Open Journal of Biological Sciences
Isolation and characterization of probiotic bacteria from fruit pulp, screening of probiotic properties and production of bacteriocin and probiotic curd
Dhanvanti B Kamaliya, Bhumi M Javia, Megha S Gadhvi and Dushyant R Dudhagara*
Cite this as
Kamaliya DB, Javia BM, Gadhvi MS, Dudhagara DR (2023) Isolation and characterization of probiotic bacteria from fruit pulp, screening of probiotic properties and production of bacteriocin and probiotic curd. Open J Biol Sci 8(1): 033-042. DOI: 10.17352/ojbs.000036Copyright
© 2023 Kamaliya DB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The present study was investigated to isolate probiotic lactic acid bacteria from Actinidia deliciosa (kiwi fruit) pulp. A total of eight isolates were found and two of them were probiotic LAB strains. The both A2 and A5 isolates strains were Gram-positive, catalase and oxidase negative, non-spore forming, non-motile, rod or short cocci shaped bacteria. The A2 and A5 strains both survived well in conditions of low pH, 0.4% phenol and high bile salt concentrations. They could grow at different temperatures and were resistant to different NaCl concentrations. A study was also conducted on the various probiotic and safety attributes of isolates. Bacteriocins, a kind of ribosomal protein produced by LAB, were estimated using the Folin-Lowery method. The formation of bacteriocins was influenced by various physical and chemical factors. The result demonstrates that the optimum conditions for the production of bacteriocins by the A2 strain were glucose as carbon source, pH 6.5, 2% NaCl and 1% bile salts. For the A5 strain, sucrose as a carbon source, pH 6.5, 1% NaCl and 1% bile salt were optimum conditions for bacteriocin production. Study was also conducted on exopolysaccharide (EPS) production, adhesion characteristics, antagonistic activity and antibiotic sensitivity. Probiotic curd (Dahi) formation was done and several parameters of curd like pH, total lactic acid concentration and water-holding capacity were all investigated.
Introduction
The term “probiotic” was coined by Lilly and Stillwell (1965) to describe the “substances secreted by one microorganism that stimulate the growth of another” [1]. The name “probiotic” comes from the Greek word “protokos” which means “for life”. According to the Food and Agriculture Organization of the United Nations (FAO) and World Organization (WHO) in 2001, probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits on the host [2,3]. In more modern definitions, the concept of action on the gut microflora and even that of live microorganisms has disappeared. Salminen, et al. (1999) defined probiotics as “food which contains live bacteria beneficial to health” [4]. Microorganisms’ efficient digestion and maximum absorption of nutrients increase the capacity of the host to exclude infectious microorganisms and prevent diseases [5].
Live bacteria called probiotics have the potential to both treat and prevent many diseases. Consuming certain meals, beverages, and dietary supplements can provide probiotics [6]. Commonly used probiotics contain microorganisms from the genus Bifidobacterium and a diverse group of lactic acid bacteria (Lactobacillus, Enterococcus) [7]. Lactic acid bacteria (LAB) play an important role in the production of probiotics. LAB comprises a wide range of genera and includes a diverse number of species [8].
They are a group of Gram-positive cocci or rods, non-spore-forming, catalase-negative, low pH tolerant. Lactic acid bacteria are generally considered safe in the food industry, so they are widely used in food preservation and to promote health. LAB is mostly used to produce a variety of fermented vegetables, meat, and dairy products [9].
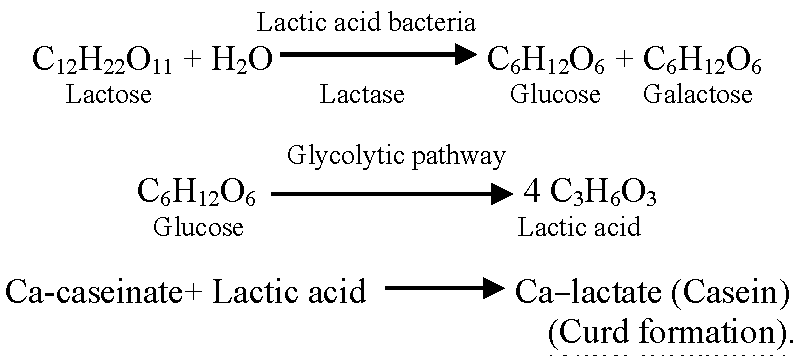
Dahi, also referred to as curd, is a traditional fermented milk product that is beneficial to humans in terms of nutrition and health [10]. The mechanism of curd formation during Dahi making is as follows [11].
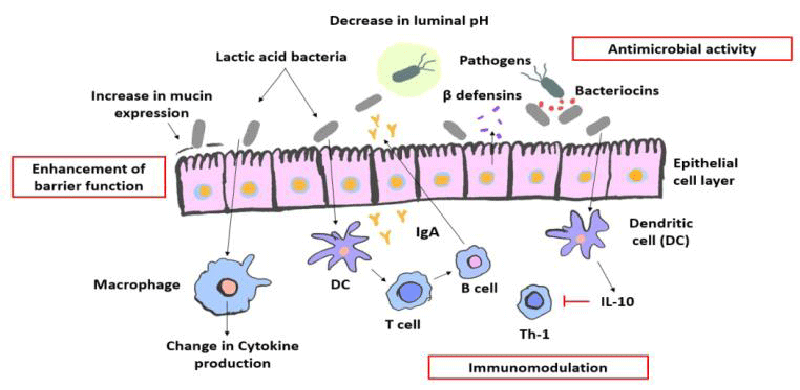
Probiotic organisms are thought to promote the health of the host. Numerous pathways have been discovered in these studies to try and explain how probiotics might protect the body from internal disease [4]. By altering gut microflora, enhancing the gut mucosal barrier, preventing pathogen adherence, pathogen inactivation, altering dietary proteins by intestinal microflora, altering bacterial enzyme activity, affecting gut mucosal permeability, and regulating the immune system, they provide particular health benefits [12]. Lactic acid bacteria produce short-chain fatty acids, viz., acetic, propionic, and butyric acids. These acids lower intestinal pH and prevent the growth of harmful microbes [13] Figure 1.
Positive effects of probiotics include effects on mineral metabolism, especially bone stability, and prevention of osteoporosis, strengthening the intestinal barriers, prevention of colon cancer and urogenital infections, management of lactose intolerance, reduction of cholesterol and blood pressure, reduction of the inflammatory actions of the body, reduction of Helicobacter pylori infection, suppression, and control of pathogenic microorganism’s growth [15,16].
Exopolysaccharides may be produced by several probiotic LAB (EPS) [17]. EPS protect cells from harmful environmental factors such as dehydration, extreme temperature, acid, osmotic stress, phagocytosis, macrophages, and antibiotics by forming layer surrounding cells [18]. The unique structural features have made bacterial EPSs of particular interest in the fields of chemistry, medicine, and the food industry [19]. EPS are frequently employed in the food industry as viscous, stabilising, and emulsifying agents due to their capacity to hold water [20]. It improves the texture and rheological texture and sensibility of bread and dairy products such as yoghurt and cheese [21].
The bacteriocins produced by Lactic Acid Bacteria (LAB) have a lot of potential as food biopreservation agents because they can suppress the growth of pathogenic and spoilage bacteria. Bacteriocins are proteinaceous molecules with ribosomal encoding [22]. However, the amount of bacteriocin produced can be impacted by environmental parameters like temperature, pH, and media composition [23]. In the current work, we examined the optimal conditions for probiotic bacteria to produce the bacteriocin.
Many fruits, including bananas, yellow and red apples, grapes, kiwis, and oranges, can contain LAB [24]. Fruits are an important factor of a balanced diet in our everyday routines. Due to its numerous benefits over the past 20 years, the kiwi fruit has grown extremely popular according to [25], kiwi fruit contains a number of important anti-cancer bioactive substances that have prooxidant, antioxidant, tumor-selective, cytotoxic, and antibacterial activities.
The purpose of the current research is to identify novel probiotic species from kiwi fruit and determine their health-promoting qualities, relevance to the food sector, and potential for use in future medical studies.
Materials and methods
Sample collection and isolation of probiotic bacteria
Actinidia deliciosa (kiwi), along with other prebioticated fruits, were collected from the local market in Junagadh, Gujarat, India. The fruits were left to ripen for 3 to 5 days. It was then cleansed with sterile, deionized water. The husk was cut off using a knife, then crushed with a sterile Mortan pestle under aseptic conditions. According to the method of Gracia, et al. 2016 [26], 25 gm of fresh fruit pulp was obtained and suspended in 225 ml of sterile peptone water (0.1 gram/100 ml), which was homogenized for 3 min at room temperature. Then, further dilutions (10-1 to 10-5) were made. 100 µl of each dilution’s aliquots were spread out on de Man Rogosa Sharpe (MRS) agar and incubated anaerobically at 37 ºC for 48–72 hours. After incubation, morphologically dissimilar colonies were selected and re-streaked on an MRS agar plate to get pure isolates. The isolated colonies were identified by colony Morphology, Gram staining, Catalase test, Oxidase test, and other biochemical tests. The identified organisms were stored at -20 ºC in a 70% glycerol stock solution for further studies.
Screening of safety and probiotic properties of LAB
Gelatinase activity: The gelatinase activity of isolates was investigated as described by Liliane, et al. 2019 [27]. Isolated bacterial strains were streaked into tubes containing nutrient gelatin agar (peptone 5 gm/L, beef extract 3 gm/Land gelatin120 gm/L) (Himedia, Mumbai, India). The inoculated tubes were incubated at 30 ºC for 7-10 days, with liquefaction being monitored daily and refrigerated at 4 ºC for 1 h. A strain of Pseudomonas was used as a positive control.
Hemolytic activity: According to Bazireh, et al. 2020 [28], the isolates were streaked on blood agar plates and incubated at 30 ºC for 24-48 h. They were observed for the zone of hemolysis surrounding the colonies.
Antibiotic sensitivity test: Antibiotic sensitivity of isolates was checked by disc diffusion method as described by Yasmin, et al. (2020) [29]. A 0.2 µl bacterial suspension was spread evenly on the surface of MRS agar plates. The inoculated plates were allowed to dry before placing the disc containing antibiotics. Standard concentrations of antibiotics including Rifampin, Streptomycin, and Chloramphenicol were utilized.
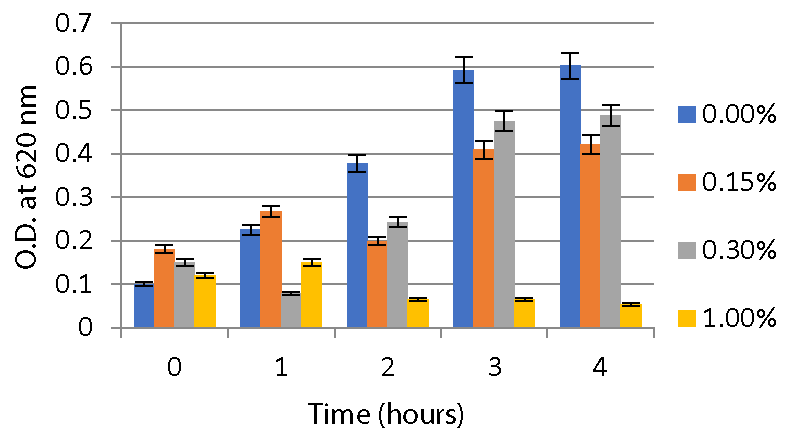
Acid and bile salt tolerance test: According to the slightly modified method described by Gracial, et al. 2016, the cell pellets were harvested by centrifugation and re-suspended in 10 ml of PBS with pH adjusted to 2.0, 3.0, 5.0 and 7.0 to check acid tolerance. For bile salt tolerance, cells were resuspended in 10 ml MRS broth containing 0%, 0.15%, 0.3%, and 1% bile salt. The growth of viable microorganisms was enumerated at 0, 1, 2, 3, and 4 h. A UV-VIS Spectrophotometer was used to measure absorbance at 620 nm, which was used to track the growth of microorganisms.
Antagonistic activity: The antagonistic activity of isolates was determined by the agar well diffusion method as described by Yasmin, et al. 2020 with slight modification. The pathogenic bacteria were spotted on the Muller Hinton agar plate. Prepare a bacterial suspension in 0.85 % NaCl (1.505 OD at 620 nm). Then add 0.5 ml probiotic bacterial culture to each well and incubate all the plates at 37 ºC for 24-48 h in the incubator. Then observe the zone of clearance.
Cell surface hydrophobicity test: The degree of hydrophobicity of the isolates was assessed using Yasmin, et al. 2020’s approach and a slightly modified version of it. The cells were washed two times with PBS having pH 7 and resuspended in 5 ml PBS having pH 7. The cell suspension was incubated at 37 ºC for 10 min. Then take absorbance at 600 nm at 0 h (A0). After that, 3 ml of cell suspension was mixed with 1 ml of hydrocarbon (xylene). For phase separation, the cell suspension and maintained for 20 min. At 600 nm, absorbance was measured after the aqueous phase (at the bottom) was collected (A1).
Hydrophobicity (%) = (1 − A1/A0) × 100
Whereas, A0 = Initial absorbance, A1 = Final absorbance
Physiological and biochemical characterization
Growth at different NaCl concentrations: According to Samedi and Charles in 2019 [30], the 0.1 ml overnight culture of probiotic strains was inoculated in test tubes containing 10 ml MRS broth containing 2%, 4%, and 6.5% (W/V) of NaCl concentration. The appropriate amount of bromocresol purple (indicator) was added to the broth and incubated at room temperature for 7 days.
Growth at different temperature: In the method described by Samedi and Charles in 2019, the 0.1 ml overnight culture of probiotic strains was inoculated in test tubes containing 10 ml modified MRS broths. Bromocresol purple (0.04 g/l) was added to the broth and incubated for 7 days at four different temperatures; 10 ºC, 30 ºC, 37 ºC, and 50 ºC.
Resistance to phenol: According to Yasmin, et al. 2020 the 0.1 ml probiotic strains were inoculated in 10 ml of 0.4% phenol-containing MRS broth and incubated at 37 ºC for 24h. Optical density was measured at 620 nm for 0 min immediately after inoculation and again after 24 h.
Carbohydrate fermentation test: The carbohydrate fermentation test was described by Kavitha and Devasena in 2013 [31]. 0.1 ml of bacterial suspension was added to a test tube containing modified MRS broth with 2% of various sugars such as glucose, fructose, lactose, mannitol, and sucrose at pH 7.4 and incubated at 30 ºC for 48 hours. A tube without inoculation was kept as a negative control. A change in colour from purple to yellow indicated a positive reaction. The pH of the broth was checked after 48 h.
Production of Exopolysaccharide (EPS)
EPS production was determined according to the method described by Yasmin, et al. 2020. Both strains were inoculated into MRS broth and incubated at 37 ºC for 24-48 h. After incubation, cell pellets were removed by centrifugation, then 4% trichloroacetic acid was added and the mixture was vortexed at 4 ºC for 3 h. Precipitated proteins were removed through centrifugation at 10,000 rpm for 20 min. The supernatant was concentrated through evaporation. The precipitated EPS was then mixed with an equivalent volume of 2-isopropylalcohol and incubated at 4 ºC overnight. The precipitates were collected after centrifuging the mixture for 20 min at 10000 rpm. The EPS is a carbohydrate, whose amount was calculated by the phenol sulfuric acid method described by Neeru, et al. 2015.
Production of bacteriocin
The probiotic strains were inoculated in MRS broth for 24-48 h. The supernatant was collected after incubation and used as a sample for estimation of bacteriocins. The concentration of soluble protein in the culture supernatant was estimated according to the [32].
Optimization of bacteriocins production
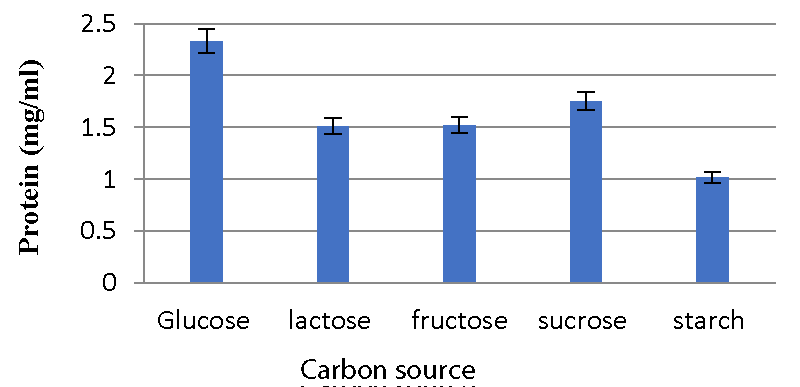
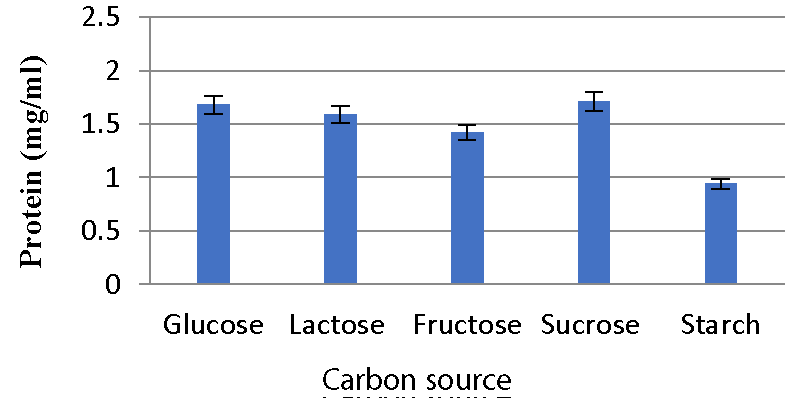
Effect of carbon sources on bacteriocin production: Meera, et al. 2012 [33] evaluated the effect of different carbon sources on bacteriocin production. The 2% dextrose, fructose, sucrose, starch, and lactose were supplemented. The 0.2 ml bacterial suspension was added to each tube containing 10 ml MRS broth with different carbon sources. Each tube was incubated at 37 ºC for 48 hours. The supernatant was collected by centrifugation. The bacteriocin content was determined by Folin-Lowry’s method.
Effect of NaCl concentration on bacteriocin production: The effect of salt on bacteriocin production was evaluated according to the method described by Meera, et al. 2012 [33], the 0.2 ml of probiotic bacteria was inoculated into 10 ml MRS broth (pH 6.5) supplemented with 1%, 2%, 3%, and 4% NaCl salt concentration. Then all the tubes were incubated at 37 ºC for 48 hours. The supernatant was collected by centrifugation at 10000 rpm for 10 min. The protein concentration was determined by Folin-Lowry’s method.
Effect of different pH on bacteriocin production: Meera, et al. 2012 [33] evaluated the effect of bile salts concentration on bacteriocin production. MRS broth with various pH values 5, 6, 7, and 8 was used to assess the effect of pH on bacteriocin production. The 0.2 ml of bacterial suspension was inoculated in 10 ml MRS broth at a different pH. The tubes were incubated at 37 ºC for 48 hours. Then the supernatant was collected by centrifugation. Folin-Lowry’s method was used to determine the protein concentration.
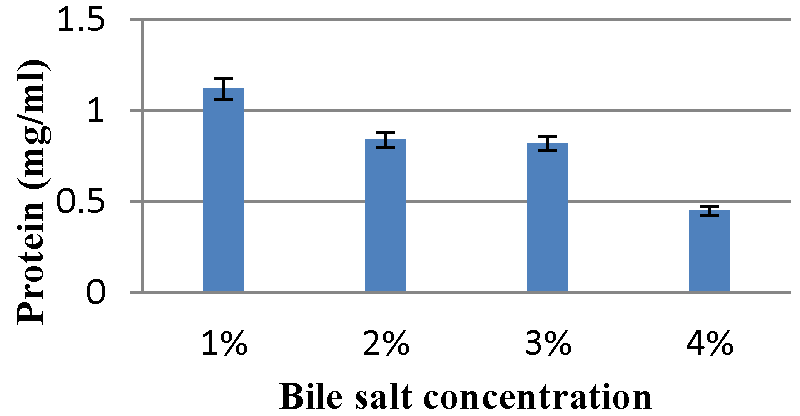
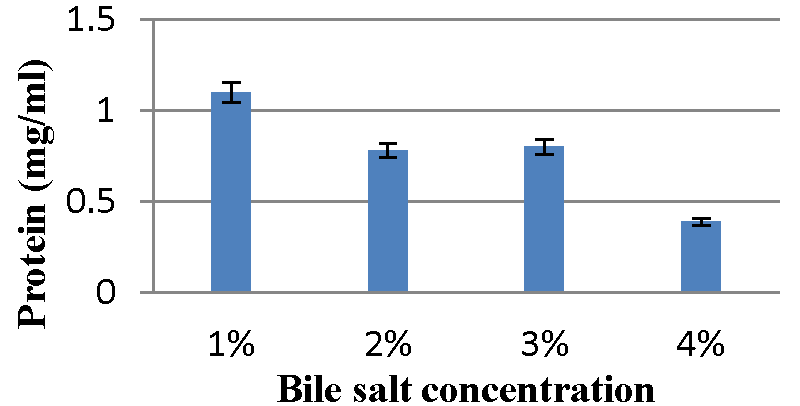
Effect of bile salt concentration on bacteriocin production: The influence of bile salt content on bacteriocin production was studied by Meera, et al. 2012 [33]. The 0.2 ml probiotic organism was inoculated in 10 ml MRS broth (pH 6.5) supplemented with 1%, 2%, 3%, and 4% bile salt respectively. Then all the tubes were incubated at 37 ºC for 48 hours. The supernatant was collected by centrifugation at 10000 rpm for 10 min. The protein concentration was determined by Folin-Lowry’s method.
Production of probiotic curd
Both probiotic strains were inoculated in MRS broth for 24-48 hours. The cell pellets were harvested by centrifugation. The 0.85% sterile NaCl solution was used to wash the cell pellets. The cells were then resuspended in sterile 0.85% NaCl solution and incubated for 10 min at 37 ºC. 50 ml of sterile milk was inoculated with 1.5 ml of the bacterial culture and it was then incubated at 37 ºC for 18 to 20 hours. Several curd properties were examined after incubation.
pH of curd: pH of curd was measured after the completion of incubation.
Total titratable acidity of curd: Titratable acidity was determined by neutralising the acid present in 10gm of the curd samples using a 0.11N NaOH solution. The titration was performed using 10 drops of phenolphthalein as an indicator until a pink endpoint was reached. The total titratable acidity is calculated by the following equation (https://agrimoon.com/estimation-of-titratable-acidity-in-curd/) [34].
Titratable acidity = 9AN/W
Where A = volume NaOH used for titration,
N= Normality of NaOH
W= weight of curd.
Water holding capacity of curd: Take 10 gm of curd in tubes and centrifuge them at 10,000 rpm for 15 min. The WHC was calculated by the following equation:
WHC (%) = (W1-W2/W1)100
Where, W1 =weight of the curd in grams, W2 = weight in grams of pellets after centrifugation.
Results
Isolation and Identification of probiotic bacteria
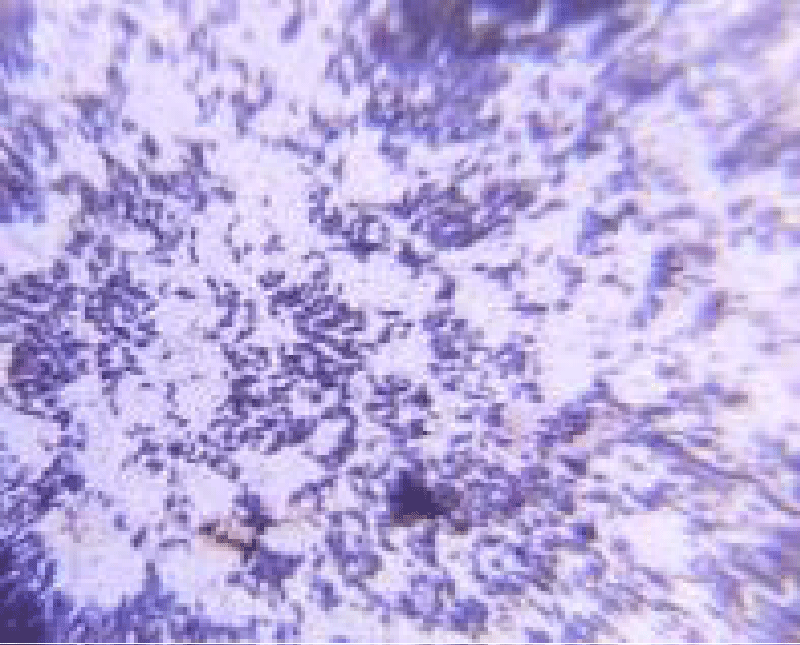
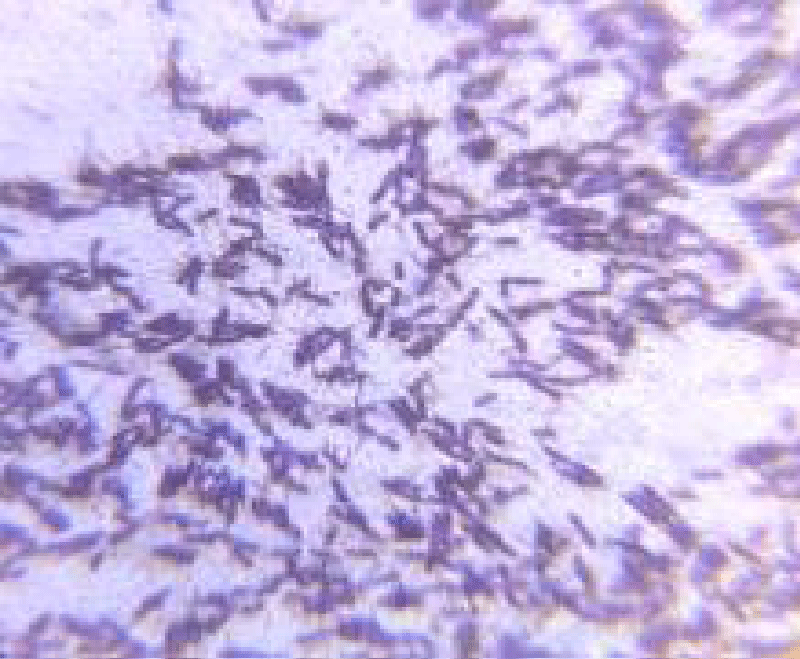
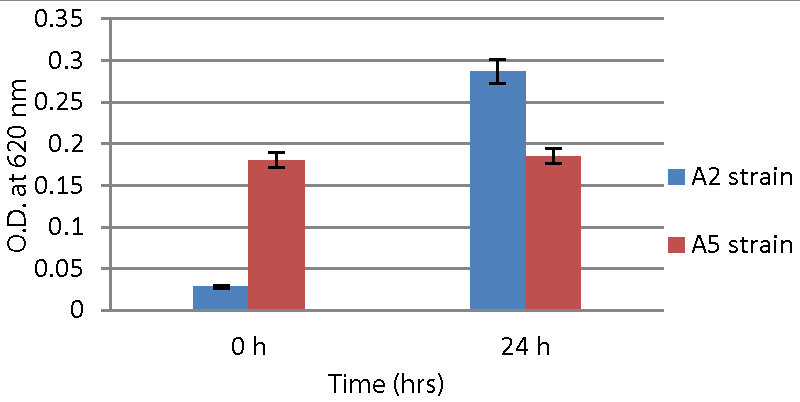
Total Eight isolates were initially obtained from kiwi fruit pulp. Out of these, A2 and A5, isolates exhibited all the characteristics of potential probiotic bacteria. They all have similar biochemical properties as LAB. The A2 strain was gram-positive, short rod-shaped, and had a pinpoint, cream-colored colony. The A5 strain appeared as a small, white colony and was gram-positive, rod-shaped as shown in Figures 2,3.
Screening of safety and probiotic properties of LAB
Gelatinase activity: The results obtained showed that organisms do not liquefy the gelatin agar, which confirmed that both strains have gelatinase-negative activity when compared with a gelatinase-positive organism Pseudomonas aeruginosa.
Hemolytic activity: Both A2 and A5 strains were evaluated for hemolytic activity on blood agar plates and the result obtained showed that no zone of hemolysis was observed around the colony thus both strains were hemolytic negative.
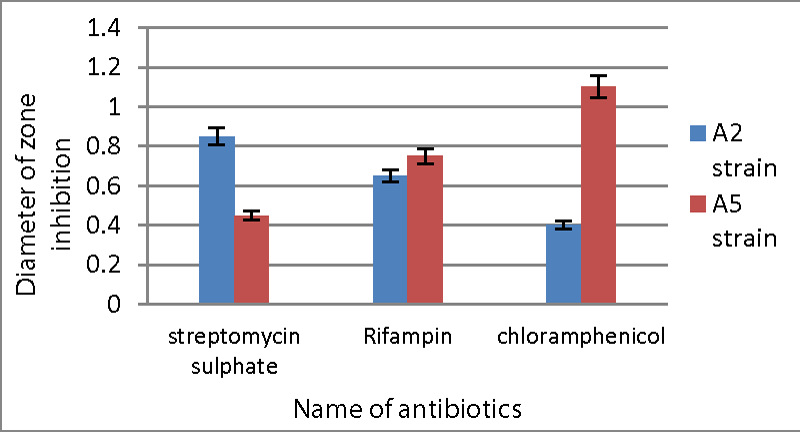
Antibiotic sensitivity test: Both strains were sensitive to antibiotics including streptomycin, chloramphenicol, and rifampin. The minimal inhibition seen with chloramphenicol for the A2 strain was 0.40 cm diameter, whereas the maximum inhibition was 0.85 cm diameter by streptomycin sulphate. While for the A5 strain, streptomycin sulphate showed the least amount of inhibition (0.45 cm), while chloramphenicol showed the maximum inhibition (1.1 cm) Graph 1.
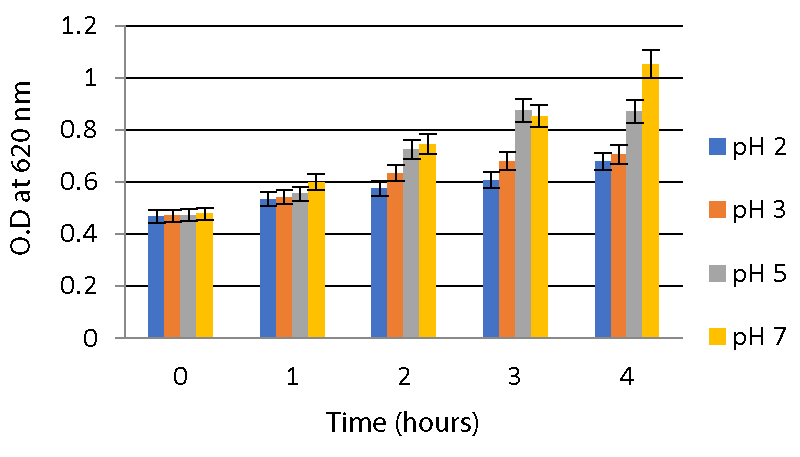
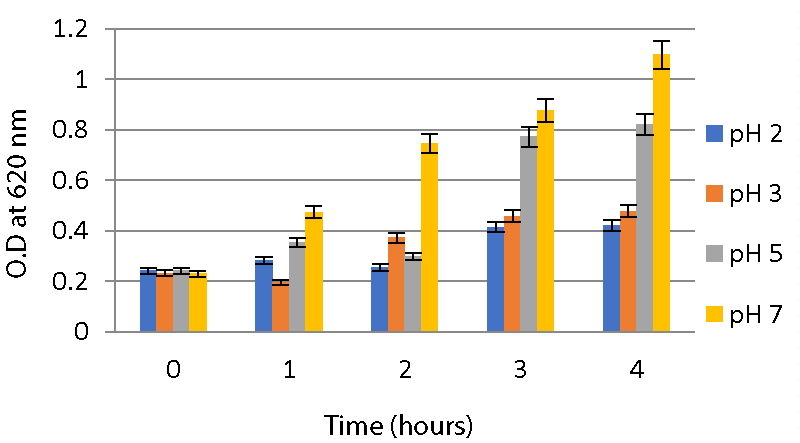
Acid and bile salt tolerance assays: As described in the method, the optical density of the sample was measured at 620nm for different time intervals to check tolerance. From this, it is clear that the isolates were able to survive at low pHs 2, 3, and 5 for 4 hours. A significant increase in O.D values was observed during the interval. Both strains were able to survive at pH 2 and pH 3. While at pH 7, both strains showed a high growth rate compared to two other ranges of pH as shown in Graphs 2,3.
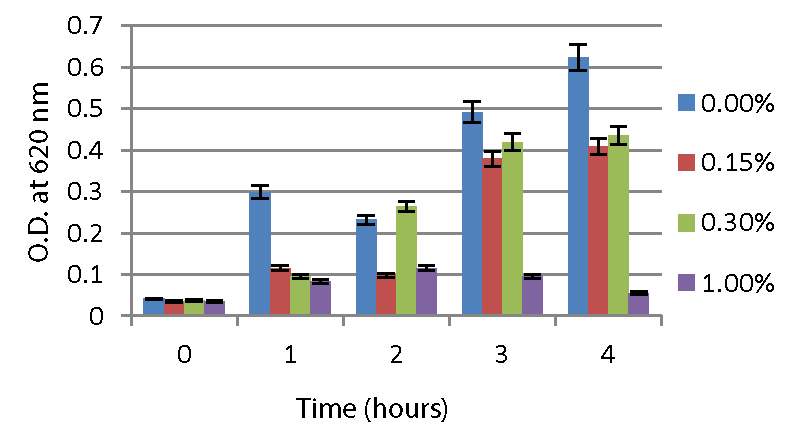
The A2 strain and A5 strain, resistant to low pH, were further screened for their ability to tolerate the bile salt. According to the results (Graphs 4,5), the A2 and A5 strains can tolerate 0.3% bile salt and are able to survive at 1 % bile salt concentration.
Antagonistic activity: This study has shown the survival potential of probiotic bacteria against pathogenic bacteria or normal intestinal microflora. Both strains showed a zone of inhibition against Staphylococcus and Pseudomonas species while no zone of inhibition was observed against E.coli. The zone of clearance is listed in Table 1.
Cell surface hydrophobicity: The affinity of the bacterial strains to hydrocarbons (hydrophobicity) was reported as adhesion percentage according to the formula:
Hydrophobicity (%) = (1 − A1/A0) × 100
For A2 strains:
A0 = 0.534; A1 = 0.477
Hydrophobicity (%) = (1 − A1/A0) × 100
Hydrophobicity = 97.9%
For A5 strains:
A0 = 0.573; A1 = 0.511
Hydrophobicity (%) = (1 − A1/A0) × 100
Hydrophobicity = 84.3%
The hydrophobicity of A2 and A5 strains was measured by xylene extraction and the result showed 97.9% and 84.3% adhesion with hydrocarbons respectively.
Physiological and biochemical characterization
Growth at different NaCl concentrations: The results showed that both A2 and A5 strains were able to survive in 2% and 4% NaCl, no growth was observed in 6.5% NaCl concentration. The growth was visualized by the change in color of the dye due to low pH from purple to yellow after 7 days of incubation, which indicates the growth of organisms at different NaCl concentrations.
Growth at different temperature: During incubation at different temperatures, growth was indicated by the change in colour of the medium containing dye from purple to yellow due to a change in pH. A2 and A5 both strains turned yellow at 10 ºC, 30 ºC, 37 ºC. Maximum growth was observed at 37 ºC and no growth was reported at 50 ºC.
Resistance to phenol: Resistance of phenol was analysed by measuring O.D. at 620 nm after incubation at 37 ºC for 24 hrs. Both strains can able to grow at 0.4% phenol Graph 6.
Carbohydrate fermentation test: The results obtained (Figure 2) showed that both strains inoculated with carbon sources such as mannitol, lactose, fructose, sucrose, and glucose showed positive results with a colour change from purple to yellow. After 48 hrs, the pH of the broth changed as shown in Tables 2,3. At 0 min, all broth had apH 7.4, but after 48 hours, the pH had dropped due to the production of acids. The A2 strain fermented glucose and produced more lactic acid as compared to the A5 strain.
Production of Exopolysaccharide (EPS)
To analyze EPS production, the carbohydrate content was estimated by the phenol sulphuric acid method. The result (Table 3) showed that both strains produce EPS. The A2 strain produces EPS at 0.056 mg/ml while the A5 strain produces 0.062 mg/ml. when compared to the A2 strain, the A5 strain produces significantly more EPS.
O.D. = 0.014 mg/ml (as per standard).
Production of bacteriocin
Bacteriocin is a protein that was estimated by the Folin-Lowary method. The result given in Table 4 revealed that both strains produce bacteriocin. The A2 strain produced bacteriocin at 1.006 mg/ml while the A5 strain produced 0.985 mg/ml. The A2 strain produced a high amount of bacteriocin compared to the A5 strain.
0.1 O.D. = 0.12 mg/ml (according to standard)
Optimization of bacteriocins production
Effect of carbon sources on bacteriocin production: The A2 strain inoculated in glucose-containing media produced a high amount of bacteriocins, while the A5 strain produced a high amount of bacteriocin in media containing sucrose. Less activity was observed with starch and fructose Graphs 7,8.
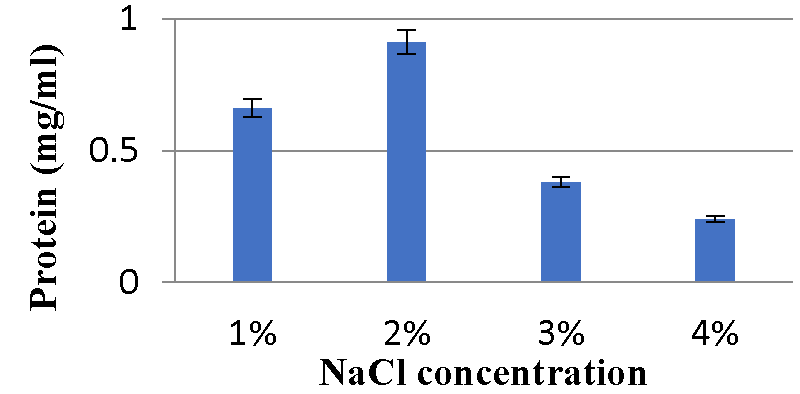
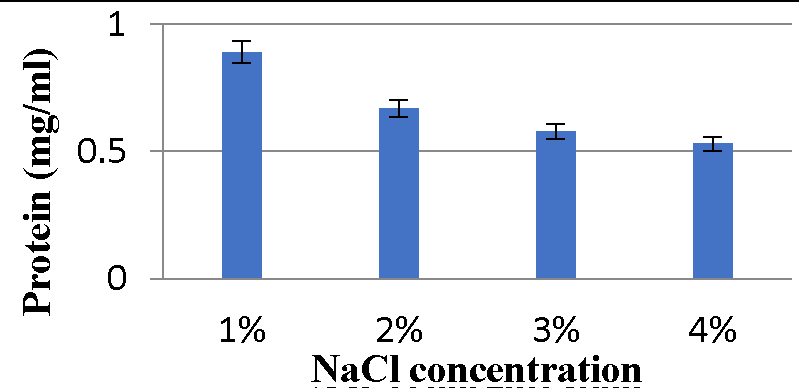
Effect of NaCl concentration on bacteriocin production: The results (Graphs 9,10) showed maximum protein content was observed with 2% NaCl and minimum bacteriocin production was observed with 4% NaCl for the A2 strain. While the A5 strain’s maximum bacteriocin production was observed with 1% NaCl, minimum bacteriocin production was observed with 4% NaCl.
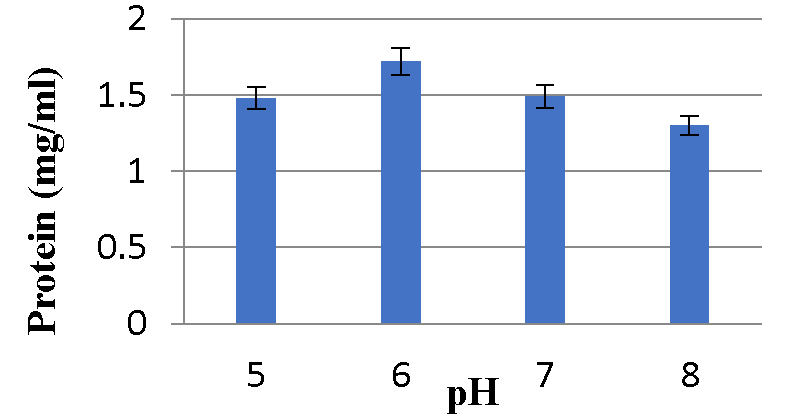
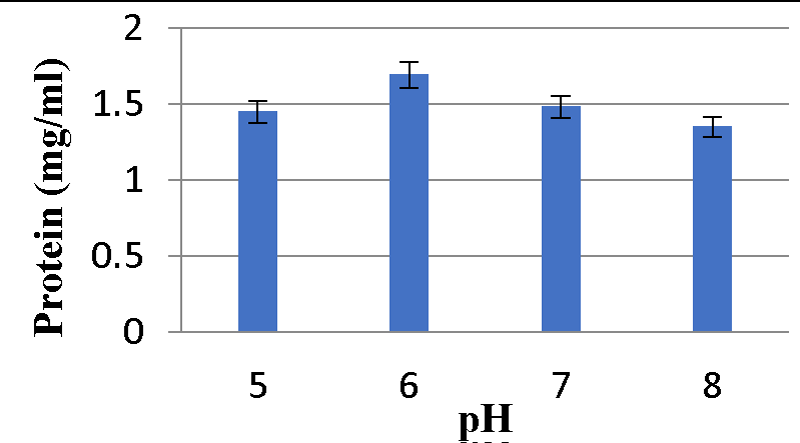
Effect of different pH on Bacteriocin production: Graphs 9,10 shows maximum protein content was observed at pH 6 and minimum bacteriocin production was observed at pH 8 for A2 and A5 strains respectively Graphs 11,12.
Effect of bile salt concentration on bacteriocin production: The Graphs 13,14 indicate maximum content of protein was observed with 1% bile salt concentration and minimum bacteriocin production was observed with 4% bile salt concentration for A2 and A5 strains, respectively.
Production of probiotic curd
Both probiotic strains were inoculated into sterile milk. After 18 hours, curd formation was observed by the A2 strain and A5 strains.
pH of curd: The 6.8 pH containing milk was inoculated by both probiotic strains and the pH of the curd formed was 4.33 for the A2 strain and 4.48 for the A5 strain. The A2 strain produces a higher amount of acid than the A5 strain.
Total Titratable acidity of curd
For A2 strain:
For A5 strain:
The titratable acidity of probiotic curd formed by the A2 strain was 0.9 while the A5 strain was 1.10.
Water holding capacity of curd: The WHC of the curd sample was calculated according to the following equation:
For A2 strain:
For A5 strain:
The A2 strain had a 71% water-holding capacity while the A5 strain had a 73 % water-holding capacity.
Discussion
The probiotic bacteria such as LAB were Gram-positive, catalase and oxidase negative, non-motile, methyl red test positive and VP, citrate utilization, indol, and urease tests were negative, according to Mamta, et al. 2017 [35]. Out of the eight strains, two strains, A2 and A5 have all expected results as putative probiotic bacteria. They all have similar biochemical properties as LAB.
In the current research finding, liquefaction of the gelatin agar was not observed by the A2 and A5 strains which indicate isolates do not produce the gelatinase enzyme. Gelatin is a protein derived from collagen, a material found in the bones, cartilage, and skin of animals that’s essential for healthy joints. Kalui, et al. 2009 [36] reported that L. plantarum strains tested negative for gelatinase enzyme. Safety is one of the suggested features in the FAO/WHO (2001) [3] criteria for the evaluation of probiotics. According to Vuyst, et al. 2003 [37], probiotic strains are chosen based on the absence of hemolytic and gelatinase activity, which indicates that these bacteria are non-virulent. Zone of hemolysis was absent in both the strain in this research also. Many probiotic Lactobacillus and Enterococcus strains exhibit antibiotic sensitivity to various antibiotics, according to Bazireh, et al. 2020 [28]. From the comparison of those results with the current result, probiotic isolated strains are susceptible to routinely used antibiotics like streptomycin, Chloramphenicol, and Rifampin.
One of the main selection criteria for probiotic bacteria is resistance to low pH [38]. In this investigation, it was discovered that both isolated probiotic strains can live at low pH and 0.3% bile salt concentration. The ability of lactic acid bacteria to resist gastrointestinal stress is dependent on their low pH tolerance [39]. For probiotic bacteria to reach the small intestine, they must navigate a variety of challenging situations in the stomach [40]. The very important characteristic of LAB is to survive in the small intestine which contains 0.3% w/v bile salt concentration and the recommended stay time is 4 hours [41]. Many bacterial strains have bile salt resistance because of the bile salt hydrolase (BSH) enzyme, which hydrolyzes conjugated bile salts and lessens their toxicity [42].
As a result of the bactericidal effects of protease-sensitive bacteriocins, LAB has been shown to have inhibitory properties, primarily against Gram-positive pathogens and closely related bacteria [43]. LAB strains are usually inactive against Gram-negative bacteria due to the resistance provided by the outer membrane. In the current study, isolates exhibited inhibition against Gam-positive bacteria but not against Gram-negative bacteria, such as E. coli. These results are similar to Jack, et al. 1995 [43]. This antagonistic activity of isolated bacterial strains might be due to acid production.
Bacterial colonisation of the GIT and adhesion to the intestinal lining depend on cell surface hydrophobicity [29]. As reported by Rijnaarts, et al. 1993 [44], as the hydrophobicity of the cell increases, the cell adhesion level also increases and the hydrophobicity of both isolates was also found in this research.
The results clearly showed that an increase in NaCl content had an effect on the growth of both strains. The LAB isolated from traditional drinking yoghurt was able to grow at a 4% NaCl concentration but not at a 6.5% NaCl concentration, according to Azadnia and Khan Nazer 2009 [45]. According to Vaibhav, et al. (2012) [46], all 15 isolated lactic acid bacteria were able to withstand 2% NaCl.
Mesophilic bacteria known as the LAB thrive best at temperatures between 10 and 45 °C. Stamer in 1979 [47] stated that 30-45 °C is the ideal temperature for LAB development. In the current investigation, the optimal growth temperature was found to be 37 °C and both strains were unable to grow at 50 °C.
The A2 isolates used in the current study demonstrated a high tolerance for phenol concentration (0.4%), indicating that they may withstand the bacteriostatic effects of phenol in the GIT. Actually, phenol is a by-product of the metabolism of aromatic amino acids, which occurs in the gut [29].
According to Vaibhav et al. (2012) [46], the probiotic bacteria isolated from human breast milk showed positive results for the sugars such as glucose, xylose, ribose, arabinose, melibiose, raffinose, galactose, maltose, sucrose, fructose, and lactose. In the current study, both strains were also capable of fermenting glucose, fructose, sucrose, lactose, and mannose.
Both isolates were capable of producing exopolysaccharides. EPS production is essential for defense, colonisation, and acting as an intermediate between the host and the bacterium [48]. EPS serves as a growth medium for the gut microbiota and provides resistance against phagocytosis and bacteriophage attack [29].
Various physicochemical parameters seem to influence bacteriocin activity. Meera, et al. (2012) [33], found that the maximum bacteriocin synthesis occurred when glucose was used as a carbon source and 1% NaCl. The current research reveals that for the A2 strain, glucose as a carbon source, 2% NaCl, 1% bile salts, and pH 6 were the ideal conditions for bacteriocin production, while sucrose as a carbon source, 1% NaCl, 1% bile salts and pH 6 were the optimal conditions for A5 strain.
The pH values for the various types of curd samples were 4.400.10, 4.230.06, 4.030.06, and 3.900.10, according to Sarker, et al. (2018) [11]. In the present research, the pH of curd produced by the A2 strain was 4.33, whereas curd produced by the A5 strain had a pH of 4.48.
Probiotic curd made by the A2 strain had a titratable acidity of 0.9 whereas that made by the A5 strain had a titratable acidity of 1.10. The titratable acidity of typical fermented milk products ranges from 0.7% to 1.2%, according to Staffolo, et al. (2004) [49]. The A5 strain in the current study was shown to be more efficient at storing water than the A2 strain.
Conclusion
Probiotics are now extremely beneficial in maintaining a healthy lifestyle. The current investigation focused on isolating probiotic lactic acid bacteria from the pulp of kiwi fruit. Out of the total isolated eight strains of bacteria found in kiwi fruit pulp, only two were shown to have all probiotic and safety properties and evaluated for their ability to form probiotic curd. These strains were capable of growing at various pH values, temperatures, bile salt concentrations, and NaCl concentrations. Both bacteria produce EPS, bacteriocins and can withstand phenol concentrations of 0.4%. An antibacterial protein molecule called bacteriocin is currently important in the medical industry and certain other industries. Various physical and chemical factors, such as the carbon supply, NaCl concentration, bile salts concentration, pH, and temperature have an impact on the production of bacteriocins. In The present study, physical and chemical parameters were optimized for the maximum production of bacteriocin protein. The findings led to the conclusion that for the A2 strain, the ideal conditions for the formation of bacteriocins were glucose as a carbon source, pH 6, 2% NaCl, and 1% bile salt concentration. The best circumstances for the A5 strain’s production of bacteriocin were sucrose as the carbon source, pH 6, 1% NaCl, and 1% bile salt concentration. Therefore, isolated bacteriocin-producing probiotic strains have the potential to be used in the food and pharmaceutical industries.
- LILLY DM, STILLWELL RH. PROBIOTICS: GROWTH-PROMOTING FACTORS PRODUCED BY MICROORGANISMS. Science. 1965 Feb 12;147(3659):747-8. doi: 10.1126/science.147.3659.747. PMID: 14242024.
- Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989 May;66(5):365-78. PMID: 2666378.
- Joint FA. Guidelines for the evaluation of probiotics in food, London, Ontario, Canada, April 30 and May 1, 2002. http://www. who.int/foodsafety/publications/fs_management/probiotics2/en/index. html. 2002.
- Salminen S, Ouwehand A, Benno Y, Lee YK. Probiotics: how should they be defined? Trends in Food Science Technology. 1999 Mar 1;10(3):107-10.
- Dal Bello F, Walter J, Hammes WP, Hertel C. Increased complexity of the species composition of lactic acid bacteria in human feces revealed by alternative incubation condition. Microb Ecol. 2003 May;45(4):455-63. doi: 10.1007/s00248-003-2001-z. Epub 2003 Apr 22. PMID: 12704557.
- Williams NT. Probiotics. Am J Health Syst Pharm. 2010 Mar 15;67(6):449-58. doi: 10.2146/ajhp090168. PMID: 20208051.
- Daneshi M, Ehsani MR, Razavi SH, Labbafi M. Effect of refrigerated storage on the probiotic survival and sensory properties of milk/carrot juice mix drink. Electronic Journal of Biotechnology. 2013; 16(5):5.
- Mathew S, Vijay S, Potty VP. Assessment of probiotic potential of lactic acid bacteria isolated from curd and its application using fruit juice. Int J CurrMicrobiolApplScie. 2017; 6:282-9.
- Van Tassell ML, Miller MJ. Lactobacillus adhesion to mucus. Nutrients. 2011 May;3(5):613-36. doi: 10.3390/nu3050613. Epub 2011 May 20. PMID: 22254114; PMCID: PMC3257693.
- Yadav SS, Longnecker N, Dusunceli F, Bejiga G, Yadav M, Rizvi AH, Manohar M, Reddy AA, Xaxiao Z, Chen W. Uses, consumption and utilization. In Chickpea breeding and management. Wallingford UK: CABI. 2007; 72-100.
- Sarker MT. Prabakusuma AS, Islam MS. Dahi (Curd) preparation from milk with different levels of carrot (Dacuscarota) juice. MOJ Food Process Technol. 2018; 6(1):66-71.
- Betoret N, Puente L, Dıaz MJ, Pagan MJ, Garcıa MJ, Gras ML, Martı́nez-Monzó J, Fito P. Development of probiotic-enriched dried fruits by vacuum impregnation. Journal of Food Engineering. 2003; 56(2-3):273-7.
- Krasaekoopt W, Bhandari B, Deeth H. Evaluation of encapsulation techniques of probiotics for yoghurt. International Dairy Journal. 2003; 13(1):3-13.
- Cerdó T, García-Santos JA, G Bermúdez M, Campoy C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients. 2019 Mar 15;11(3):635. doi: 10.3390/nu11030635. PMID: 30875987; PMCID: PMC6470608.
- Corr SC, Hill C, Gahan CG. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv Food Nutr Res. 2009;56:1-15. doi: 10.1016/S1043-4526(08)00601-3. PMID: 19389605.
- Dugas B, Mercenier A, Lenoir-Wijnkoop I, Arnaud C, Dugas N, Postaire E. Immunity and probiotics. Immunol Today. 1999 Sep;20(9):387-90. doi: 10.1016/s0167-5699(99)01448-6. PMID: 10462737.
- Silva LA, Lopes Neto JH, Cardarelli HR. Exopolysaccharides produced by Lactobacillus plantarum: technological properties, biological activity, and potential application in the food industry. Annals of Microbiology. 2019; 69: 321-8.
- Degeest B, De Vuyst L. Indication that the nitrogen source influences both amount and size of exopolysaccharides produced by streptococcus thermophilus LY03 and modelling of the bacterial growth and exopolysaccharide production in a complex medium. Appl Environ Microbiol. 1999 Jul;65(7):2863-70. doi: 10.1128/AEM.65.7.2863-2870.1999. PMID: 10388677; PMCID: PMC91430.
- Ras M, Lefebvre D, Derlon N, Paul E, Girbal-Neuhauser E. Extracellular Polymeric Substances diversity of biofilms grown under contrasted environmental conditions. Water Res. 2011 Feb;45(4):1529-38. doi: 10.1016/j.watres.2010.11.021. Epub 2010 Nov 24. PMID: 21193214.
- Singh P, Saini P. Food and health potentials of exopolysaccharides derived from Lactobacilli. Microbiology Research Journal International. 2017 Jan 10;22(2):1-4.
- Garai-Ibabe G, Dueñas MT, Irastorza A, Sierra-Filardi E, Werning ML, López P, Corbí AL, Fernández de Palencia P. Naturally occurring 2-substituted (1,3)-beta-D-glucan producing Lactobacillus suebicus and Pediococcus parvulus strains with potential utility in the production of functional foods. Bioresour Technol. 2010 Dec;101(23):9254-63. doi: 10.1016/j.biortech.2010.07.050. Epub 2010 Jul 16. PMID: 20691585.
- Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005 Oct;3(10):777-88. doi: 10.1038/nrmicro1273. PMID: 16205711.
- Abo-Amer AE. Optimization of bacteriocin production by Lactobacillus acidophilus AA11, a strain isolated from Egyptian cheese. Annals of microbiology. 2011 Sep;61:445-52.
- Saif FA. Efficacy of Lactic Acid Bacteria isolated from some fruits and vegetables. Egyptian Journal of Microbiology. 2016; 51(1):13-28.
- Motohashi N, Shirataki Y, Kawase M, Tani S, Sakagami H, Satoh K, Kurihara T, Nakashima H, Mucsi I, Varga A, Molnár J. Cancer prevention and therapy with kiwifruit in Chinese folklore medicine: a study of kiwifruit extracts. J Ethnopharmacol. 2002 Aug;81(3):357-64. doi: 10.1016/s0378-8741(02)00125-3. PMID: 12127237.
- Garcia EF, Luciano WA, Xavier DE, da Costa WC, de Sousa Oliveira K, Franco OL, de Morais Júnior MA, Lucena BT, Picão RC, Magnani M, Saarela M, de Souza EL. Identification of Lactic Acid Bacteria in Fruit Pulp Processing Byproducts and Potential Probiotic Properties of Selected Lactobacillus Strains. Front Microbiol. 2016 Aug 30;7:1371. doi: 10.3389/fmicb.2016.01371. PMID: 27625647; PMCID: PMC5003889.
- da Silva LA, Lopes Neto JH, Cardarelli HR. Safety and probiotic functionality of isolated goat milk lactic acid bacteria. Annals of Microbiology. 2019 Dec; 69:1497-505.
- Bazireh H, Shariati P, Azimzadeh Jamalkandi S, Ahmadi A, Boroumand MA. Isolation of Novel Probiotic Lactobacillus and Enterococcus Strains From Human Salivary and Fecal Sources. Front Microbiol. 2020 Dec 4;11:597946. doi: 10.3389/fmicb.2020.597946. PMID: 33343539; PMCID: PMC7746552.
- Yasmin I, Saeed M, Khan WA, Khaliq A, Chughtai MFJ, Iqbal R, Tehseen S, Naz S, Liaqat A, Mehmood T, Ahsan S, Tanweer S. In vitro Probiotic Potential and Safety Evaluation (Hemolytic, Cytotoxic Activity) of Bifidobacterium Strains Isolated from Raw Camel Milk. Microorganisms. 2020 Mar 2;8(3):354. doi: 10.3390/microorganisms8030354. PMID: 32131456; PMCID: PMC7143641.
- Samedi L, Charles AL. Isolation and characterization of potential probiotic Lactobacilli from leaves of food plants for possible additives in pellet feeding. Annals of Agricultural Sciences. 2019 Jun 1; 64(1):55-62.
- Yavuzdurmaz H. Isolation, characterization, determination of probiotic properties of lactic acid bacteria from human milk (Doctoral dissertation, Izmir Institute of Technology (Turkey)).
- https://www.researchgate.net/profile/Arvind_Singh56/post/Which_is_the_economical_and_easy_method_to_estimate_protein_oil_and_sugar_content_in_maize_corn_seeds/attachment/59d63c1279197b8077999105/AS%3A413819290570753%401475673615545/download/1.pdf.
- Meera NS, Devi MC. Partial characterization and optimization of parameters for Bacteriocin production by Probiotic Lactic acid bacteria. Journal of Microbiology and Biotechnology Research. 2012;2(2):357-65.
- https://agrimoon.com/estimation-of-titratable-acidity-in-curd/.
- Thakur M, Deshpande HW, Bhate MA. Isolation and identification of lactic acid bacteria and their exploration in non-dairy probiotic drink. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:1023-30.
- Zernadji W, Chebchoub F, Dairi SE. Test of the manufacture of a fermented drink based on strawberry juice and whey (Doctoral dissertation, University of Jijel).
- De Vuyst L, Foulquié Moreno MR, Revets H. Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int J Food Microbiol. 2003 Aug 1;84(3):299-318. doi: 10.1016/s0168-1605(02)00425-7. PMID: 12810293.
- Cakir I. Determination of some probiotic properties on Lactobacilli and Bifidobacteria. Ankara Ankara University. 2003.
- Wang CY, Lin PR, Ng CC, Shyu YT. Probiotic properties of Lactobacillus strains isolated from the feces of breast-fed infants and Taiwanese pickled cabbage. Anaerobe. 2010 Dec;16(6):578-85. doi: 10.1016/j.anaerobe.2010.10.003. Epub 2010 Oct 15. PMID: 20951815.
- Chou LS, Weimer B. Isolation and characterization of acid- and bile-tolerant isolates from strains of Lactobacillus acidophilus. J Dairy Sci. 1999 Jan;82(1):23-31. doi: 10.3168/jds.S0022-0302(99)75204-5. PMID: 10022003.
- Prasad J, Gill H, Smart J, Gopal PK. Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. International Dairy Journal. 1998 Dec 1;8(12):993-1002.
- du Toit M, Franz CM, Dicks LM, Schillinger U, Haberer P, Warlies B, Ahrens F, Holzapfel WH. Characterisation and selection of probiotic lactobacilli for a preliminary minipig feeding trial and their effect on serum cholesterol levels, faeces pH and faeces moisture content. Int J Food Microbiol. 1998 Mar 3;40(1-2):93-104. doi: 10.1016/s0168-1605(98)00024-5. PMID: 9600615.
- Jack RW, Tagg JR, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995 Jun;59(2):171-200. doi: 10.1128/mr.59.2.171-200.1995. PMID: 7603408; PMCID: PMC239359.
- Rijnaarts HH, Norde W, Bouwer EJ, Lyklema J, Zehnder AJ. Bacterial Adhesion under Static and Dynamic Conditions. Appl Environ Microbiol. 1993 Oct;59(10):3255-65. doi: 10.1128/aem.59.10.3255-3265.1993. PMID: 16349063; PMCID: PMC182446.
- Azadnia PK, Khan Nazer AH. Identification of lactic acid bacteria isolated from traditional drinking yoghurt in tribes of Fars province. Iranian Journal of Veterinary Research. 2009 Sep 1;10(3):235-40.
- Bhatt VD, Vaidya YH, Kunjadia PD, Kunjadia AP, Patel R. Isolation and characterization of probiotic bacteria from human milk. Int J Pharm Sci Health Care. 2012;3(2):62-70.
- Stamer JR. The lactic acid bacteria: microbes of diversity.
- Bengoa AA, Llamas MG, Iraporda C, Dueñas MT, Abraham AG, Garrote GL. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018 Feb;69:212-218. doi: 10.1016/j.fm.2017.08.012. Epub 2017 Aug 19. PMID: 28941904.
- Staffolo MD, Bertola N, Martino M. Influence of dietary fiber addition on sensory and rheological properties of yogurt. International Dairy Journal. 2004 Mar 1;14(3):263-8.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
