei K, Li X. Ultrasound-Chargeable Persistent Luminescence Nanoparticles to Generate Self-Propelled Motion and Photothermal/NO Therapy for Synergistic Tumor Treatment. ACS Nano. 2023 Aug 22;17(16):16089-16106. doi: 10.1021/acsnano.3c04906. Epub 2023 Jul 29. PMID: 37515593.
- Lahoti HS, Jogdand SD. Bioimaging: Evolution, Significance, and Deficit. Cureus. 2022 Sep 8;14(9):e28923. doi: 10.7759/cureus.28923. PMID: 36225412; PMCID: PMC9541884.
- Pratiwi FW, Kuo CW, Chen BC, Chen P. Recent advances in the use of fluorescent nanoparticles for bioimaging. Nanomedicine (Lond). 2019 Jul;14(13):1759-1769. doi: 10.2217/nnm-2019-0105. Epub 2019 Jul 12. PMID: 31298068.
- le Masne de Chermont Q, Chanéac C, Seguin J, Pellé F, Maîtrejean S, Jolivet JP, Gourier D, Bessodes M, Scherman D. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc Natl Acad Sci U S A. 2007 May 29;104(22):9266-71. doi: 10.1073/pnas.0702427104. Epub 2007 May 21. PMID: 17517614; PMCID: PMC1890483.
- Maldiney T,Scherman D,Richard C. Persistent Luminescence Nanoparticles for Diagnostics and Imaging, In Functional Nanoparticles for Bioanalysis, Nanomedicine, and Bioelectronic Devices, ACS Publications. 2012; 2:1-25. https://hal.science/hal-03828340
- Liu J, Lécuyer T, Seguin J, Mignet N, Scherman D, Viana B, Richard C. Imaging and therapeutic applications of persistent luminescence nanomaterials. Adv Drug Deliv Rev. 2019 Jan 1;138:193-210. doi: 10.1016/j.addr.2018.10.015. Epub 2018 Nov 7. PMID: 30414492.
- Sun M, ZJ Li. Li, Liu CL, Fu HX, Shen JS, Zhang HW. Persistent Luminescent Nanoparticles for Super-Long Time in Vivo and in Situ Imaging with Repeatable Excitation, J. Lumin. 2014; 145:838-842. https://doi.org/10.1016/j.jlumin.2013.08.070
- Calatayud DG, Jardiel T, Cordero-Oyonarte E, Caballero AC, Villegas M, Valle-Noguera A, Cruz-Adalia A, Peiteado M. Biocompatible Probes Based on Rare-Earth Doped Strontium Aluminates with Long-Lasting Phosphorescent Properties for In Vitro Optical IMAGING. Int J Mol Sci. 2022 Mar 21;23(6):3410. doi: 10.3390/ijms23063410. PMID: 35328831; PMCID: PMC8954243.
- Zhou Y, Lu S, Zhi J, Jiang R, Chen J, Zhong H, Shi H, Ma X, An Z. Microscopic Afterglow Bioimaging by Ultralong Organic Phosphorescent Nanoparticles in Living Cells and Zebrafish. Anal Chem. 2021 Apr 27;93(16):6516-6522. doi: 10.1021/acs.analchem.1c00423. Epub 2021 Apr 14. PMID: 33852275.
- Terraschke H, Franzreb M, Wickleder C. Magnetism and Afterglow United: Synthesis of Novel Double Core-Shell Eu2+ -Doped Bifunctional Nanoparticles. Chemistry. 2020 May 26;26(30):6833-6838. doi: 10.1002/chem.201904551. Epub 2020 Apr 30. PMID: 31922631; PMCID: PMC7318628.
- Harish V, Tewari D, Gaur M, Yadav AB, Swaroop S, Bechelany M, Barhoum A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials (Basel). 2022 Jan 28;12(3):457. doi: 10.3390/nano12030457. PMID: 35159802; PMCID: PMC8839643.
- Wu S, Li Y, Ding W, Xu L, Ma Y, Zhang L. Recent Advances of Persistent Luminescence Nanoparticles in Bioapplications. Nanomicro Lett. 2020 Mar 10;12(1):70. doi: 10.1007/s40820-020-0404-8. PMID: 34138268; PMCID: PMC7770784.
- Liu H, Xu CT, Lindgren D, Xie H, Thomas D, Gundlach C, Andersson-Engels S. Balancing power density based quantum yield characterization of upconverting nanoparticles for arbitrary excitation intensities. Nanoscale. 2013 Jun 7;5(11):4770-5. doi: 10.1039/c3nr00469d. Epub 2013 Apr 22. PMID: 23604490.
- Paterson AS, Raja B, Garvey G, Kolhatkar A, Hagström AE, Kourentzi K, Lee TR, Willson RC. Persistent luminescence strontium aluminate nanoparticles as reporters in lateral flow assays. Anal Chem. 2014 Oct 7;86(19):9481-8. doi: 10.1021/ac5012624. Epub 2014 Sep 23. PMID: 25247754; PMCID: PMC4188266.
- Danthanarayana AN, Finley E, Vu B, Kourentzi K, Willson RC, Brgoch J. A multicolor multiplex lateral flow assay for high-sensitivity analyte detection using persistent luminescent nanophosphors. Anal Methods. 2020 Jan 21;12(3):272-280. doi: 10.1039/c9ay02247c. Epub 2019 Dec 10. PMID: 32577135; PMCID: PMC7310964.
- Jiang Y, Wang F, Zhou H, Fan Z, Wu C, Zhang J, Liu B, Wang Z. Optimization of strontium aluminate-based mechanoluminescence materials for occlusal examination of artificial tooth. Mater Sci Eng C Mater Biol Appl. 2018 Nov 1;92:374-380. doi: 10.1016/j.msec.2018.06.056. Epub 2018 Jul 20. PMID: 30184763.
- Fathi-Achachelouei M, Knopf-Marques H, Ribeiro da Silva CE, Barthès J, Bat E, Tezcaner A, Vrana NE. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front Bioeng Biotechnol. 2019 May 24;7:113. doi: 10.3389/fbioe.2019.00113. PMID: 31179276; PMCID: PMC6543169.
- Hasan A, Morshed M, Memic A, Hassan S, Webster TJ, Marei HE. Nanoparticles in tissue engineering: applications, challenges and prospects. Int J Nanomedicine. 2018 Sep 24;13:5637-5655. doi: 10.2147/IJN.S153758. PMID: 30288038; PMCID: PMC6161712.
- Sepahvandi A, Eskandari M, Moztarzadeh F. Fabrication and characterization of SrAl2O4: Eu(2+)Dy(3+)/CS-PCL electrospun nanocomposite scaffold for retinal tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2016 Sep 1;66:306-314. doi: 10.1016/j.msec.2016.03.028. Epub 2016 Apr 11. PMID: 27207067.
- Cerro PRD, Teittinen H, Norrbo I, Lastusaari M, Massera J, Petit L. Novel borosilicate bioactive scaffolds with persistent luminescence. Biomed. Glas. 2020; 6(1):1-9. https://doi.org/10.1515/bglass-2020-0001
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018 Sep 19;16(1):71. doi: 10.1186/s12951-018-0392-8. PMID: 30231877; PMCID: PMC6145203.
- Vega-Vásquez P, Mosier NS, Irudayaraj J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front Bioeng Biotechnol. 2020 Feb 18;8:79. doi: 10.3389/fbioe.2020.00079. PMID: 32133353; PMCID: PMC7041307.
- Qin X, Wang J, Yuan Q. Synthesis and Biomedical Applications of Lanthanides-Doped Persistent Luminescence Phosphors With NIR Emissions. Front Chem. 2020 Dec 14;8:608578. doi: 10.3389/fchem.2020.608578. PMID: 33381494; PMCID: PMC7767859.
- Shi J, Sun X, Li J, Man H, Shen J, Yu Y, Zhang H. Multifunctional near infrared-emitting long-persistence luminescent nanoprobes for drug delivery and targeted tumor imaging. Biomaterials. 2015 Jan;37:260-70. doi: 10.1016/j.biomaterials.2014.10.033. Epub 2014 Oct 23. PMID: 25453956.
- Can-Uc B, Montes-Frausto JB, Juarez-Moreno K, Licea-Rodriguez J, Rocha-Mendoza I, Hirata GA. Light sheet microscopy and SrAl2 O4 nanoparticles codoped with Eu2+ /Dy3+ ions for cancer cell tagging. J Biophotonics. 2018 Jun;11(6):e201700301. doi: 10.1002/jbio.201700301. Epub 2018 Mar 5. PMID: 29316331.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
