Journal of Biology and Medicine
Antimicrobial activities of untreated and grape vinegar treated selected vegetables against common food borne pathogens
Jyotsana Singh# and Amar P Garg*
Cite this as
Singh J, Garg AP (2023) Antimicrobial activities of untreated and grape vinegar treated selected vegetables against common food borne pathogens. J Biol Med 7(1): 001-007. DOI: 10.17352/jbm.000035Copyright License
© 2023 Singh J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.In the post-Covid-19 era, plant-based diets and products have become more popular. Fruit vinegar is considered a healthy drink, rich in bioactive compounds like organic acids, polyphenols, flavonoids and alkaloids that provide several beneficial properties. Fruit vinegar also contains several beneficial nutrients like amino acids, sugars, vitamins and minerals. They provide energy, regulate cell metabolism, immunity, antioxidation, anticoagulation and help in the improvement of brain development. Vinegar is also known for its strong antimicrobial properties against foodborne pathogens. The aim of the present investigation was to find out the antimicrobial activities of grape vinegar against common food-borne pathogens and to see whether common vegetable lose their antimicrobial activities when soaked with grape vinegar or gain. The vegetables used in the present investigation were randomly collected from the local market. We have evaluated the antimicrobial properties of untreated and grape vinegar (GV) treated selected vegetables against Escherichia coli (ATCC8739), Bacillus subtilis, Staphylococcus aureus (ATCC 6539), Shigella flexneri (ATCC 12022), Salmonella typhi (ATCC 14028), Cronobacter sakazakii (ATCC 29544), Vibrio parahaemolyticus (ATCC 17802), Vibrio cholera (ATCC 3906) and Pseudomonas aeruginosa. Based on the randomized survey, commonly used vegetable ginger (Zingiber officinale), garlic (Allium sativum), onion (Allium cepa), raw papaya (Carica papaya), white radish (Raphanus sativus) and green chilli (Capsicum annuum) were selected for the assessment of their antimicrobial activities. Different phytochemicals were found in water extract and grape vinegar-treated vegetables. Our results suggested that in general, grape vinegar-treated vegetables showed higher antimicrobial activities against all tested food-borne pathogens, but onion, garlic, green chillies and white radish revealed drastically higher activities. Hence, it is suggested that onion, garlic, green chillies and white radish should preferably be used after soaking with grape vinegar. All vegetables except garlic and white radish showed very little antimicrobial activity against B. subtilis. V. cholera was highly sensitive to grape vinegar-treated vegetables except for garlic. Similarly, V. parahaemolyticus was highly sensitive to grape vinegar-treated green chillies, white radish, raw papaya, and onion. Shigella flexneri also showed greater sensitivity to GV-treated green chillies and white radishes. Pseudomonas aeruginosa was sensitive to ginger and green chillies only. It may safely be concluded that grape vinegar-treated vegetables are beneficial to fight against food-borne infections. The vegetable when soaked with grape vinegar did not lose their antimicrobial activities rather they gained the antimicrobial components from grape vinegar. Our studies revealed that white radish, ginger, green chilies, and onion should be used after soaking in grape vinegar while garlic and raw papaya should be eaten without treatment with grape vinegar.
Introduction
Vinegar is a fermentation product of acetic acid bacteria and fruit vinegar is becoming an important ingredient of the diet of the common man. Fermentable sugars from natural fruit juices are first converted to ethanol by yeasts under anaerobic conditions which is subsequently transformed into acetic acid bacteria of the genus Acetobacter. Acetic acid is the main product of the fermentation process, however, small quantities of tartaric and citric acids are also produced during the fermentation process [1,2]. Bioactive compounds in grape vinegar possess antioxidant activities [3] and have been used in various nutritional, food, medicinal, and pharmaceutical industries [4]. Several studies have reported that various effects of vinegar on the physiology of the human body are related to its phytochemical composition and its concentration [5]. Almost all traditional and industrial vinegar possess antimicrobial activities against various food-borne pathogens including Klebsiella pneumonia, Escherichia coli, Bacillus cereus, Salmonella typhi, Pseudomonas aeruginosa, and Staphylococcus aureus [2,6-12].
The quality and origin of vinegar are related to the chemical composition and sensory properties of vinegar that may contain diverse vitamins and bioactive compounds including gallic acid, catechin, epicatechin, chlorogenic acid, caffeic acid, coumaric acid and ferulic acid that healing properties along with antioxidant, antibacterial, anti-obesity, antihypertensive and cholesterol-lowering and are helpful in fighting with various illnesses [12-14].
Fruits vinegar also contains microbial additives consisting of Lipopolysaccharides (LPS) which are generated at some point in vinegar aging through the destruction of microorganisms through an elevation of acidity [15]. LPS is known to modulate the host macrophage community to modify severe issues in patients having diabetes, dyslipidemia, allergy and cancer [16]. Vinegar beautifies phagocyte pastime to ingest resistant microbes like Staphylococcus aureus and Escherichia coli which toughen the immune device to eliminate pathogen microbes and manage the infection process [17,18].
Various vegetables like ginger, garlic, onion, white radish, raw papaya and green chilies are included in the daily dose of the diet of the common man. Garlic is naturally a source with numerous therapeutic properties. Garlic is an ethnos pharmaceutical drug and is known to have allicin an organosulfur emulsion, which prevents lipids from biosynthesis [19]. In medicine, ginger is considered an aphrodisiac and has been used to repel mosquitoes. Oil from ginger is known to be therapeutic [20].
As a food item, onion is generally served as a vegetable component in frying, riding, etc., and is also eaten raw in salads, made into juice, pickled in vinegar, or used as a spice. As an herbal medicine, onion is recommended to relieve or prevent several common conditions, similar to atherosclerosis, asthma, bronchitis and coughs. The health benefits of onion are mainly attributed to its different ingredients, similar to organosulfur composites, phenolic composites, polysaccharides, and saponins [21,22].
Papaya is considered one of the important fruits because it is a rich source of antioxidants, phytochemicals, nutrients similar as carotenes, vitamin C and flavonoids, the B vitamins including folate and pantothenic acid, minerals such as potassium and magnesium and salutary fiber [23,24].
Green chili is a source of natural antioxidants containing many bioactive components which play a significant role in the prevention of free radical formation by scavenging or promoting their decomposition [25]. Green chilies are one of the widely consumed vegetables because of the combination of color, flavor, taste and nutritional value.
These vegetables are routinely used with or without vinegar. The antimicrobial properties of vegetables and vinegar are well known but the antimicrobial properties of vinegar-treated vegetables are not adequately studied. In this paper, we report the antimicrobial activities of grape vinegar with and without selected vegetables against common food-borne pathogens to find out the better usable form of the vegetables.
Materials and methods
Samples
Vegetables were purchased from the local market of Meerut in a randomized manner and were cleaned thoroughly under running tap water wiped with a clean dry cloth, cut into small pieces and dried under sunlight for 2 to 4 hours. When dried, these were soaked into 25 ml of grape vinegar for 7 days. Thereafter, these eatables were crushed with vinegar and filtered through Whatman filter paper no. 1. The filtrate was stored in a glass jar at room temperature for further experimental use [4].
Grape vinegar produced by the company namely Village Natural Vinegar, Village Food Products, Saradhana, District: Meerut was used. The manufacturer of the Company is working in close association with our University and we found that the manufacturer used a mixture culture of Acetobacter strain for the production of grape vinegar. The crude vinegar was filtered and centrifuged at 10,000xg for 30 minutes and used during the entire study.
Qualitative phytochemical analysis of grape vinegar
Qualitative analysis of untreated and grape vinegar-treated vegetables was made using the AOAS protocol (1990) as described by Trease and Evans [26] and [4]. These were used to detect the presence of alkaloids, saponins, tannins, flavonoids, anthraquinones, terpenoids and glycosides.
(a) Detection of alkaloids: for qualitative analysis of alkaloidMayer’s Test - The filtrate (1 mL) was placed in a test tube along with 5 - 6 drops of Mayer’s reagent, which was prepared by dissolving mercuric chloride (1.36 g) and potassium iodide (5.00 g) in 100 ml of water. The cream-colored precipitate confirmed the presence of an alkaloid.
(b) Dragendroff’s Test - Dragendroff’s reagent (potassium bismuth iodide solution) was added to the filtrate (1 mL) in the test tube and the reddish-brown precipitate was the indicator of the presence of alkaloid.
Detection for flavonoids
Alkaline reagent test- 1 mL of extracts (water and vinegar) were treated with a few drops of sodium hydroxide (NaOH) and examined for significant yellow colour that vanished upon the addition of dilute HCl, confirming the presence of flavonoids.
Detection of flavanoids
Shinoda test: 1 mL of test vinegar and/or extract was dissolved in 2 mL of 95% ethanol. One to two drops of concentrated hydrochloric acid (HCl) were added, a piece of magnesium was added and then the mixture was heated. Both the water and vinegar extracts of every edible showed the presence of a magenta colour. The presence of flavones is tested using magnesium. Flavonoids in the test sample are converted to anthocyanidins if they are present. While the longer conjugation in the resulting anthocyanin moves the colour further out into the red portion of the visible spectrum, the conjugation in the flavonoid compound results in yellow colour. This is a straightforward visible test for the presence of flavonoids due to the striking shift in colour.
Detection for phenol
Ferric chloride test: 1mL of water and/or vinegar extracts from vegetables was mixed with 5 - 6 drops of 5% ferric chloride solution. A few drops of lead acetate solution were added to 1 mL of water and vinegar extract from various foods. Both vinegar extract and water extracts showed the development of yellow precipitate in the presence of phenol.
Detection for Tannin
(a) Ferric chloride test: To 1 mL of each of the two extracts, 2 mL of 5% FeCl3 was added. The presence of tannins in the test samples was established by the emergence of a greenish-black colour.
(b) Vanillin hydrochloride test: In 1 mL of water and /or vinegar extract of eatables, a few drops of 2% vanillin hydrochloride were added. Purplish-red coloured precipitation was detected, which indicated the presence of tannins.
Detection for terpenoids
Horizon test: Trichloroacetic Acid (TCA) was added to 1mL of vegetable extracts (water and vinegar) and the development of yellow to crimson precipitation suggested the presence of terpenoids
Detection of carbohydra
(a) Molisch’s test: 2 mL of concentrated sulfuric acid was added to a test tube’s sidewalls after the extracts (1 mL) were treated with 2 - 3 drops of 1% alcoholic-naphthol solution. The formation of brown to violet rings confirmed the presence of carbohydrates. With the addition of hydrochloric acid, it leads to the creation of an aldehyde.
(b) Benedict’s test: Benedict’s reagent (5 mL) was mixed in 1mL of the extract, and the mixture was heated for 2 minutes before cooling. Production of scarlet precipitate was the indication of carbohydrates.
Detection of glycosides
Keller Killiani test: 0.5 mL glacial acetic acid + a few drops of 5% ferric chloride + two to three drops of concentrated sulphuric acid were added to 1 mL of each extract. At the intersection of the two liquids of the extracts, a formation of reddish-brown colour confirmed the presence of glycosides.
Detection for saponins
(a) Foam test: In double-deionized water, the extracts were dissolved and the mixture was vortexed for 15 minutes that produced stable foam, which indicated the presence of saponins.
(b) Olive test: When three drops of extra virgin olive oil were added to the foam test’s foaming and violently shaken, an emulsion was created that could be seen in both the water and vinegar extracts of the edible.
Antimicrobial activity using agar well diffusion method
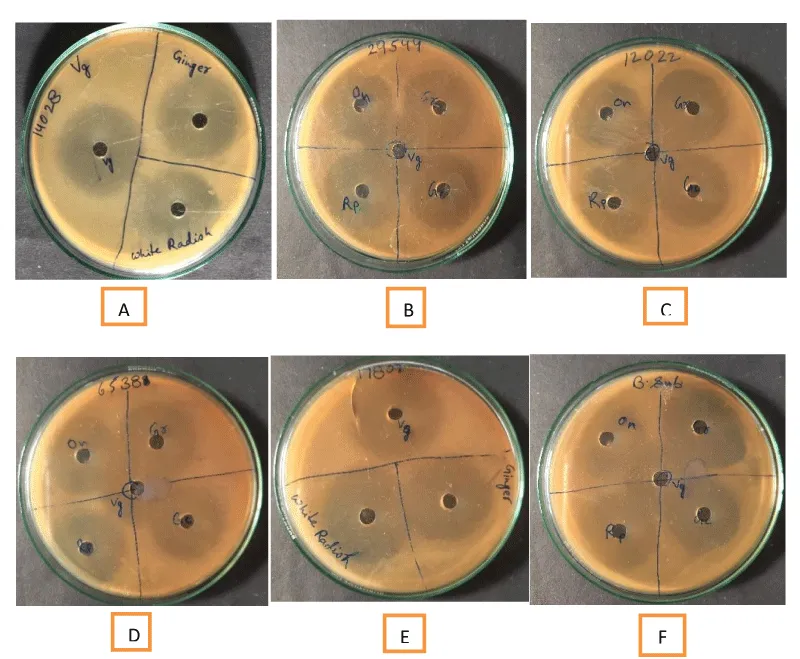
The antimicrobial activity of different vegetables with and without Grape Vinegar (GV), was assessed using agar well diffusion method as described earlier [4]. The selected food-borne bacterial pathogens were inoculated into 10 mL of nutrient broth and incubated at 37 ± 1 ℃ for 24 h. The Mueller Hinton agar plates were inoculated separately with 100 µL of test bacterial pathogen and evenly spread aseptically on the entire surface of the plate using an “L” shaped glass spreader. The wells were cut aseptically using a sterile crock borer (6mm diam.) and 50µLof extract of each of the vegetables with and without grape vinegar (GV) was added carefully and any outflow of the test material was avoided. The plates were incubated in an upright position at 37 ± 1 ℃ for 48 h in the BOD incubator to allow the radial diffusion of the test substances. The diameter of the zone of inhibition (in mm) was measured against Escherichia coli (ATCC8739), Bacillus subtilis, Staphylococcus aureus (ATCC 6539), Shigella flexneri (ATCC 12022), Salmonella typhi (ATCC 14028), Cronobacter sakazakii (ATCC 29544), Vibrio parahaemolyticus (ATCC 17802), V. cholera (ATCC 3906) and Pseudomonas aeruginosa. Each experiment was conducted in triplicate. Standard error was calculated using the standard formula.
Results and discussions
The main objective of this research was to compare the antimicrobial activity of bioactive compounds of common vegetables with and without soaking in grape vinegar. Qualitative analysis of untreated and grape vinegar treated common vegetable - ginger (Zingiber officinale), garlic (Allium sativum), onion (Allium cepa), raw papaya (Carica papaya), white radish (Raphanus sativus) and green chilli (Capsicum annuum) is presented in Table 1 which revealed that grape vinegar did not contain anthraquinones while garlic, onion, green chillies, and white radish has sufficient quantities of anthraquinones. Similarly, ginger and white radish did not produce positive color reactions for tannins but after treatment with grape vinegar, these possess the beneficial effects of tannins (Figure 1). Terpenoides were found absent in onion and raw papaya but after soaking in grape vinegar, showed the positive reaction to terpenoids (Figure 1). The study further showed that alkaloids are present in grape vinegar as well as common vegetables. Alkaloids are important bioactive compounds that are known for their antibacterial, antiviral and insecticidal effects on various food-borne pathogens besides the positive health effects. Anthraquinones contain antibacterial, antiviral, antifungal and antioxidant properties and are also known to counter the negative effects of the chemicals found in tobacco, a nightshade plant, which can cause cancer [2,27].
Qualitative phytochemical analysis revealed that saponins were absent in green chili water extract but soaked with grape vinegar it shows a positive result (Table 1, Figure 1) and the bioactive saponins of grape vinegar were transferred to green chillies. The saponins are also well known for their antimicrobial activities both against Gram +ve or Gram -ve food-borne pathogens [28,29].
Flavonoids are an important class of natural products, particularly because they belong to plant secondary metabolites having a polyphenolic structure, widely found in fruits, vegetables, and certain beverages. Flavonoids are used by vegetables for their growth and defense against plaques. Flavonoids act in plants as antioxidants, antimicrobials, photoreceptors, visual attractors, feeding repellents and light screening. Flavonoids have antiviral, anti-inflammatory, and vasodilating properties. However, the antioxidant activity of flavonoids is due to their ability to reduce free radical formation and scavenge free radicals [30]. Flavonoids actively interact with the gut microbiota, inhibit the growth of various pathogens and increase the populations of beneficial genera such as Bifidobacterium and Lactobacillus [31]. Bifidobacterium is known as a happy bacterium and helps recover from depression as well [32]. Our findings support the health-promoting and food safety-related potentials of grape vinegar [1].
The present study revealed that the grape vinegar-treated vegetables showed high antimicrobial activity against all test food-borne pathogens (Table 2). Each test was performed in triplicate and the zone of inhibition (mm in diameter) was calculated on the x and y axis both from which the diameter of the disc (x2) was subtracted and divided by 4 to get the final zone of inhibition. A simple standard error was calculated which showed that there was a significant difference in the zone of inhibition between vegetables and test food-borne pathogens. Complicated statistical analyses were avoided. On comparison of the antimicrobial activity of grape vinegar (control) with water extract of ginger, garlic, onion, raw papaya, green chillies and white radish, it was found that the latter produced a greater zone of inhibition almost against all test pathogens which suggest vegetables possess significant antimicrobial activities and when these were soaked in grape vinegar their antimicrobial activities enhanced significantly (Table 2). The agar well diffusion method showed that both eatables with and without grape vinegar had antibacterial activity against selected test food-borne pathogens (Figure 2). The antimicrobial activity of the vinegar varied for each bacterial species and with each vegetable but the overall comparison made it very clear that grape vinegar-treated vegetables had better antimicrobial activities against food-borne pathogens. Grape vinegar contains good amounts of organic acids and phenolic compounds that may be attributed to its higher antibacterial activity against food-borne pathogens. A very high zone of inhibition was recorded against Vibrio parahaemolyticus (ATCC17082) by GV-treated onion, raw papaya, green chilles, and white radish (Table 2). Pseudomonas aeruginosa was highly sensitive to GV-treated onion while Staphylococcus aureus was inhibited by all GV-treated vegetables. Shigella flexneri and Salmonella typhi showed a high zone of inhibition with GV-treated onion, green chilles and white radish. Bacillus subtilis was sensitive to GV-treated garlic and white radish while Escherichia coli showed significant inhibition by GV-treated onion and white radish. Vibrio cholera (ATCC 3906) showed significant inhibition by all GV-treated vegetables except garlic.
White radish is well known for its antimicrobial activity it also shows high antimicrobial activity and is being used in the treatment of various diseases as traditional medicine [33]. Raw papaya is very useful for the treatment of cough, bronchitis, chest asthma and colds since ancient times and its medicinal value has been attributed to the presence of natural metabolites found in its leaf, bark, and twigs that possess both antitumor and pesticidal properties [34]. Chemical analysis of green chili has revealed that it contains high concentrations of several essential nutrients, including vitamin C which plays an important role in human health. Capsaicin is a well-studied chemical component of the Capsicum species and one of the pungent capsaicinoids that have a high degree of biological activity affecting the nervous, cardiovascular, and digestive systems [35].
The consumption of vinegar is associated with several health benefits [36] and is known for its strong antimicrobial properties [37]. These properties are mainly due to the content of acetic acid, which inhibits the development of pathogenic and food spoilage organisms. Bacteria such as E. coli and Staphylococcus aureus, and yeasts like Candida albicans are part of normal human microbiota [38] that are well-known opportunistic pathogens and may contribute to disorders of the gastrointestinal tract, skin and soft tissue, circulatory system, respiratory tract, and urogenital system. A new medical approach suggests that the identification of non-antibiotic antimicrobial agents may help reduce the use of antibiotics in the treatment of certain conditions and prevent further development of antibiotic resistance [1].
Current estimates indicate that about 80 million people worldwide still depend on plants for their health needs and approximately 95% of modern drugs have been isolated from traditional medicinal plants. The World Health Organization (WHO) estimated that around 80% of rural patients seek alternative treatment options in many countries including India and are dependent on different medicinal plants and biomolecules for the management of different diseases [34].
Conclusion
The findings of the present study suggest that GV-treated vegetables possess higher antimicrobial activities than untreated. It is concluded that in case of cholera infections garlic is not helpful while GV-treated white radish is most effective. In the case of Salmonella, GV-treated ginger, onion, green chillies and white radish may be used. Overall studies suggest that white radish, ginger, green chilies and onion should be used after soaking in grape vinegar while garlic and raw papaya should be eaten without treatment with grape vinegar.
Authors are thankful to Shobhit Institute of Engineering and Technology (Deemed University), Modipuram, Meerut (UP) India for providing facilities for conducting this work and the firm Vigour of Village Natural Vinegar, Village Food Products, Saradhana, District: Meerut for making research-grade pure vinegar for us.
- Antoniewicz J, Jakubczyk K, Kwiatkowski P, Maciejewska-Markiewicz D, Kochman J, Rębacz-Maron E, Janda-Milczarek K. Analysis of Antioxidant Capacity and Antimicrobial Properties of Selected Polish Grape Vinegars Obtained by Spontaneous Fermentation. Molecules. 2021 Aug 4;26(16):4727. doi: 10.3390/molecules26164727. PMID: 34443313; PMCID: PMC8397985.
- Kahraman HA, Tutun H, Keyvan E, Balkan BM. Investigation of Chemical, Antibacterial and Antiradical Properties of Home-Made Apple and Grape Vinegars. Ankara Üniversitesi Veteriner Fakültesi Dergisi. 2021. DOI: htpp://doi.org/10.33988/auvfd.865309
- Kelebek H, Kadiroğlu P, Demircan NB, Selli S. Screening of bioactive components in grape and apple vinegars: Antioxidant and antimicrobial potential. Journal of the Institute of Brewing. 2017; 123(3):407-416. doi:10.1002/jib.432.
- Singh J, Garg AP. Antimicrobial activity of Apple cider vinegar treated selected vegetables against Common foodborne bacterial pathogens. Bioscience Biotechnology Research Communications. 2022; 15:(2). DOI: http://dx.doi.org/10.21786/bbrc15.2.13
- Petsiou EI, Mitrou PI, Raptis SA, Dimitriadis GD. Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight. Nutr Rev. 2014 Oct;72(10):651-61. doi: 10.1111/nure.12125. Epub 2014 Aug 28. PMID: 25168916.
- Zhang J, Zhou K, Zheng B, Zhao L, Shen P, Ji J, Wei Z, Li L, Zhou J, Xiao Y. High Prevalence of ESBL-Producing Klebsiella pneumoniae Causing Community-Onset Infections in China. Front Microbiol. 2016 Nov 15;7:1830. doi: 10.3389/fmicb.2016.01830. PMID: 27895637; PMCID: PMC5109008.
- Rasool MH, Siddique AB, Saqalein M, Asghar MJ, Zahoor MA, Aslam B, Shafiq HB, Nisar MA. Occurrence and antibacterial susceptibility pattern of bacterial pathogens isolated from diarrheal patients in Pakistan. Saudi Med J. 2016 Mar;37(3):274-9. doi: 10.15537/smj.2016.3.14449. PMID: 26905349; PMCID: PMC4800891.
- Bakir S, Devecioglu D, Kayacan S, Toydemir G, Karbancioglu-Guler F, Capanoglu E. Investigating the Antioxidant and Antimicrobial Activities of Different Vinegar. Eur. FoodRes. Technol. 2017; 243:2083–2094. DOI: http://doi.org/10.1007/s00217.017-2908-0
- Al-Nabulsi AA, Olaimat AN, Osaili TM, Shaker RR, Zein Elabedeen N, Jaradat ZW, Abushelaibi A, Holley RA. Use of acetic and citric acids to control Salmonella Typhimurium in tahini (sesame paste). Food Microbiol. 2014 Sep;42:102-8. doi: 10.1016/j.fm.2014.02.020. Epub 2014 Mar 15. PMID: 24929724.
- Sengun IY, Kilic G, Ozturk B. Screening physicochemical, microbiological and bioactive properties of fruit vinegars produced from various raw materials. Food Sci Biotechnol. 2019 Oct 14;29(3):401-408. doi: 10.1007/s10068-019-00678-6. PMID: 32257524; PMCID: PMC7105562.
- Ozturk I, Caliskan O, Tornuk F, Ozcan N, Yalcin H, Baslar M, Sagdic O. Antioxidant, Antimicrobial, Mineral, Volatile, Physicochemical and Microbiological Characteristics of Traditional Home-Made Turkish Vinegars. LWT Food Sci. Technol. 2015; 63: 144-151. DOI: http: doi.org/10.1016/j.lwt.2015.03.003
- Kahraman HA, Tutun H, Kaya MM, Soner Tutun S, Usluer MS, Rugji J, Yurdakul Ö. Total phenolic content, antiradical, antimicrobial and antibiofilm properties of grape and apple vinegar. Journal of Advances in VetBio Science and Techniques. 2021; 6(2):150-158. https://doi.org/10.31797/vetbio.960155
- Budak NH, Aykin E, Seydim AC, Greene AK, Guzel-Seydim ZB. Functional properties of vinegar. J Food Sci. 2014 May;79(5):R757-64. doi: 10.1111/1750-3841.12434. PMID: 24811350.
- Tasdemir SS, Sanlier N. An Insight into the Anticancer Effects of Fermented Foods: A Review. J. Funct. Foods 2020; 75:104281. DOI: htpp://doi.org/10.1016/j.j.ff 2020. 104281
- Hashimoto M, Obara K, Ozono M, Furuyashiki M, Ikeda T, Suda Y, Fukase K, Fujimoto Y, Shigehisa H. Separation and characterization of the immunostimulatory components in unpolished rice black vinegar (kurozu). J Biosci Bioeng. 2013 Dec;116(6):688-96. doi: 10.1016/j.jbiosc.2013.05.029. Epub 2013 Jun 27. PMID: 23810669.
- Bravo MN, Silva S, Coelho AV, Boas LV, Bronze MR. Analysis of phenolic compounds in Muscatel wines produced in Portugal, Anal. Chim. Acta. 2006; 563:84-92.
- Yagnik D, Ward M, Shah AJ. Antibacterial apple cider vinegar eradicates methicillin resistant Staphylococcus aureus and resistant Escherichia coli. Sci Rep. 2021 Jan 20;11(1):1854. doi: 10.1038/s41598-020-78407-x. PMID: 33473148; PMCID: PMC7817673.
- Özdemir GB, Özdemir N, Ertekin-Filiz B, Gökırmaklı Ç, Kök-Taş T, Budak NH. Volatile aroma compounds and bioactive compounds of hawthorn vinegar produced from hawthorn fruit (Crataegus tanacetifolia (lam.) pers.). J Food Biochem. 2022 Mar;46(3):e13676. doi: 10.1111/jfbc.13676. Epub 2021 Mar 2. PMID: 33650149.
- Ashfaq F, Ali Q, Haider MA, Hafeez MM, Malik A. Therapeutic activities of garlic constituent phytochemicals. Biological and Clinical Sciences Research Journal1. 2021; 2708-2261.DOI:http://doi.org/10.47264/bcsrj0201007
- Kumar R, Saha P, Lokare P, Datta K, Selvakumar P, Chourasia A. A Systemic Review of Ocimum sanctum (Tulsi): Morphological Characteristics, Phytoconstituents, and TherapeuticApplications. International Journal for Research in Applied Sciences and Biotechnology. 2022; 9(2):221-226. DOI: https://doi.org/10.31033/ijrasb.9.2.15
- Marrelli M, Amodeo V, Statti G, Conforti F. Biological Properties and Bioactive Components of Allium cepa L.: Focus on Potential Benefits in the Treatment of Obesity and Related Comorbidities. Molecules. 2018 Dec 30;24(1):119. doi: 10.3390/molecules24010119. PMID: 30598012; PMCID: PMC6337254.
- Teshika JD, Zakariyyah AM, Zaynab T, Zengin G, Rengasamy KR, Pandian SK, Fawzi MM. Traditional and modern uses of onion bulb (Allium cepa L.): a systematic review. Crit Rev Food Sci Nutr. 2019;59(sup1):S39-S70. doi: 10.1080/10408398.2018.1499074. Epub 2018 Oct 4. PMID: 30040448.
- Murcia MA, Jiménez AM, Martínez-Tomé M. Evaluation of the antioxidant properties of Mediterranean and tropical fruits compared with common food additives. J Food Prot. 2001 Dec;64(12):2037-46. doi: 10.4315/0362-028x-64.12.2037. PMID: 11770635.
- Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. FoodChemistry.2002; 76(1):69-75 DOI: http://dx.doi.org/10.1016/S0308-8146(01)00251-5
- Dimitrios B. Sources of natural phenolic antioxidants. Food Science and Technological Research. 2006; 17(9),505-512. DOI: https://doi.org/10.101016/j.tifs.2006.04.004
- Singh J, Amar P Garg. Antimicrobial Activity of Sugarcane Vinegar with Eatables Against Selected Food Borne Pathogens. Acta Scientific Microbiology. 2023; 6:2; 02-08.
- Fouillaud M, Venkatachalam M, Girard-Valenciennes E, Caro Y, Dufossé L. Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities. Mar Drugs. 2016 Mar 25;14(4):64. doi: 10.3390/md14040064. PMID: 27023571; PMCID: PMC4849068.
- Jain AS, Surana SJ, Gokhale SB, Tatiya AU, Bothara RC. Antimicrobial properties of Eranthemum roseum (Vahl) R. Br. Iranian Journal of Pharmaceutical Research. 2007; 6(2):131-133.
- Soetan kO, Oyekunle MA, Aiyelaagbe OO, Fafunso MA. Evaluation of the antimicrobial activity of saponins extract of Sorghum Bicolor L. Moench. African Journal of Biotechnology. 2006; 5(23):2405-2407.
- Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016 Dec 29;5:e47. doi: 10.1017/jns.2016.41. PMID: 28620474; PMCID: PMC5465813.
- Pei R, Liu X, Bolling B. Flavonoids and gut health. Curr Opin Biotechnol. 2020 Feb;61:153-159. doi: 10.1016/j.copbio.2019.12.018. Epub 2020 Jan 15. PMID: 31954357.
- Siddique SP, Garg AP. Isolation, Characterization and Quantitative Enumeration of Lactic Acid Bacteria from Human Faeces. Bioscience Biotechnology Research Communications. 2022; 15:1. DOI: http://dx.doi.org/10.21786/bbrc/15.1.33
- Lim HW, Song KY, ChonJW, JeongD, Seo KH. Antimicrobial Action of Raphanusraphanistrum subsp. sativus (radish) Extracts against Foodborne Bacteria Present in Various Milk Products: A Preliminary Study. Journal of Milk Science and Biotechnology. 2019; 37(3):187-195. DOI:htpp://doi.org/10.22424/jmsb.2019.37.3.187
- Dwivedi MK, Sonter S, MishraS, Kumar D, Patel, Kumar Singh P. Antioxidant, antibacterial activity, and phytochemical characterization of Carica papaya flowers. Journal of Basic and Applied Sciences. 2020; 9(23). DOI:https://doi.org/10.1186/s43088-020-00048w
- Omolo AM, Wong ZZ, Mergen AK, Hastings JC, Le NC, Reiland HA, Case KA, Baumler DJ. Review article on Antimicrobial Properties of Chili Peppers. Journal of Infectious Diseases and Therapy. 2014; 2(4). DOI: https://doi.org/10.4172/2332-0877.1000145
- Ashchyan H, Jen M, Elenitsas R, Rubin AI. Surreptitious apple cider vinegar treatment of a melanocytic nevus: Newly described histologic features. J Cutan Pathol. 2018 Apr;45(4):307-309. doi: 10.1111/cup.13102. Epub 2018 Feb 2. PMID: 29393529.
- Zhang XL, Zheng Y, Xia ML, Wu YN, Liu XJ, Xie SK, Wu YF, Wang M. Knowledge Domain and Emerging Trends in Vinegar Research: A Bibliometric Review of the Literature from WoSCC. Foods. 2020 Feb 10;9(2):166. doi: 10.3390/foods9020166. PMID: 32050682; PMCID: PMC7074530.
- Thomas S, Izard J, Walsh E, Batich K, Chongsathidkiet P, Clarke G, Sela DA, Muller AJ, Mullin JM, Albert K, Gilligan JP, DiGuilio K, Dilbarova R, Alexander W, Prendergast GC. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017 Apr 15;77(8):1783-1812. doi: 10.1158/0008-5472.CAN-16-2929. Epub 2017 Mar 14. PMID: 28292977; PMCID: PMC5392374.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
