Journal of Biology and Medicine
Bacteriological pattern of urinary tract infection in men with symptomatic benign prostatic hyperplasia at a tertiary hospital in Nigeria
Ngwu PE1,3*, Ihedoro IE1 and Kalu EI2
2Department of Microbiology, Federal Medical Centre, Umuahia, Nigeria
3Department of Surgery, College of Medicine, Gregory University, Uturu, Nigeria
Cite this as
Ngwu PE, Ihedoro IE, Kalu EI (2022) Bacteriological pattern of urinary tract infection in men with symptomatic benign prostatic hyperplasia at a tertiary hospital in Nigeria. J Biol Med 6(1): 024-028. DOI: 10.17352/jbm.000032Copyright License
© 2022 Ngwu PE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction/Background: Benign Prostatic Hyperplasia (BPH) is characterized by narrowing of the prostatic urethra with resultant difficulty in passing urine, stasis, and a predisposition to urinary tract infection. The objective of this study is to identify the prevalence of urinary tract infections, common organisms isolated, their antimicrobial sensitivity pattern, and the relationship of co-morbidities with urinary tract infection in this population.
Materials and methods: All patients who presented to our urology team with bladder outlet obstruction secondary to benign prostatic hyperplasia between January 2020 and January 2021 were included. Information on age, occupation, co-morbid conditions, urine microscopy, culture, and sensitivity patterns were obtained and analyzed using SPSS version 25. Midstream urine samples were collected from 172 BPH patients. Microscopy, culture, and antibiotic susceptibility tests were carried out.
Results: From our study, the prevalence of bacteriuria was 67.9% with the 65-74 and 45-54 age groups having the highest and the least prevalence of bacteriuria (88.9% and 33.3% respectively). The most common organisms cultured from their urine were Pseudomonas (17.9%), E. coli (14.3%), Coliforms (10.7%), and Klebsiella (10.7%). Sensitivity patterns of these microorganisms revealed the highest sensitivity to the fluoroquinolones (25.9%) followed by Nitrofurantoin (14.8%) and Ceftriaxone and Cefoxitin (7.4%). The least sensitivity was to Augmentin and Gentamycin.
Conclusion: Bacteriuria is common in patients with BPH. Pseudomonas spp was the commonest isolated organism in our study and most isolated organisms were susceptible to the fluoroquinolones.
Introduction
Benign prostatic hyperplasia (BPH) is characterized by progressive enlargement of the prostate [1]. It is a nonmalignant neoplastic process secondary to increased cellular growth and one of the most common causes of Lower Urinary Tract symptoms (LUTS) [2]. About half of the male population over the age of 50 can be diagnosed with histological BPH, and this prevalence increases with age to about 90% over the age of 80 [3].
As the lumen of the Prostatic urethra becomes compromised by fibroadenomatous growth in the Periurethral region of the prostate, urine outflow is obstructed progressively resulting in incomplete bladder emptying causing stasis and may predispose patients to infection [4].
Bacterial presence in the Urine within its tract is known as bacteriuria and can result in a microbial invasion of the organs responsible for the manufacture (kidney), transport (ureters and urethra), and storage (bladder) of urine. Infection of the kidney is known as pyelonephritis.
Infection of the lower urinary tract that involves the bladder is called Cystitis while it is called urethritis when the urethra is involved [4]. Urinary tract infection is one of the commonest infections encountered in our environment [5].
In a study done by Pourmand, et al. the incidence of bacteriuria following prostatectomy was 10.8% [6]. In a meta-analysis conducted by Qiang, et al, bacterial contamination was detected in 44% of the BPH prostatic specimens. They reported satisfactory results and reduced infection rate following preoperative antibiotic prophylaxis in TURP patients [7]. A Research done by Akobi, et al. showed a high incidence rate of 62.5% of UTIs among patients with an indwelling urinary catheter and diagnosed of benign prostate hyperplasia [8], while a study done by Agbugui, et al. noted bacteriuria in 44.7% of the patients [9].
UTIs occur when normal protective mechanisms fail. These mechanisms include: dislodging of bacteria during urination, high urea concentration in the urine, antibacterial secretions from the prostate, high urine osmolality and white blood cells [10].
According to Oni, et al. in their study on patients with the indwelling urinary catheter at Ibadan, Nigeria reported that the common agents of infections are Klebsiella species, Escherichia coli, Proteus species, and Staphylococcus aureus, in order of frequency [11]. Taiwo and Aderounmu reported that in Osogbo, Nigeria, Klebsiella specie is the commonest pathogen isolated with 46(36.6%), followed by Pseudomonas specie 34(27.8%), Escherichia coli 26(20.6%), Staphylococcus aureus 12(9.5%), Proteus mirabilis 4(3.2%), Candida albicans 4(3.2%) and coagulate negative staphylococcus 2(1.6%) [12]. Oshodi, et al reported that Proteus mirabilis is the leading cause of UTI among the subjects of the study closely followed by E. coli and Pseudomonas aeruginosa, while Akobi, et al demonstrated that Escherichia coli 247(67.7%) was the most prevalence uropathogen in North Central Nigeria [8].
The antimicrobial susceptibility pattern confirms that most of the urinary isolates in our environment are resistant to the commonly used antibiotics including cephalosporin and fluoroquinolones [12]. Akobi, et al observed that the most susceptible antimicrobial agent in their locality was Nitrofurantoin (61.9%) and Levofloxacin (44.1%). The bacterial agents of infection were noted to be resistant to ampicillin, cotrimoxazole, and nitrofurantoin commonly used for the patient with a urological problem, however, ceftazidime, ceftriazone, pefloxacin and ofloxacin showed good sensitivity against the bacteria [11].
Materials and methods
All patients who presented to our urology team with bladder outlet obstruction secondary to benign prostatic hyperplasia between January 2020 and January 2021 were included. Information on age, occupation, co-morbid conditions and urine microscopy, culture, and sensitivity patterns were obtained and analyzed using SPSS version 25. Midstream urine samples were collected from 172 BPH patients. Microscopy, culture and antibiotic susceptibility tests were carried out.
This study was carried out at the Federal Medical Center, Umuahia, Nigeria over a period of one year (January 2020 to Jan 2021). Approval was obtained from the ethical Review Committee of the Hospital. It was a single-center cross-sectional study. Patients who presented to our urology team with features suggestive of bladder outlet obstruction secondary to benign prostatic hyperplasia formed the study population.
- Inclusion criteriPatients presenting with lower urinary tract symptoms secondary to BPH.
Exclusion criteria
- Patients on steroid therapy
- Recent antibiotics use.
Methods
The minimum sample size was calculated using the Fisher’s formula for a single sample prevalence cross-sectional study [13].
, where: n = minimum sample size, Z = standard deviation at 99% confidence limit is equivalent to 2.576, P = Expected prevalence of UTI among BPH patients = 93% (estimated from the prevalence of UTI among BPH patients in Enugu) [14], e = level of precision desired = 0.05
= (2.576)2 x0.93 (1-0.93)/0.052 = 172.8
Adjusting for a population size less than 10,000
Since the target population in this study is less than 10,000, we adjust the sample size
Nf = n/ (1+n/N)
nf = desired sample size when population is less than 10,000;
n = the desired sample size when population is more than 10,000;
N = the estimate of the population size (300 BPH patients)
Nf = 173/ (1+100/300)
Nf = 173/(1+0.333)
Nf = 173/1.333
Nf = 129.7 approximately 130
To account for non-response (NR) of 20% due to the poor data quality of hospital-based record
N= 162.5
Total minimum sample size of 163 participants.
Following informed consent, a proforma was opened for each patient, with information on occupation, co-morbid conditions, urine microscopy and culture and sensitivity patterns. Using purposive standard sampling technique, clean catch midstream urine samples were collected from non-catheterized patients and catheter specimens after clamping the catheter for 30minutes from catheterized patients into a sterile universal container [15] and submitted for analysis within an hour of collection. The specimen were subjected to microscopy, culture and sensitivity. The centrifuged urine specimens were examined under a microscope for the presence of bacteria, white blood cells, red blood cells, casts, pus cells and crystals and recorded. The specimen using a standard wire loop of 4mm, on CLED, MacConkey and Blood agar and incubated at 37˚C for 18 - 24 hours. Plates were examined for bacterial growth with growths of ≥105 cfu/ml taken to be significant. The isolates were identified by colony morphology, Gram staining, motility testing and necessary biochemical tests [16]. Antibiotic susceptibility tests were carried out by adopting Kirby Bauer method and CLSI guidelines [17].
Data analysis
Data were presented in the form of tables, frequencies and percentages. Statistical analysis was done using SPSS version 25.
Results
From Table 1, the mean age of the 172 recruited BPH patients was 66.4 years (± 8.8) with 39.5% within the 65-74 year age category. Most were drivers or retired (21.4% each). Prevalence of bacteriuria was 67.9% with the 65-74 year and 45-54 year age group having the highest and the least prevalence of bacteriuria (88.9% and 33.3% respectively). 41.5% of patients had either suprapubic discomfort or pain on micturition Table 2.
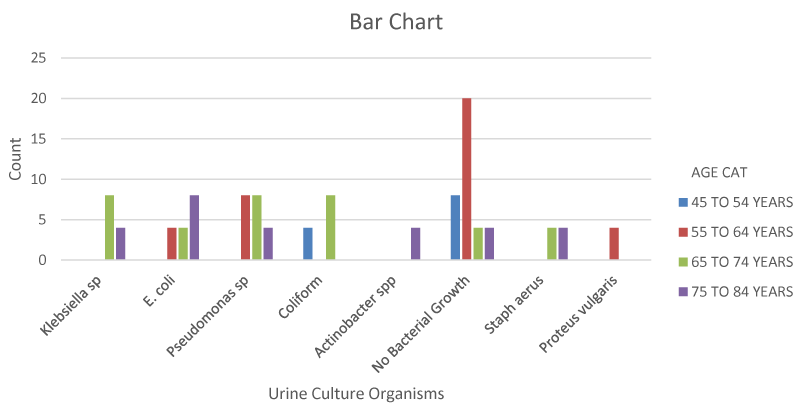
Tables 3,4 shows that the most common organisms cultured from their urine were Pseudomonas (17.9%), E.coli (14.3%), Coliforms (10.7%) and Klebsiella (10.7%). Other organisms isolated include Staphylococcus aerus (7.1%), Proteus vulgaris (3.6%) and Actinobacter spp. (3.6%).
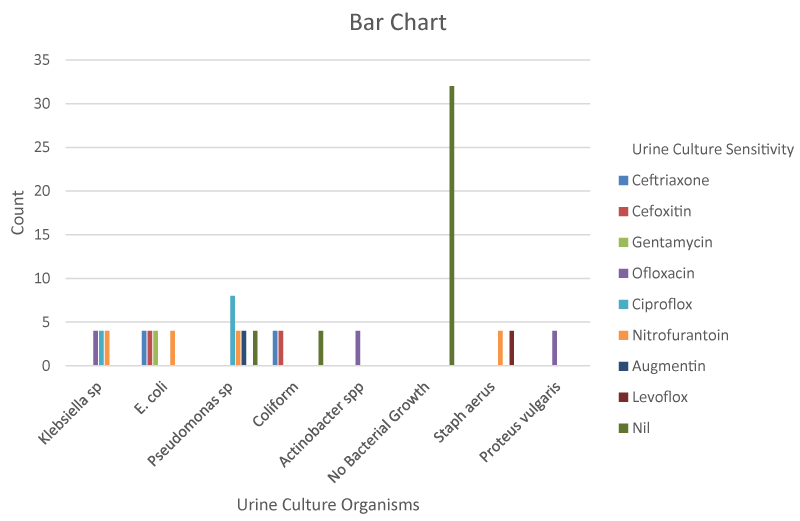
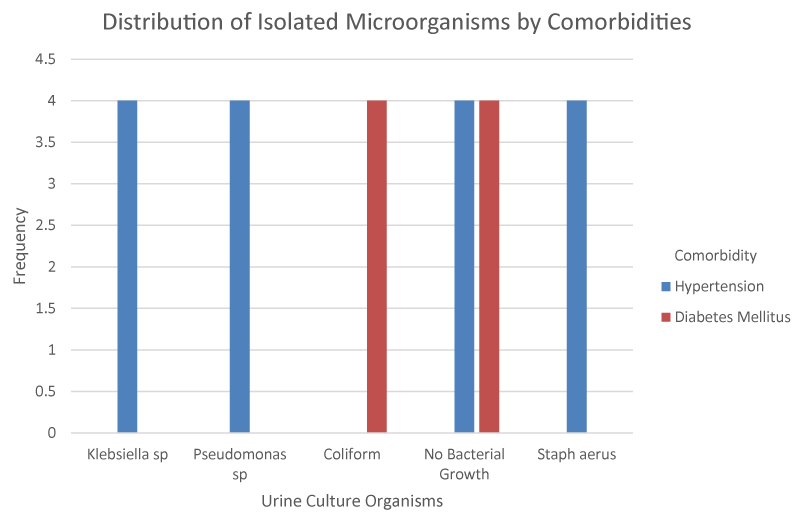
Figure 1 shows that the patients at the lowest age group (45-54) had the least incidence of UTI followed by those in the 55 to 64 age group. Sensitivity patterns of these microorganisms revealed the highest sensitivity to the fluoroquinolones (25.9%) followed by Nitrofurantoin (14.8%), Ceftriaxone and Cefoxitin (7.4%). The least sensitivity was to Augmentin, Gentamycin. From Table 5,6, 20.9% (36 patients) had co-morbidities with p-value less than 0.05 which is statistically significant Tables 1-6, Figures 1-3.
Discussion
Our study sought to define the urine bacteriology of patients with symptomatic BPH in our area. Bladder outlet obstruction due to BPH is a major cause of bacteriuria in aging males. This is as a result of increased bacterial colonization of the urinary tract due to stasis and incomplete bladder emptying as well as urethral catheterization in these category of patients.
The prevalence of bacteuria was 67.9% in this study which is similar to a value of 62.8% in a study by Mishra PP, et al. [15] and 62.5% by Akobi OA, et al. [8].
Among the pathogens isolated, Pseudomonas (17.9%) had the highest percentage of occurence,this was followed by E.coli (14.3%), Coliforms (10.7%) and Klebsiella (10.7%). Proteus Spp and actinobacter Spp with a percentage of 3.6%. In the studies by Mishra PP, et al. and Akobi, et al. the most common organism isolated was E. coli with a percentage of 31.81% and 67.7% respectively.
The incidence of UTI seems to be higher in the elderly (65 years and above) as seen in Table 2 probably as a result of worsening stasis in the elderly group. This is in agreement with a study done by Oshodi AJ, et al. and Mishra, et al. which showed a steady rise in the prevalence of UTI among BPH patients [4,15].
The most common organism cultured in our study is Pseudomonas (17.9%) and this is followed by E. coli (14.3%). In a study by Oni, et al. Klebsiella Spp (36%) was the most common organism isolated followed by Pseudomonas aeroginosa at 15.1%. A study by Oshodi, et al. put Pseudomonas Spp and E.coli as the second and 3rd predominant causes of UTI while a study by Agbugui e al had E.coli (47.6%) as the most common organism isolated [4,9,11].
Sensitivity patterns of these microorganisms revealed the highest sensitivity to the fluoroquinolones(25.9%) followed by Nitrofurantoin (14.8%). A study done by Agbugui, et al. [9] revealed a highest sensitivity to the Carbapenems followed by Nitrofurantoin while in the study done by Chedi, et al. [5] the pathogens showed highest sensitivity to the fluoroquinolones followed by cephalosporins.
Regarding co-morbidities, a significant number of patients in this study had co-morbidities. A number of previous studies have demonstrated the association of lower urinary tract infections with co-morbidities [18,19].
Fluoroquinolones should therefore be a major consideration when thinking of the class of antibiotic to be used before culture result is out in our region. Broader studies are however needed to strengthen this submission.
- Chan SW. Pathology and Medical Therapy of Benign Prostatic Hyperplasia. The Hong Kong Medical Diary, 16, 4-7. - References - Scientific Research Publishing [cited 2021 Sep 24]. Available from: 2011; https://scirp.org/reference/ReferencesPapers.aspx?ReferenceID=1418905
- (PDF) Benign prostatic hyperplasia and lower urinary tract symptoms: evidence and approaches for best case management . [cited 2021 Sep 24]. Available from: https://www.researchgate.net/publication/51059325_Benign_prostatic_hyperplasia_and_lower_urinary_tract_symptoms_evidence_and_approaches_for_best_case_management
- Edlin RS, Heyns CF, Van Vuuren SP, Zarrabi AD. Prevalence of histological prostatitis in men with benign prostatic hyperplasia or adenocarcinoma of the prostate presenting without urinary retention. S Afr J Surg. 2012 Nov 12;50(4):127-30. doi: 10.7196/sajs.1095. PMID: 23217554.
- Oshodi AJ, Nwabuisi C, Popoola AA, Edungbola LD, Agbede OO, Ii AAA, et al. Bacterial Uropathogen among Benign Prostatic Hyperplasia Patients at a Tertiary Hospital in Nigeria. 2015;(March):22–7.
- Chedi B, Wannang N, Halliru M, Bichi L. Seven months retrospective study on Urinary Tract Infection among patients at Aminu Kano Teaching Hospital, Kano - Nigeria. Bayero J Pure Appl Sci . 2011 Feb 23 ,2021 ;Sep 24,2(2):95–8. Available from: https://www.ajol.info/index.php/bajopas/article/view/63791
- Urinary infection before and after prostatectomy Pourmand G, Abedi AR, Karami AA, Khashayar P, Mehrsai AR - Saudi J Kidney Dis Transpl . 2021; Sep 24. Available from: https://www.sjkdt.org/article.asp?issn=1319-2442;year=2010;volume=21;issue=2;spage=290;epage=294;aulast=Pourmand
- Qiang W, Jianchen W, MacDonald R, Monga M, Wilt TJ. Antibiotic prophylaxis for transurethral prostatic resection in men with preoperative urine containing less than 100,000 bacteria per ml: a systematic review. J Urol. 2005 Apr;173(4):1175-81. doi: 10.1097/01.ju.0000149676.15561.cb. PMID: 15758736.
- Akobi O, Inyinbor H, Akobi E, Emumwen E, Ogedengbe S, Uzoigwe E. Lower Urinary Tract Infections among Patients Diagnosed of Benign Prostate Hyperplasia in Federal Medical Centre, Bida, North Central, Nigeria. Br J Med Med Res. 2016;14(7):1–9.
- Agbugui JO, Obarisiagbon EO, Osaigbovo II. Bacteriology of Urine Specimens Obtained from Men with Symptomatic Benign Prostatic Hyperplasia. Niger J Surg. 2016 Jul-Dec;22(2):65-69. doi: 10.4103/1117-6806.177415. PMID: 27843267; PMCID: PMC5013744.
- Kasper DL, Fanci AS, Longo DL, Braunwald SL, HanserJameson JL. Stamm WE. Urinary Tract Infections and Pyelonephritis. Harrison Principles of Internal Medicine,16th Edition , McGraw Hill, New York,. 1715–1721,2005.
- Oni AA, Mbah GA, Ogunkunle MO, Shittu OB, Bakare RA. Nosocomial infections: urinary tract infection in patients with indwelling urinary catheter. African J Clin Exp Microbiol . 2003 Jan 1 2021 Sep 25;4(1):63–71. Available from: https://www.ajol.info/index.php/ajcem/article/view/7326
- Taiwo S, Aderounmu A. Catheter associated urinary tract infection: Aetiologic agents and antimicrobial susceptibility pattern in Ladoke Akintola University Teaching Hospital, Osogbo, Nigeria. African J Biomed Res . 2009 Dec 10 [cited 2021 Sep 25];9(3). Available from: https://www.ajol.info/index.php/ajbr/article/view/48897
- Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013 Apr;35(2):121-6. doi: 10.4103/0253-7176.116232. PMID: 24049221; PMCID: PMC3775042.
- Adaeze E, Philip I, Francis O. Prevalence of urinary tract infection and antibiotic resistance pattern of isolates in benign prostatic hyperplasia patients with urinary catheter seen at a tertiary hospital in enugu state, nigeria. 2020 cited 2021 Dec 15;1:92–104. Available from: http://pharmacologyonline.silae.it
- Mishra PP, Prakash V, Singh K, Mog H, Agarwal S. Bacteriological Profile of Isolates From Urine Samples in Patients of Benign Prostatic Hyperplasia and or Prostatitis Showing Lower Urinary Tract Symptoms. J Clin Diagn Res. 2016 Oct;10(10):DC16-DC18. doi: 10.7860/JCDR/2016/21973.8734. Epub 2016 Oct 1. PMID: 27891339; PMCID: PMC5121677.
- Tille PM. Bailey & Scott’s Diagnostic Microbiology. 13th editi. Missouri Mosby: Elsevier Inc. 2014; 193–335 .
- Forbes BA, Sahm DF, Weissfeld AS. Bailey and Scott’s Diagnostic Microbiology. 12th Editi. Mosby: Elsevier, China. 2007; 824–855 .
- Coyne KS, Kaplan SA, Chapple CR, Sexton CC, Kopp ZS, Bush EN, Aiyer LP; EpiLUTS Team. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int. 2009 Apr;103 Suppl 3:24-32. doi: 10.1111/j.1464-410X.2009.08438.x. PMID: 19302499.
- Odoki M, Almustapha Aliero A, Tibyangye J, Nyabayo Maniga J, Wampande E, Drago Kato C, Agwu E, Bazira J. Prevalence of Bacterial Urinary Tract Infections and Associated Factors among Patients Attending Hospitals in Bushenyi District, Uganda. Int J Microbiol. 2019 Feb 17;2019:4246780. doi: 10.1155/2019/4246780. PMID: 30906323; PMCID: PMC6397969.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
