Journal of Biology and Medicine
Uses of Intravenous Immunoglobulin: A 13-Year Evaluation of Guillain–Barré Syndrome in Fez, Morocco
F El Hilali1*, H El Hilali1, M F Belahsen2, A El Idrissi3 and H Mazouz1
2University Hospital Hassan II of Fez, Morocco, North Africa
3City University of New York, College of Staten Island, USA
Cite this as
El Hilali F, El Hilali H, Belahsen MF, El Idrissi A, Mazouz H (2017) Uses of Intravenous Immunoglobulin: A 13-Year Evaluation of Guillain–Barré Syndrome in Fez, Morocco. J Biol Med 1(1): 001-005. DOI: 10.17352/jbm.000001Plasma-derived medicines in Morocco have been of great demand, and often on a short supply. Moroccan Intravenous Immunoglobulin (IVIG) formulation is still using sugar as stabilizer, while some other industries are discontinuing sugar and currently using amino acid as stabilizer. This is due to the fact that amino acid is rapidly metabolized/cleared from the blood circulation and has fewer adverse effects. The term Guillain-Barré syndrome (GBS) defines a recognizable clinical entity that is characterized by rapidly evolving symmetrical limb weakness, loss of tendon reflexes, absent or mild sensory signs, and variable autonomic dysfunction. Few studies have been conducted to investigate the use of IVIG products for GBS in Morocco. The objective of this study was to evaluate the use of IVIG and its adverse effects in Moroccan hospital. This retrospective study was conducted to evaluate the demographic and clinical features in 74 patients with GBS, and to assess the use of IVIG in these patients at University Hospital Hassan II of Fez - Neurology Department from 2003 to 2015. Forty-seven (64 %) male and 27 (36 %) female cases were studied, among whom nine patients had an infection. Eighty eight 88% of total patients had GBS and 12% had chronic inflammatory demyelinating polyneuropathy (CIDP). Among the 74 patients, 14 % patients showed spontaneous recovery. Twenty eight % of total patients received corticosteroid therapy, 1% of patients underwent Plasma Exchange (PE), 18% (13 patients) received IVIG. Among the 18% that received IVIG, 1 out of 13 patients developed symptoms of hypersensitivity, including fever and chills. Since the use of IVIG resulted in only 1 out 13 patients developed symptoms of hypersensitivity, we concluded that IVIG therapy had a better outcome. IVIG therapy provides patients with GBS, a safer and effective alternative to standard therapies.
Introduction
Plasma-derived medicines in Morocco have been of great demand, and often on a short supply. Moroccan Intravenous Immunoglobulin (IVIG) formulation is still using sugar as stabilizer, while some other industries are discontinuing sugar and currently using amino acid as stabilizer. This is due to the fact that amino acid is rapidly metabolized/cleared from the blood circulation and has fewer adverse effects. The term Guillain-Barré syndrome (GBS) defines a recognizable clinical entity that is characterized by rapidly evolving symmetrical limb weakness, loss of tendon reflexes, absent or mild sensory signs, and variable autonomic dysfunction. Few studies have been conducted to investigate the use of IVIG products for GBS in Morocco. The objective of this study was to evaluate the use of IVIG and its adverse effects in Moroccan hospital. This retrospective study was conducted to evaluate the demographic and clinical features in 74 patients with GBS, and to assess the use of IVIG in these patients at University Hospital Hassan II of Fez - Neurology Department from 2003 to 2015. Forty-seven (64 %) male and 27 (36 %) female cases were studied, among whom nine patients had an infection. Eighty eight 88% of total patients had GBS and 12% had chronic inflammatory demyelinating polyneuropathy (CIDP). Among the 74 patients, 14 % patients showed spontaneous recovery. Twenty eight % of total patients received corticosteroid therapy, 1% of patients underwent Plasma Exchange (PE), 18% (13 patients) received IVIG. Among the 18% that received IVIG, 1 out of 13 patients developed symptoms of hypersensitivity, including fever and chills. Since the use of IVIG resulted in only 1 out 13 patients developed symptoms of hypersensitivity, we concluded that IVIG therapy had a better outcome. IVIG therapy provides patients with GBS, a safer and effective alternative to standard therapies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculo-neuropathy, manifested by progressive flaccid paralysis, with a reported annual incidence of 0.5–2 cases per 100,000 people [5]. Molecular mimicry of pathogen-borne antigens, leading to generation of cross-reactive antibodies which are autoantibodies against ganglioside, is part of the pathogenesis of GBS [6]. Most patients recover spontaneously. In a small number of patients the inflammation in the nerve can be severe enough to cause degeneration of the whole nerve fibers. In those patients, the recovery is often slower and incomplete. A chronic form of this illness may present with progressive symptoms and result in chronic inflammatory demyelinating polyneuropathy (CIDP) [7, 8]. GBS is usually a post-infectious disorder occurring outside the context of pre-existing disease, and represents a model for post-infectious autoimmune disorders [9]. The criteria for a diagnosis of GBS are summarized on clinical features, lumbar puncture, nerve conduction studies, magnetic resonance imaging, and serum antibodies [10].
There is no known cure for GBS. But treatments can help improve symptoms of GBS and shorten its duration. Esposito et al. [10] reported three therapeutic approaches to GBS [10]. First, the general medical care such as; monitoring of respiratory, cardiac and hemodynamic function, prophylaxis for deep vein thrombosis, management of possible bladder and bowel dysfunction, early initiation of physiotherapy and rehabilitation. Second, the psychosocial support such as; pain management using opioids or non-steroidal anti-inflammatory drugs, immunological treatment with documented efficacy, IVIG therapy, and Plasma exchange (PE). Third, the new treatments under evaluation, such as Interferon-beta, Cyclophosphamide, Rituximab and Eculizumab. The US Food and Drug Administration (FDA) approved IVIG for use in GBS and CIDP [11].
Since there are limited studies on the use of IVIG and its adverse effects on patients, therefore, the purpose of this retrospective study was to investigate and evaluate the use of IVIG by reporting a 13-year period from 2003 to 2015 at the University Hospital Hassan II of Fez - Neurology Department, Morocco, in 74 patients with GBS and CIDP.
Materials and Methods
Sources and preparation of IVIG
In Morocco, the plasma is prepared from whole blood donation. It is separated from cellular elements by centrifugation and frozen at - 25°C within 6 to 24 h after collection. Required tests are the determination of ABO blood-Rh (D) phenotype Rh, Kell, screening haemolysin anti-A and anti-B, antierythrocyte antibodies, antigens and antibodies to HIV1 and HIV2, antibodies to HCV (for the detection of the hepatitis C virus), (HBsAg) (surface antigen of hepatitis B virus), syphilis (TPHA) and the determination of Alanine Amino Transferase level.
The first batches of IVIG were received from LFB fractionator in 2005. The contract defined the obligations and the respective administrative and technical responsibilities of both parties in the procedures for collecting, monitoring, plasma preparation and storage. One of the main requirements of the fractionator was to set up a quality system in all the Regional Blood Transfusion Centers (CRTS) that meet the fractionator’s requirements. Plasma for fractionation is a pool of plasma from non-remunerated donors including males and females both [4]. The manufacturing process is divided into three parts; 1- Protocol of purification; Ethanol precipitation, depth filtration. 2- Viral inactivation step; pH4/pepsin and 3- Inactivation of infectious agents step; Nanofiltration. The protein concentration of Moroccan IVIG preparations is 50 mg/ml, sucrose is the stabilizing agent, and it contains also sodium 8mg/ml, IgA 17mg/g, and traces of pepsin. Moroccan IVIG preparations are medical product for hospital prescription only. They are available only as a powder and solvent for solution for infusion.
Methods
The retrospective chart review of 74 consecutive patients, aged between 10-80 years and admitted with the Symptoms of GBS, was done at University Hospital Hassan II of Fez; Neurology Department, between 2003 and 2015. The diagnosis of GBS was made on well-established criteria, excluding other neurological disorders. IVIG dose is 0.4g/kg of body weight per day for 5 days. The study was formally approved by the ethical committee at the University of Fez on June 2015. Data were collected from patient registers. Data used in this study also included the following methods: Demographic and clinical data; age, gender, type of therapy used, adverse effects, in hospital outcomes, and associated pathologies.
Results
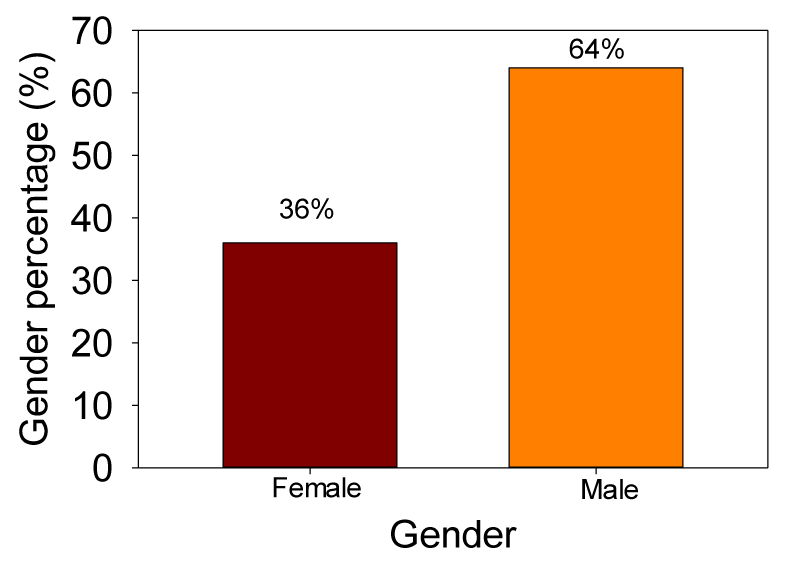
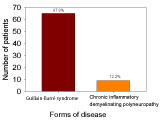
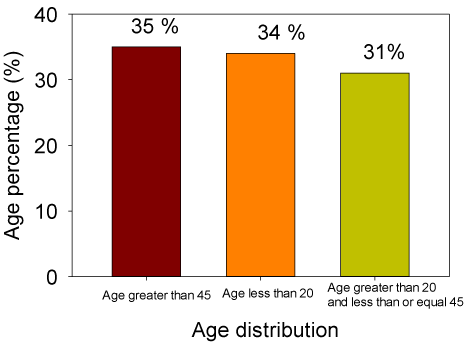
This retrospective study showed that there were 47 (64 %) male and 27 (36 %) female cases, thereby indicating a significantly higher proportion of males (Figure 1), among them nine patients had an infection. Varied clinical observations were found; however the most common clinical feature was the severe weakness. Eighty eight % of patients were diagnosed with GBS and 12% of patients had CIDP (Figure 2). GBS is more common in adults aged 45 and over (Figure 3). A total of 28 (38%) patients had other associated pathologies; vasculitis, tuberculosis, pneumopathy, pruritus, pressure ulcers, Wegener’s granulomatosis, epitaxy, pancytopenia, urinary infection, arterial hypertension, diabetes, dermatophyte, cardiac failure, functional colopathy, cholecystectomy, chronic gastritis, prostatic hypertrophy, urethritis, poliomyelitis, icterus, facial palsy, flu, cardiopathy, diarrhea, and sinusitis.
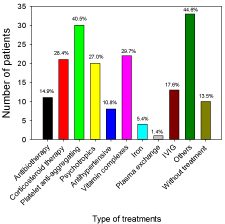
Fourteen % of patients showed spontaneous recovery. The medical treatments administered to patients were; 15% of patients received antibiotherapy, 28% of patients received corticosteroid therapy, 41% of patients received platelet anti-aggregating, 27% of patients received psychotropics, 11% of patients received antihypertensive, 30% of patients received vitamin complexes, 5% of patients received iron, 1% of patients underwent Plasma Exchange (PE), 45% of patients received other treatments, 18% of patients received IVIG (Figure 4). No significant toxicity and/or adverse events related to the IVIG therapy was observed, except 8 % of patients who developed symptoms of hypersensitivity, including fever and chills. Seventy six 76 % of patients were treated with more than one medication. All patients have been released from hospital after a real improvement in their daily life. Physical therapy has been described for all patients before and during recovery.
Discussion
Guillain-Barré syndrome (GBS) is the most common and most severe acute paralytic neuropathy, with about 100 000 people developing the disorder every year worldwide. GBS is often preceded by an infection. This could be a bacterial or viral infection. It may also be triggered by vaccine administration or surgery. There is no known cure for GBS. But treatments can help improve symptoms of GBS and shorten its duration. GBS is typically treated with PE to remove antibodies from the blood or IVIG [5].
This study did not include the patients who developed respiratory failure and treated in intensive-care units. Results indicated that there were 47 (64 %) male and 27 (36 %) female cases, thereby indicating a male preponderance coinciding with many studies on GBS [12, 13]. In our study, nine patients had an infection. Current reviews revealed that Campylobacter jejuni is the most widely reported infection associated with GBS. Other infections are those due to cytomegalovirus, Epstein–Barr virus, measles, influenza A virus and Mycoplasma pneumoniae, as well as enterovirus D68 and Zika virus. GBS has also been observed in patients who had recently been vaccinated against rabies, influenza A/H1N1 [10]. Eighty eight % of patients had GBS and 12% of patients had chronic inflammatory demyelinating polyneuropathy which is even more insidious and which behaves like a chronic form of GBS, creeping up more slowly, and typically without an inciting event. CIDP is closely related to GBS. CIDP isn’t linked to illness. With GBS, once treated, most people recover fairly quickly. CIDP, on the other hand, tends to be a longer-term problem. In rare cases, people who don’t recover from GBS may develop CIDP. The diagnosis of acute-onset CIDP should be considered when a patient thought to have GBS deteriorates again after 8 weeks from onset or when deterioration occurs 3 times or more [14].
A total of 28 (38%) patients had other associated pathologies and the most of them were infection. An interesting study sought to report one case of the association between GBS with Salmonella paratyphi A, the patient was treated with ceftriaxon (2 g administered intravenously, daily for 10 days) [15]. Fourteen % of patients showed spontaneous recovery. Lundkvist et al. [16] investigated the spontaneous recovery from the GBS by analyzing the ability of post recovery serum to inhibit anti-neuroblastoma cell line antibody binding in sera from seven patients in the pre-recovery phase or with a chronic form of the disease. They reported its association with anti-idiotypic antibodies recognizing a cross-reactive idiotype on anti-neuroblastoma cell line antibodies [16]. In this study, many medical treatments were administered to patients with GBS and CIDP, physical therapy has also been described for all patients before and during recovery, this is consistent to the Johns Hopkins Hospital treatment regimen; it is about rehabilitation during the recovery, corticosteroids for patients with CIDP, IVIG or PE for acute exacerbations of symptoms, and anti-seizure medications, antidepressants and analgesics including opiate drugs for neuropathic pain due to CIDP [17].
Our study reported that 18% of patients were treated with IVIG and 1 % of patients were treated by PE. Kuwahara et al. [18], reported that the efficacy of PE and IVIG therapies for GBS has been established, but IVIG is slightly safer and much easier to use than PE. Traditionally, treatments in our region were corticosteroid, PE, vitamin complexes, iron. However, recently, IVIG, has been introduced in this region, and its use is expensive for the patients. Although, we do not have any record in the patients clinical record, our observation was that the patient’s health was improved. Research on comparing IVIG group with other group is needed in the future. IVIG therapy provides our patients, a safer and effective alternative to standard therapies.
The efficiency of Moroccan IVIG was reported in previous prospective and non-randomized study. The study included all patients with GBS who required mechanical ventilation (MV). There were two groups: group 1 (group treated by IVIG) and group 2 (group treated by Plasma Exchange P E). Results suggested that IVIG have more benefits to patients than PE; For the IVIG group, the ICU stay was shorter, weaned earlier of MV and the beginning of motility recuperation was precocious than PE group [19].The low percentage of patients who received IVIG, that may be due to the fact that in Morocco, IVIG is very expensive and it is not covered by the health care, and disadvantaged patients cannot afford this therapy. On the other hand, the shortage of blood and blood donors in Morocco [20], makes IVIG a mainstay therapeutic of various inflammatory autoimmune diseases, scarce, and expensive product. Zuercher et al. [21] discussed the potential next generation biologics to replace IVIG [21]. Furthermore, Cherin et al. [22] reported that subcutaneous immunoglobulin (SCIg) therapy could be an alternative of IVIG in patients with autoimmune neuromuscular disorders who have difficult venous access or with insufficient response, and for patients preferring home care setting [22].
In our series, GBS is more common in adults aged 45 and over. Anyone can develop GBS; however, it is more common among older adults. The incidence of GBS increases with age, and people older than 50 years are at greatest risk for developing GBS [5, 23].
In our study, we experienced lower incidence of adverse effects of IVIG (1 out of 13) compare to other studies [24, 25].
Conclusion
Conclusively, in this retrospective regional study, we studied the demographic and clinical data on patients with GBS and CIDP and evaluated the use of IVIG therapy and its adverse effects. While the ultimate decision of IVIG therapy can depend on the possibility of procuring a mostly expensive IVIG that is not covered by the Moroccan social security system for health, IVIG therapy provides patients with GBS, a safer and effective alternative to standard therapies.
Although, we do not have statistical data to prove that IVIG worked better comparing with other treatments, our observation was that the patients’ health was improved, and the good and precocious recovery was observed. Research on comparing IVIG group with other groups is needed in the future.
- Shoenfeld Y, Katz U (2005) IVIg therapy in autoimmunity and related disorders: our experience with a large cohort of patients. Autoimmunity 38: 123-37. Link: https://goo.gl/WxKwQq
- Hooper JA (2008) Intravenous immunoglobulins: evolution of commercial IVIG preparations. Immunol Allergy Clin North Am 28: 765-778. Link: https://goo.gl/K5pcVn
- Burnouf T, Seghatchian J (2014) Go no Go in plasma fractionation in the world’s emerging economies: still a question asked 70 years after the COHN process was developed!. Transfus Apher Sci 51: 113-119. Link: hsssttps://goo.gl/MRXzsz
- Boulahdid S, Benahadi A, Adouani B, Laouina A, Mehdaoui FE, et al. (2014) Improvement of national blood safety profile: effect of contract fractionation of plasma in resource limited countries, the Moroccan experience. Haemophilia 20: e359–362. Link: https://goo.gl/MZpnWf
- World Health Organization (WHO), accessed 24.01.2017. Link: https://goo.gl/uGO8ru
- Van Sorge NM, van der Pol WL, Jansen MD, van den Berg LH (2004) Pathogenicity of anti-ganglioside antibodies in the Guillain-Barré syndrome. Autoimmunity Reviews. 3: 61-68. Link: https://goo.gl/PPdSFR
- Bradshaw DJ, Jones HR (1992) Guillain-Barre syndrome in children: clinical course, electrodiagnosis, and prognosis. Muscle Nerve 15: 500–506. Link: https://goo.gl/EOlzpm
- Hartung HP, Kieseier BC, Kiefer R (2001) Progress in Guillain-Barre syndrome. Curr Opin Neurol 14: 597– 604. Link: https://goo.gl/Spk5y7
- Samarkos M, Vaiopoulos G (2005) The role of infections in the pathogenesis of auto-immune diseases. Current Drug Targets - Inflammation & Allergy 4: 71-75. Link: https://goo.gl/w5iqyG
- Esposito S, Longo MR (2017) Guillain–Barré syndrome. Autoimmunity Reviews 16: 96–101. Link: https://goo.gl/bUbI6O
- Food and Drug Administration (FDA), accessed 24.01.2017. Link: https://goo.gl/Na7RRn
- Yakoob MY, Rahman A, Jamil B, Syed N (2005) Characteristics of Patients with Guillain Barre Syndrome at a Tertiary Care Centre in Pakistan, 1995-2003. J Pak Med Assoc 55: 493-496. Link: https://goo.gl/q3Wat1
- Sharma G, Sood S, Sharma S (2013) Seasonal, Age & Gender Variation of Guillain Barre Syndrome in a Tertiary Referral Center in India. Neuroscience and Medicine 4: 23-28. Link: https://goo.gl/hpcHcP
- Ruts L, Drenthen J, Jacobs BC, van Doorn PA (2010) Distinguishing acute-onset CIDP from fluctuating Guillain-Barre syndrome: a prospective study. Neurology 74: 1680-1686. Link: https://goo.gl/ayPuOY
- Khan FY, Kamha AA, Abbas MT, Miyares F, Elshafie SS (2007) Guillain-Barré syndrome associated with Salmonella paratyphi A. Clin Neurol Neurosurg 109: 452-454. Link: https://goo.gl/OMExtB
- Lundkvist I, van Doorn PA, Vermeulen M, Brand A (1993) Spontaneous recovery from the Guillain-Barré syndrome is associated with anti-idiotypic antibodies recognizing a cross-reactive idiotype on anti-neuroblastoma cell line antibodies. Clin Immunol Immunopathol 67: 192-198. Link: https://goo.gl/e1ydPH
- Johns Hopkins Medicine, accessed 24.01.2017. Link: https://goo.gl/MtOiGj
- Kuwahara M, Kusunoki S (2016) Novel Therapy in Guillain-Barré Syndrome. Brain Nerve 68: 1423-1429. Link: https://goo.gl/3eMvcI
- Charra B, Hachimi A, Benslama A, Motaouakkil S (2014) Intravenous immunoglobulin vs plasma exchange in treatment of mechanically ventilated adults with Guillain-Barré syndrome. Pan Afr Med J 18: 35. Link: https://goo.gl/nAJdHJ
- El-Hilali F, El-Hilali H, Dheeb BI, Traore BM, Messouak M, et al. (2016) Blood Transfusion Utility During Cardiopulmonary Bypass and Correlation with Key-Biochemical Laboratory Findings: A New Approach to Identify Preventive and Risk Factors (1-Year Practice at University Hospital Hassan-II of Fez). Biochem Anal Biochem 5: 290. Link: https://goo.gl/KFOc6m
- Zuercher AW, Spirig R, Baz Morelli A, Käsermann F (2016) IVIG in autoimmune disease - Potential next generation biologics. Autoimmun Rev 15: 781-785. Link: https://goo.gl/zuyrmk
- Cherin P, Belizna C, Cartry O, Lascu-Dubos G, de Jaeger C, et al. (2016) Long-term subcutaneous immunoglobulin use in inflammatory myopathies: A retrospective review of 19 cases. Autoimmun Rev 15: 281-286. Link: https://goo.gl/MpirGc
- Centers for Disease Control and Prevention (CDC), accessed 24.01.2017. Link: https://goo.gl/QJI6yj
- Gürcan HM, Ahmed AR (2007) Frequency of adverse events associated with intravenous immunoglobulin therapy in patients with pemphigus or pemphigoid. Ann Pharmacother 41: 1604-1610. Link: https://goo.gl/YIIO5e
- Frances Rose R Palabrica, Shirley L Kwong, Padua F R (2013) Adverse events of intravenous immunoglobulin infusions: a ten-year retrospective study. Asia Pac Allergy 3: 249–256. Link: https://goo.gl/9XNZiN
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
