Open Journal of Biological Sciences
Dairy and Nondairy Probiotic Microorganisms: A Literature Review
Department of Microbiology (Centre of Excellence), Dr Rammanohar Lohia Avadh University,Ayodhya-224001, India
Author and article information
Cite this as
Kushwaha TN, Kumar S. Dairy and Nondairy Probiotic Microorganisms: A Literature Review. Open J Biol Sci. 2025; 10(1): 001-010. Available from: 10.17352/ojbs.000039
Copyright License
© 2025 Kushwaha TN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Consumers who scrutinize good probiotic alternatives have a stronger desire for health knowledge. This review presents a discussion of recent findings related to dairy and nondairy probiotics. Probiotic bacteria have gained popularity over the past two decades due to growing scientific evidence indicating their beneficial effects on human health. As a result, they have been incorporated into a variety of food items by various food industries that are actively researching and promoting them. Therefore, probiotics are used in a variety of items, mostly fermented dairy products.
Since humanity began consuming fermented milk, probiotic civilizations have existed. For instance, Metchnikoff recommended that gut microbiota have a positive impact on fitness due to the presence of beneficial bacteria and suggested that consuming fermented milk could be helpful in this regard [1]. In 1953, Kollath used the term “probiotic,” derived from the Greek word “for life,” for the first time to describe bio-supplements necessary to restore the health of patients suffering a form of malnutrition due to overeating highly refined food [2-4].
Probiotics are defined as the effects generated by bacteria that induce the growth of other microorganisms. The host animals benefit from having a better balance of gut microbes. Havenaar, et al. [5] broadened the definition to include both food and non-food items, as well as the use of mono- and mixed cultures [5,6]. Probiotics are explained as “living microorganisms that, when supplied in reasonable dosages, confer a health benefit on the host” [7]. To reevaluate the idea of probiotics, the International Scientific Association for Probiotics and Prebiotics (ISAPP) arranged a meeting of clinical and scientific experts on probiotics (with specializations in gastroenterology, pediatrics, family medicine, gut microbiota, microbiology of probiotic bacteria, microbial genetics, immunology, and food science) on October 23, 2013. The meeting kept the FAO/WHO definition in place for probiotics, with a slight grammatical rectification as “live microorganisms that, when supplied in suitable proportions, confer a health benefit on the host” [7]; inconsistencies between the expert consultation and clarifications were made to the FAO/WHO Guidelines include in the framework for the definition of probiotic bacterial species that demonstrate to have health benefits in research that were conducted under controlled conditions [7]. So far, a recent probiotic definition is given below, as per FAO/WHO guidelines:
- Any specific claim beyond “contains probiotics” must be further substantiated.
- Maintain live cultures traditionally associated with fermented foods, for which there is no evidence of health benefits outside the probiotic framework.
- Keep undefined fecal microbiota transplants outside the framework of probiotics.
- New commensals and consortia comprising specified strains from human samples, with enough evidence of safety and efficiency, are ‘probiotics [8-10].
The other two important conditions are synbiotics and prebiotics. Prebiotic components are non-digestible but help stimulate the activity of beneficial bacteria positively, thereby supporting the host. Prebiotics are considered stimulants for microbial colonies, supporting public health, and also provide textural details to meals. For example, synbiotic acidophilus milk, added with prebiotic inulin, induced physicochemical and sensory quality improvements in probiotic acidophilus milk. The prebiotic component, Galacto-Oligosaccharides (GOS) (non-digestible by human digestive enzymes), has been reportedly used in juice and other probiotic liquids to benefit colonic microorganisms such as Bifidobacterium spp. [11].
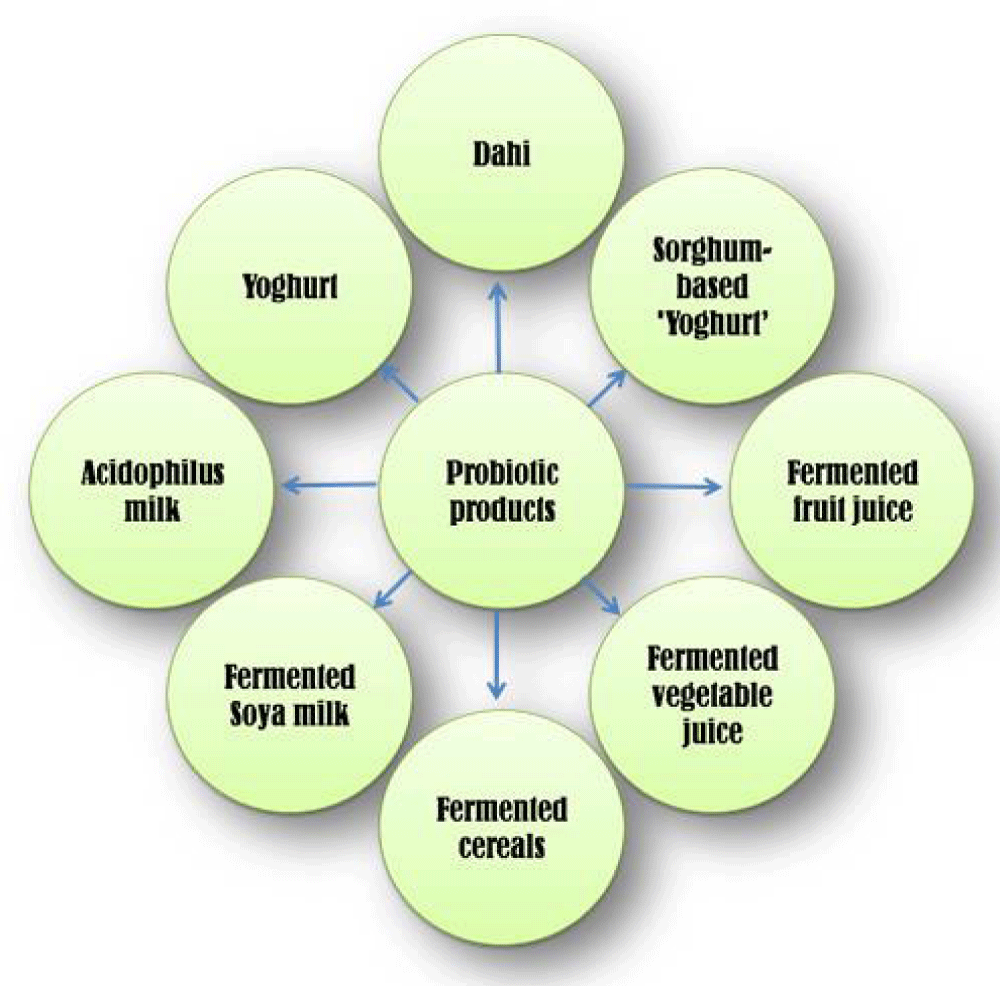
Probiotics are widely available economically all over the world, primarily in fermented foods and dairy byproducts, which are the major sources of probiotics [12]. Yogurt, Dahi, and other fermented dairy products are traditionally used as probiotics to make them functional foods for health [13-16]. Probiotic meals made without dairy are consequently becoming more widespread. In recent times, a shift towards non-dairy foods, like probiotic fermented cereals, vegetable juice, and fruit juices, has been observed as a result of the growing health issues caused by the presence of a high cholesterol content, a lot of saturated fatty acids, lactose intolerance, and milk protein allergy in dairy-based diets [17,18]. Some of the dairy and nondairy products are shown in Figure 1.
The matrix in which the microorganisms are enclosed has a significant impact on the power and interactions of the bacteria in commercial probiotic products. Commercial probiotic formulations must maintain their stability during storage. When preparing functional probiotic meals, it’s crucial to use the proper food distribution method [19,20]. The main requirements for the success of these effects on the market are that they maintain viability and sensory components [21]. Due to high temperature, chemical loss, or cell injury caused by osmotic pressure, technological conditions during the preparation of probiotic food can severely reduce the lifetime of probiotic cells [22,23]. Probiotics’ capacity to survive long-term storage and processing depends in large part on the kind of food matrix, moisture content, and cell state [24]. The survival of probiotics under intense heat stress during processing and long-term storage is substantially impacted by moisture and cell conditions. The probiotic dairy products with their physiological effects on health are listed in Table 1.
Probiotic products
Since ancient times, food fermentation has been practiced and evolved via changes in substrates, procedures, and technology. It is achieved by microbial cultures using techniques such as enrichment and back-slopping to enhance the organoleptic quality, nutrient availability, and storage life of food. In many cases, this also adds healthy microorganisms to the consumer’s diet. Lactic acid bacteria, many of which are known to have probiotic properties, are one of the principal groups of microbes used in conventional and industrial cereal fermentation, followed by yeast and mold. While nondairy foods, particularly millets and cereal mixes, have not been well studied. Beneficial microorganisms in dairy fermented foods have attracted attention as a source of probiotic microbes. Probiotics have many potential health advantages, notably for immunity and gut health, but there are also risks to be aware of, especially for those with certain food sensitivities or medical disorders. The health issues related to the use of dairy food products are given below:
Health risks associated with probiotic dairy products
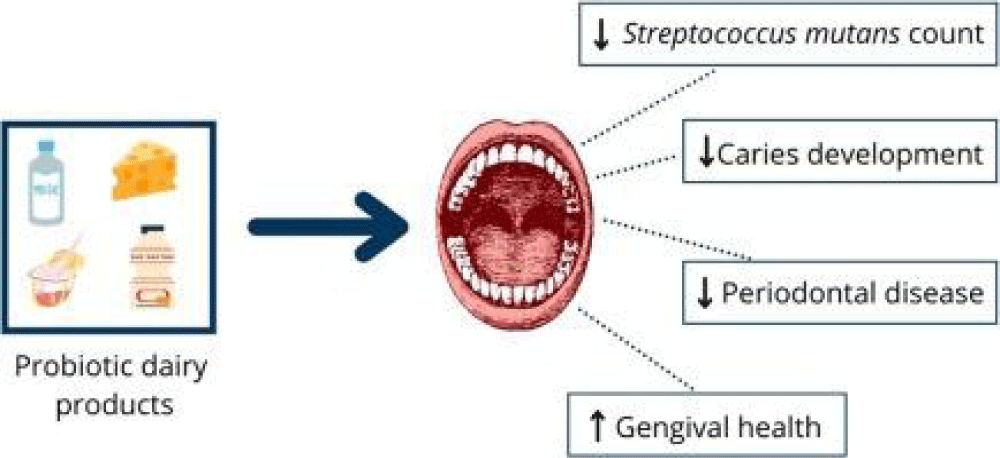
Probiotic meals derived from milk are associated with some health hazards. They mostly consist of anti-lactose prejudice, allergies to milk protein, oral disease (Figure 2), gastroesophageal reflux disease, and high cholesterol and fat levels. Risks from these are reported below.
In individuals having lactose intolerance caused by insufficient amounts of the enzyme lactase, it can cause stomachache and other related gastric issues. Moreover, allergic reactions ranging from minor discomfort to severe allergies can be brought on by sensitivities to milk protein. Products containing probiotics derived from milk have been linked to oral health issues such as periodontal disorders and dental cavities. Probiotics and oral bacteria may combine to cause these disorders, which might exacerbate pre-existing oral health problems. In addition, individuals suffering from Gastroesophageal Reflux Disease (GERD) could feel worse when they eat probiotic meals made from milk since these products might make acid reflux and heartburn worse. So, eating these kinds of meals has been linked to higher cholesterol levels, and over time, it may raise the risk of cardiovascular disease [32].
High cholesterol range and fat range
The amount of fat in milk varies depending on where the milk comes from. Cow milk has a fat content of 4% to 5%, whereas the fat content of up to 7% to 8% is in the milk of buffalo. It has a 0.05 ratio of polyunsaturated to saturated fatty acids. According to Levy and Feinleib, drinking a lot of milk increases the blood’s total and LDL cholesterol levels [33]. The high plasma cholesterol levels caused by dietary fat are also responsible, which is a dangerous factor in heart disease. This type of dangerous factor can be decreased by reducing saturated fats in the diet and lowering low-density lipoproteins (LDL) cholesterol [8]. The effect of probiotics on the dangerous factors for cardiovascular disorders, especially its implications for hyperlipidemia, has just recently been studied [34].
Lactose intolerance
As well as comprehend that lactose maldigestion is one of the most common kinds of carbohydrate maldigestion. Due to low lactase, enzyme groups are associated with the incapacity to break down lactose into its components, glucose and galactose [35]. In the beginning, the enzymatic activity of lactose is high, and weaning is reduced. As a result, colonic bacteria metabolize the unabsorbed lactose in the production of gases, for example, hydrogen and methane, and short-chain fatty acids (SCFAs ) [36].
Related signs include flatulence and lax stool, bloating, cramping, and a few details also suggest that guide to an irritable syndrome known as bowel syndrome [37,38]. Lactase non-persistence is detected in 2% of people in Northern Central Europe. It accounts for roughly 40% of the population in Mediterranean countries (it is most frequent in Italy, where it is found on average in 56% of the population and is predicted to reach peaks of up to 70% in some places), 65% - 75% in a major portion of Africa, and up to more than 90% in Asia). Treatment for patients with lactose intolerance may involve altering the intestinal microbial ecosystem by encouraging the colonization of the gut by strains with β-galactosidase activity. The β-galactosidase activity of several probiotic strains is Bifidobacterium lactis W52, Bifidobacterium lactis W51, Lactobacillus acidophilus W22, Lactobacillus acidophilus W70, Lactobacillus brevis W78, Lactobacillus casei W20, Lactobacillus casei W79, Lactiplantibacillus plantarum W21, Lacticaseibacillus rhamnosus W71, Ligilactobacillus salivarius W24, Lactococcus lactis W19, Streptococcus thermophilus W69 [39].
Working sites for probiotic microorganisms
There are several possible mechanisms by which the probiotic microorganism is effective; these include competition for nutrients and probiotics, bioconversion, production of the growth substrate, direct antagonism, competitive exclusion, barrier function, reduction of inflammation, and immune stimulation. Dairy Products in probiotics may cause various effects on mental health, mouth and teeth, lungs, stomach, liver, and skin, reduced digestive discomfort, increased nutrient absorption, veginal, immunity, and general health [40]. Although the precise process by which probiotics exert their effects is not yet fully understood, they employ a variety of mechanisms of action. These encompass everything from the creation of short-chain fatty acids and bacteriocin to the lowering of gut pH, the competition for nutrients, and the activation of mucosal barrier function and immunomodulation. Numerous studies have focused on the latter in particular, and there is strong evidence that probiotics influence several aspects of the innate and acquired immune response by triggering phagocytosis and IgA secretion, altering T-cell responses, boosting Th1 responses, and suppressing Th2 responses [41]. When compared to a placebo, Lactobacillus significantly reduced both the systolic and diastolic blood pressure levels. A subgroup study revealed that a single strain of Lactobacillus reduced blood pressure more than probiotics with several strains. Despite these significant results, the studies included had significant heterogeneity even after sensitivity analysis [42].
Nondairy probiotic products
Rivera-Espinoza and Gallardo-Navarro (2010) examined a variety of non-dairy-based probiotic foods produced all over the world. Fruit-based, cereal-based, soy-based, and vegetable-based fermented foods are becoming more popular among non-dairy-based diets [18,19,43,44] (Table 2).
Products made from fruit and vegetables
Alternatives to dairy-based probiotic products are still being researched, and consumers might choose nondairy probiotic products, particularly those that use fruit and vegetable juice as their main ingredients. Fruit juices provide a wealth of nutrients, which is only one of their many advantages. It avoids the need for starter cultures. Thus, probiotic cultures do not compete for nutrients with dairy products. Additionally, nondairy sources are supplemented with acidulants that might lengthen life by scavenging oxygen from the air to create an anaerobic environment that is better for probiotic microorganisms.
The species of probiotics spend significantly not so much time in an acidic condition of the abdomen since these liquids don’t linger as long in the stomach. For this objective, a variety of fruits and vegetables are being used (Anacardium occidentale L.), such as raspberries, cantaloupe melon, juice of pomegranate, beetroot, carrot, and so on, [45,46]. Probiotic cells may now be protected from the acidic condition of juices by being encapsulated in easily accessible, harmless alginates that also increase the partitioning capability during shelf life [48,49]. These alginate beads are covered in chitosan to provide probiotic cells with prolonged protection [46]. Heidebach, et al. [47] have examined probiotic microencapsulation and its effect on applications in nutrition. They become good substrates for the development of probiotics after matrix modification because they conveniently contain healthy components such as minerals, dietary fibers, vitamins, and different types of antioxidants [50]. Additionally, they do not include dairy allergies, which are avoided by some segments of the inhabitants [51].
Fruit juices’ alluring flavors and nutritional qualities have sparked serious interest in the tale of probiotic fruit drinks [50,52,53].
However, it was shown that several probiotic bacteria can boost the matrices of fruit. According to some theories, factors influencing cell survival include the substrate, the amount of oxygen present, and how acidic the matrix is [54]. When it comes to the acid resistance of Lactobacillus spp and Bifidobacterium spp in the juices of pineapple, orange, and cranberry, [53] Sheehan, et al. [54] discovered significant variances. In comparison to cranberry juice, pineapple juice, and orange juice have seen greater and longer survival rates.
Higher resistance has been demonstrated by Paracasei strains, which can endure over 7.0 log colony-forming units/milliliter in the juice of an orange and over 06.0 log colony-forming units/milliliter in the juice of pineapple for a minimum of 3 months. Particularly earlier, the water of the coconut was fermented with Lactiplantibacillus plantarum to create a probiotic beverage [55].
Another non-dairy-based creation
Grains have a complex nutritional makeup and can outweigh the drawbacks of fermented dairy products. These are regarded as advantageous non-dairy carriers for producing probiotic meals [19]. Consuming fermented meals made from cereals has another benefit: nutritional supplements are easily accessible. Oligosaccharides, a kind of non-digestible carbohydrate, can function as a prebiotic and encourage the development of probiotic LAB [56]. One of the oldest methods of processing grains still in use in the continents of Asia and Africa for the production of gruels, porridge, and drinks is fermentation. For this endeavor, cereal grains, including millet, oats, barley, wheat, and rye, are employed. Furthermore, consuming whole grains reduces the risk of type 2 diabetes, cardiovascular disease, obesity, and a specific type of cancer [57] the utilization of cereal grains in the creation of functional meals is growing in popularity in Western nations [58].
The probiotic potential of cereal-based diets that undergo spontaneous fermentation was recently evaluated [59]. Mixed cereals and single cereals (malt and barley) are based on probiotic drinks, including Lactiplantibacillus plantarum and Lactobacillus acidophilus, as an alternative to dairy-based probiotic meals. In describing the function of the laboratory in the fermentation of grains used to make drinks, Waters, et al. [60] crystallised the findings of several researchers. Alginate microencapsulated Lb. is also employed as a substitute for dairy-based probiotic foods, primarily in the form of wursts. Reuters and B. Long reported that they used the flesh to make sausages [61]. In a study conducted in 2010, Rivera-Espinoza and Gallardo-Navarro studied the microorganisms found in probiotic products made from beef [62].
Phenotypic and genotypic resemblances between dairy and nondairy probiotics
The similarities between species of dairy and nondairy outweigh the differences in their genotypic and phenotypic characteristics. The important probiotic utilized in the fermentation of dairy products is Lactococcus lactis. Lactococcus lactis, however, is not just present in dairy products; it may also be found in other sources, including the surfaces of a plant [63,64]. L. lactis subsp. Lactis and L. lactis subsp. Lactis biovar diacetylactis naturally occurs on green plant matter [63]. Additional sources of L. lactis have included dirt [65] and the hindgut of termites [66]. Morphological and genotypic features of dairy isolates and nondairy isolates were identified based on 16S rRNA examinations of almost 106 LAB isolates to evaluate each other in their effectiveness in providing sources of food. Cluster analysis using randomly amplified polymorphic DNA profiles was used to examine these isolates. According to reports, there were also no appreciable variations in the profiles of enzymes such as phosphatases, peptidases, and lipases. Another trustworthy finding was that the fermented milk made with plant-derived strains tasted similar to that made with milk-derived strains [66].
Support for partition functionality and feasibility in a matrix
The physicochemical makeup of milk, which is high in lipids (fats) and protein, serves as a defensive matrix used for probiotics in dairy-based diets. These components aid probiotics in surviving challenging conditions in the intestine and abdomen [67,68]. Additionally, milk proteins can function efficiently as a carrier matrix to protect probiotic cells until they reach the site of action in the small intestines [69]. Fresh cheeses and other fermented dairy products have been the food vehicles for probiotic infusion, with the greatest scientific and economic success of microorganisms [68,70]. However, according to Ouwehand and Salminen [71], cell viability is a crucial component of cell functioning, and the elements of the food matrix primarily affect cell functionality [20]. The maturation inhibitory ability of widely available nondairy drinks was investigated using a well diffusion agar experiment to assess the viability of Lb. Casey sells the drinks. Only citric orange juice, out of a total of 13 nondairy beverages, had an inhibitory effect on the growth of both strains, with an inhibition zone measuring 6 to 7 mm from the well’s edge for both strains [72]. Probiotic bacteria may be present in fruits like bananas and melons, and researchers have shown that these bacteria cling strongly to fruit tissue [44].
First, it is necessary to determine the effectiveness of bacteria used in dairy and nondairy drinks in stimulating resistance to stomach absorption in in vitro investigations. However, there are reports that contradictory results were accepted while researching the gastric antagonistic effects of probiotic strains, such as strains with a well-reported capacity to operate favorably in the gut of humans, did not appear as favorable in Vitro assays of stomach acid resistance. These findings support the need for much more precise testing to assess both in vitro and in vivo resistance to stomach absorption [73].
However, it cannot be completely ruled out that in vitro testing will be used to examine some intrinsic aspects, such as the effects of the food matrix on the stomach resistance of probiotic bacteria [74]. According to the phenomenon known as “Bross adaptation,” what type of conditions are pre-exposure to the sublethal level of the focus factor will allow cells to adjust to subsequent exposure to higher levels of the same pressure element or kinds of stresses. It has been observed that cell viability and functionality operate at [75]. Bifidobacteria kept at 4 to 20 °C for six weeks in another investigation showed decreased stomach digesting resistance [76]. The resistance of Bifidobacteria and Lactobacilli to the acid in juices of pineapple, cranberry, and orange has varied greatly [53]. Compared to fermented milk, fruit juice is a more varied dietary source with unique physicochemical characteristics. These unexpected outcomes are occasionally predicted because cell survival and functioning vary and rely on the ultimate output.
Rehearsal and viability of probiotics: The capacity of probiotic strains to survive throughout the upper GI, colonize, and proliferate in the human intestine is what determines the majority of their health advantages [77]. Therefore, if an appropriate quantity of potential probiotic bacteria did not permeate the marked region, the product of the probiotic would not be beneficial.
Many probiotic evaluations emphasize how probiotic viability is lost through processing, storage, and digestion [78]. Therefore, the biggest obstacle to the efficiency of a probiotic food product is maintaining the pressure of probiotics, which is necessary to achieve health benefits. Since probiotic food has health benefits, the impacts depend on the number of potential cells present at the time of ingestion [79].
WHO/FAO (2002) has demonstrated that any food claimed to contain probiotic effects must include at least a minimum of 106 to 107 colony-forming units/mL of live probiotic bacteria, emphasizing the individual. Various environmental factors can also influence the survival and persistence of probiotic bacteria. Storage, handling, transportation, and shelf life of probiotic nutrition are the primary phases involved in maintaining probiotic viability and survival. The probiotics must withstand the harsh conditions of the stomach and the bile salts in the small intestine before they can have a positive effect on the lower gastrointestinal tract.
Environmental, food, and processing parameters like pH, sugar, and chemicals like H2O2, bacteriocins, and molecular oxygen, as well as strain species, rate and amount of inoculation, packaging of materials and requirements, conditions, and storage methods, are the important factor that affects the capability and activity of probiotic cultures [80]. Separate from the production and storage components, the bile salts, intestinal current, toxic metabolites, bacteriophage, antibiotics, including phenols released during digestion, and anaerobic environments can all affect the probiotics’ ability to survive [81].
Probiotics’ growth and survivability in new food products have been improved by several initiatives [82]. The appropriate choice of acid and bile immune strains, use of oxygen-impervious containers, two-step fermentation, microencapsulation, and integration of micronutrients such as peptides and amino acids are methods to increase the survival of probiotic organisms [83].
List of probiotic microorganisms and their chemicals and physiological effects
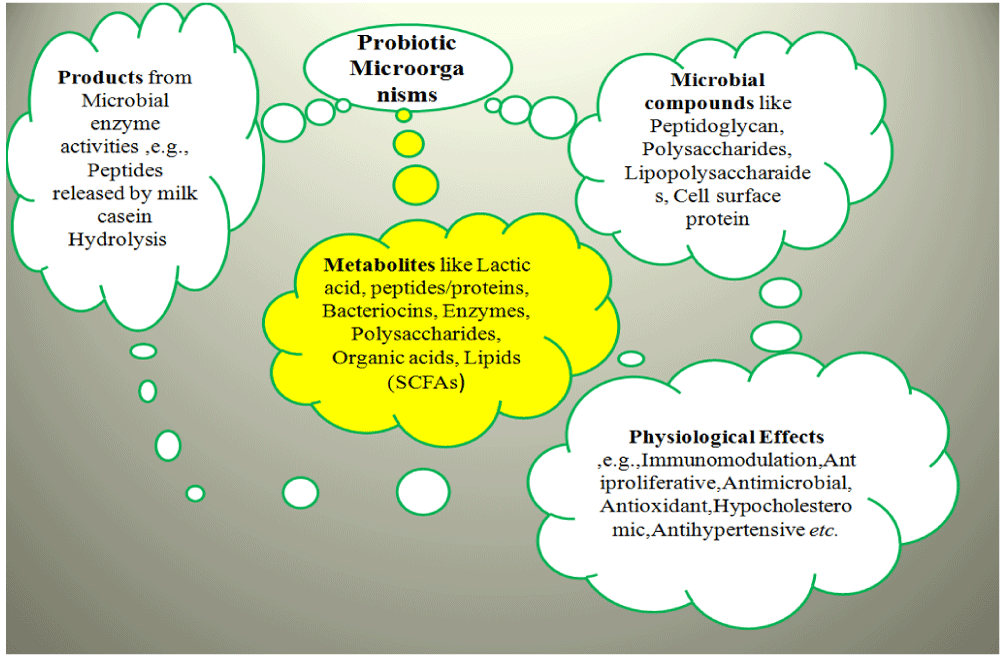
Chemicals like propionic acid, lactic acid, SCFAs, and other organic acids are synthesized by the microorganisms where they are growing, and they can nearby inhibit or support the growth of other organisms. A list of microorganisms exhibiting probiotic effects is presented in Table 3.
Potential mechanism distribution among probiotics
Some mechanisms might be widespread among commonly studied probiotic genera for example, colonization resistance, normalization of disturbed microbiota, acid and fatty acid production, increased turnover of enterocytes, regulation of intestinal transit, and competitive exclusion of pathogen; others might be frequently observed among the most strain of a probiotic species, for example, vitamin synthesis, Bile salt metabolism, direct antagonism, enzyme activity, gut barrier reinforcement, and neutralization of carcinogens; others maybe rear and present only a few strain of a given species, for example, neurological effect, immunological effect, endocrinological effects and production of specific bio-actives [8]. By fostering a hostile environment for pathogenic microbes in the intestine, probiotic microorganisms perform a crucial function. The intestinal epithelium’s shape and functionality undergo some changes. They fight with each other for the surface area of the gut epithelial layer. To prevent the adhesion and proliferation of pathogenic bacteria in the intestinal lumen, they produce specific chemicals, such as organic acids, bacteriocins, and dipicolinic acids. Probiotic mode of action includes modification of the microbial population, aggregation with the pathogenic bacteria, competitive adhesion to the epithelial receptors, modification of the structural and functional properties of the intestinal epithelium, competition for nutrients, production of specific substances, such as organic acids, bacteriocins, dipicolinic acids, etc. [85].
Challenges of probiotics in nondairy products
Storage temperature, dissolved oxygen levels, pH, and the presence of chemical inhibitors all influence stability [86]. Room temperature storage, which is common for many nondairy products, can be detrimental to probiotic stability [87]. A big obstacle is the incorporation of probiotic civilizations in nondairy products. Choosing a food matrix is crucial for the survival of the probiotic throughout the process and storage system. It has been confirmed that the food matrix, pH, and water activity of the results, and the probiotic pressures of choice all affect the probiotics’ survival and potency throughout the presentation and preservation of probiotic vegetables, fruits, and cereals. Fruit and vegetable juices include certain necessary nutrients, but other elements, such as low pH, which is linked to higher amounts of organic acids and dissolved oxygen, may affect the viability of probiotics 78. Furthermore, dairy products are frequently stored at temperatures around 5 °C, which suggests that probiotic cell viability is likely guaranteed during the development of shelf life. However, when kept at ambient temperature, typical for many nondairy food types such as cereal products, fruit juices, beverages, confectionery, and more, can make an excellent challenge for probiotic viability [88].
In comparison to dairy probiotics, the state of nondairy probiotic beverages and the viability and impact of adaptive technology for their presentation are not moving simultaneously, at least not yet. However, as a result of the new economic, technological, and economic matrix, there is an urgent need to match the demand for natural, nutritious probiotic dairy food options with nondairy probiotic meals. Few claims exist regarding the preparation and presentation of fruit juices, despite an excellent potential for employing them as a probiotic. Therefore, there is a need for more research in this field. While expanding, it’s essential to maintain a reasonable level of accessibility, stability, and sensory acceptance, particularly concerning flavor, appeal, and affordability, as these factors are imperative to their successful commercialization.
According to [89] Lee, et al. (1995), the “therapeutic minimum” for probiotics is 1 x 105 colony-forming units per gram or millilitre of the finished product. For any positive effects to manifest in humans, viable cell numbers ranging from 1 x 106 to 1 x 109 c.f.u. must be consumed every day. Even in cold storage, Lactobacillus acidophilus and Bifidobacterium bifidum have a brief stationary phase that is followed by rapid cell viability losses. As a result, the short shelf-life of these probiotic microorganisms poses a logistical challenge for researchers and manufacturers alike. Compared to Lactobacillus acidophilus and Bacillus spp., Lacticaseibacillus casei and Lactiplantibacillus plantarum have a longer shelf-life. Typical cultured milk has lactic acid at 0.5 - 1.5 w/v and a pH between 3.5 and 4.5.
To have a competitive edge, functional impacts are crucial. Therefore, caution should be used while verifying the starter’s accessible qualities before merging them into the product. The benefits of nondairy probiotic products for society can be understood and leveraged by expanding research into these items. Prebiotics can also be used to provide synbiotic effects when combined with nondairy probiotic products.
All age groups should include milk and its derivatives in their diets because they are essential for healthy eating habits. The presence of physical, biological (including pathogenic and spoilage bacteria), and chemical (such as metals, pesticides, and mycotoxins) pollutants in milk and dairy products, however, has become a growing source of concern. Milk and dairy products must be free of harmful substances, given the high consumption rates. A developing biotechnological method for chemical decontamination is microbial degradation, which is regarded as a low-risk and affordable procedure [90] (Figure 3).
There are significant challenges during manufacturing: maintaining probiotic viability during product shelf life and after ingestion into the gastrointestinal tract, and maintaining the physicochemical and sensory characteristics of conventional products [91].
For nutraceutical applications, stable preparations with intact functional properties can be achieved using microencapsulated probiotics. The study investigated the morphology, tolerance to heat and mechanical stress, storage stability, and release stability of bacteria in a simulated gastrointestinal fluid. When Lactobacillus spp. were microencapsulated, their survival rates following various treatments were much higher than those of freeze-dried cells. Most remarkably, encapsulated samples proved to be the most protective throughout gastrointestinal conditions; survival rates were 25% at 100 °C, 41% after 20 days at 70% relative humidity, and 32% following exposure to a 3-ton mechanical force. On the other hand, after being subjected to mechanical, thermal, and storage treatments, non-encapsulated samples did not survive in the gastrointestinal environment. On the other hand, after being subjected to mechanical, thermal, and storage treatments, non-encapsulated samples did not survive in the gastrointestinal environment. As a result, the study emphasizes the essential probiotic characteristics that were mostly preserved by microencapsulation, including temperature, humidity, mechanical stress, and pH tolerance [92].
Conclusion
Probiotic microorganisms exhibited health-promoting properties and were primarily utilized in fermented dairy matrices. Due to limitations such as lactose intolerance, hypercholesterolemia, and casein sensitivity, nondairy substrates—fruits, cereals, soy, and vegetables—were investigated as alternative delivery systems. These matrices provided prebiotic components and suitable pH environments but presented challenges in maintaining microbial viability. Factors including water activity, temperature, food matrix composition, and strain specificity influenced cell survival. Encapsulation techniques and controlled fermentation parameters were explored to enhance stability. Ensuring therapeutic colony-forming unit levels throughout shelf life remained critical for functional efficacy.
The authors are grateful to the Ministry of Science and Technology of the Government of India for providing funds for infrastructure improvement under DST-FIST, which enabled us to conduct research in our department.
Author contributions
Shailendra Kumar conceptualized the topic, and Triyugi Narain Kushwaha conducted the literature review and prepared the first draft. Shailendra Kumar finalized the Manuscript.
- Gasbarrini G, Bonvicini F, Gramenzi A. Probiotics History. J Clin Gastroenterol. 2016;50:S116–S119. Available from: https://doi.org/10.1097/mcg.0000000000000697
- Kollath W. Nutrition and the tooth system; general review with special reference to vitamins. Dtsch Zahnärztl Z. 1953;8:7–16. Available from: https://pubmed.ncbi.nlm.nih.gov/13068115/
- Hamilton-Miller J, Gibson G, Brück W. Some insights into the derivation and early uses of the word 'probiotic'. Br J Nutr. 2003;90:845. Available from: https://doi.org/10.1079/bjn2003954
- Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147:747–748. Available from: https://doi.org/10.1126/science.147.3659.747
- Havenaar R, Brink BT, Huis In’T Veld JHJ. Selection of strains for probiotic use. In: Fuller R, editor. Probiotics. The Scientific Basis. London: Chapman and Hall; 1992;209–224. Available from: https://link.springer.com/chapter/10.1007/978-94-011-2364-8_9
- Salminen S, von Wright A, Morelli L, Marteau P, Brassart D, de Vos WM, et al. Demonstration of safety of probiotics — A review. Int J Food Microbiol. 1998;44:93–106. Available from: https://doi.org/10.1016/s0168-1605(98)00128-7
- FAO/WHO. Report of a Joint FAO/WHO Expert Consultation on evaluation of health and nutritional properties of probiotics in food, including powder milk with live LAB. Food and Agriculture Organization of the United Nations World Health Organization; 2001. Available from: https://openknowledge.fao.org/server/api/core/bitstreams/382476b3-4d54-4175-803f-2f26f3526256/content
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. Available from: https://www.nature.com/articles/nrgastro.2014.66
- Saad N, Delattre C, Urdaci M, Schmitter JM, Bressollier P. An overview of the last advances in probiotic and prebiotic field. LWT Food Sci Technol. 2013;50:1–16. Available from: https://doi.org/10.1016/j.lwt.2012.05.014
- Amiri ZR, Khandelwal P, Aruna BR. Development of acidophilus milk via selected probiotics and prebiotics using artificial neural network. Adv Biosci Biotechnol. 2010;1:224–231. Available from: https://pdfs.semanticscholar.org/4b26/81c3ae1c8dcd5acd608ebbb3d6c506f530b8.pdf
- Sangwan V, Tomar SK, Singh RRB, Singh AK, Ali B. Galactooligosaccharides: novel components of designer foods. J Food Sci. 2011;76:R103–R111. Available from: https://doi.org/10.1111/j.1750-3841.2011.02131.x
- Heller KJ. Probiotic bacteria in fermented foods: product characteristics and starter organisms. Am J Clin Nutr. 2001;73:374–379. Available from: https://doi.org/10.1093/ajcn/73.2.374s
- Laroia S, Martin JH. Methods for enumerating and propagating bifidobacteria. Cult Dairy Prod J. 1991;26:32–33.
- Penna ALB, Rao-Gurram S, Barbosa-Ca’novas GV. Effect of milk treatment on acidification, physicochemical characteristics, and probiotic cell counts in low-fat yogurt. Milchwissenschaft. 2007;62:48–52. Available from: https://repositorio.unesp.br/entities/publication/c1b39c85-ca81-485e-9318-bb423a6187d9
- Vijayendra SVN, Gupta RC. Associative growth behavior of dahi and yoghurt starter cultures with Bifidobacterium bifidum and Lactobacillus acidophilus in buffalo skim milk. Ann Microbiol. 2013a;63:461–469. Available from: https://annalsmicrobiology.biomedcentral.com/articles/10.1007/s13213-012-0490-z
- Vijayendra SVN, Gupta RC. Performance evaluation of bulk freeze dried starter cultures of dahi and yoghurt along with probiotic strains in standardized milk of cow and buffalo. J Food Sci Technol. 2013b;51:4114–4119. Available from: https://doi.org/10.1007/s13197-013-0944-8
- Peres CM, Peres C, Hernández-Mendoza A, Malcata FX. Review on fermented plant materials as carriers and sources of potentially probiotic LAB -With an emphasis on table olives. Trends Food Sci Technol. 2012;26:31–42. Available from: https://doi.org/10.1016/j.tifs.2012.01.006
- Gupta S, Abu-Ghannam N. Probiotic fermentation of plant based products: possibilities and opportunities. Crit Rev Food Sci Nutr. 2012;52:183–199. Available from: https://doi.org/10.1080/10408398.2010.499779
- Prado FC, Parada JL, Pandey A, Soccol CR. Trends in nondairy probiotic beverages. Food Res Int. 2008;41:111–123. Available from: https://doi.org/10.1016/j.foodres.2007.10.010
- Ranadheera RDCS, Baines SK, Adams MC. Importance of food in probiotic efficacy. Food Res Int. 2010;43:1–7. Available from: http://dx.doi.org/10.1016/j.foodres.2009.09.009
- Rouhi M, Sohrabvandi S, Mortazavian AM. Probiotic fermented sausage: viability of probiotic microorganisms and sensory characteristics. Crit Rev Food Sci Nutr. 2013;53:331–348. Available from: https://doi.org/10.1080/10408398.2010.531407
- Liu B, Fu N, Woo MW, Chen XD. Heat stability of Lactobacillus rhamnosus GG and its cellular membrane during droplet drying and heat treatment. Food Res Int. 2018;112(January):56–65. Available from: https://doi.org/10.1016/j.foodres.2018.06.006
- Bustos P, Bórquez R. Influence of osmotic stress and encapsulating materials on the stability of autochthonous Lactobacillus plantarum after spray drying. Dry Technol. 2013;31:57–66. Available from: http://dx.doi.org/10.1080/07373937.2012.717325
- Endo A, Terasjarvi J, Salminen S. Food matrices and cell conditions influence survival of Lactobacillus rhamnosus GG under heat stresses and during storage. Int J Food Microbiol. 2014;174:110–112. Available from: https://doi.org/10.1016/j.ijfoodmicro.2014.01.006
- Farag MA, El Hawary EA, Elmassry MM. Rediscovering acidophilus milk, its quality characteristics, manufacturing methods, flavor chemistry and nutritional value. Crit Rev Food Sci Nutr. 2019;0(0):1–18. Available from: https://doi.org/10.1080/10408398.2019.1675584
- Janiszewska J, Ostrowska J, Szostak-Węgierek D. Milk and Dairy Products and Their Impact on Carbohydrate Metabolism and Fertility-A Potential Role in the Diet of Women with Polycystic Ovary Syndrome. Nutrients. 2020 Nov 13;12(11):3491. Available from: https://doi.org/10.3390/nu12113491
- Rabah H, Rosa do Carmo F, Jan G. Dairy Propionibacteria: Versatile Probiotics. Microorganisms. 2017;5(2):24. Available from: https://doi.org/10.3390/microorganisms5020024
- Karaca OB, Güzeler N, Tangüler H, Yaşar K, Akın MB. Effects of apricot fibre on the physicochemical characteristics, the sensory properties and bacterial viability of nonfat probiotic yoghurts. Foods. 2019;8(1):1–15. Available from: https://doi.org/10.3390/foods8010033
- Shandilya UK, Sharma A, Kapila R, Kansal VK. Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum modulates immunoglobulin levels and cytokines expression in whey proteins sensitised mice. J Sci Food Agric. 2016;96(9):3180–3187. Available from: https://doi.org/10.1002/jsfa.7497
- Kaushal D, Kansal VK. Dahi containing lactobacillus acidophilus and Bifidobacterium bifidum improves phagocytic potential of macrophages in aged mice. J Food Sci Technol. 2014;51(6):1147–1153. Available from: https://doi.org/10.1007/s13197-012-0637-8
- da Cruz MF, Magno MB, Jural LA, Pimentel TC, Ferreira DMTP, Esmerino EA, et al. Probiotics and dairy products in dentistry: A bibliometric and critical review of randomized clinical trials. Food Res Int. 2022;157:111228. Available from: https://doi.org/10.1016/j.foodres.2022.111228
- Liu X, Zhao H, Wong A. Heliyon Accounting for the health risk of probiotics. Heliyon. 2024;10(6):e27908. Available from: https://doi.org/10.1016/j.heliyon.2024.e27908
- Levy RI, Feinleib M. Risk factors for coronary artery disease and their management. In: Braunwald E, editor. Heart disease. Philadelphia: Saunders WB; 1980. p. 1246–1278.
- Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, et al. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr. 2014;54:175–189. Available from: https://doi.org/10.1080/10408398.2011.579361
- Hauck FR, Thompson JM, Tanabe KO, Moon RY, Vennemann MM. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103–110. Available from: https://doi.org/10.1542/peds.2010-3000
- Lee BH. Fundamentals of food biotechnology. New York: Wiley; 2015.
- Joachim G. The relationship between habits of food consumption and reported reactions to food in people with inflammatory bowel disease–testing the limits. Nutr Health. 1999;13:69–83. Available from: https://doi.org/10.1177/026010609901300203
- Vesa TH, Marteau P, Korpela R. Lactose intolerance. J Am Coll Nutr. 2000;19:165S–175S. Available from: https://doi.org/10.1080/07315724.2000.10718086
- Fassio F, Facioni MS, Guagnini F. Lactose maldigestion, malabsorption, and intolerance: a comprehensive review with a focus on current management and future perspectives. Nutrients. 2018;10(11):1–12. Available from: https://doi.org/10.3390/nu10111599
- Colombo M, Todorov SD, Eller M, Nero LA. The potential use of probiotic and beneficial bacteria in the Brazilian dairy industry. J Dairy Res. 2018;85(4):487–496. Available from: https://doi.org/10.1017/S0022029918000845
- Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, et al. Health benefit of probiotic: A review. Hindawi Publishing Corporation. 2013:1–7. Available from: https://doi.org/10.5402/2013/481651
- Lugito H, Djuwita R, Adisasmita, Simadibrata M. Blood Pressure Lowering Effect of Lactobacillus-Containing Probiotic. Int J Probiotics Prebiotics. 2022;17(1):1–13. Available from: https://scholar.ui.ac.id/en/publications/blood-pressure-lowering-effect-of-lactobacillus-containing-probio
- Gawkowski D, Chikindas ML. Nondairy probiotic beverages: the next step into human health. Benefic Microbes. 2013;4:127–142. Available from: https://doi.org/10.3920/bm2012.0030
- Martins EMF, Ramos AM, Vanzela ESLB, Stringheta PC, Pinto CLO, Martins JM. Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Res Int. 2013;51:764–770. Available from: https://doi.org/10.1016/j.foodres.2013.01.047
- Pereira ALF, Maciel TC, Rodrigues S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res Int. 2011;44:1276–1283. Available from: https://doi.org/10.1016/j.foodres.2010.11.035
- Nualkaekul S, Lenton D, Cook MT, Khutoryanskiy VV, Charalampopoulos D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr Polym. 2012;90:1281–1287. Available from: https://doi.org/10.1016/j.carbpol.2012.06.073
- Heidebach T, Först P, Kulozik U. Microencapsulation of Probiotic Cells for Food Applications. Crit Rev Food Sci Nutr. 2012;52(4):291–311. Available from: https://doi.org/10.1080/10408398.2010.499801
- Anekella K, Orsat V. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT Food Sci Technol. 2013;50:17–24. Available from: https://doi.org/10.1016/j.lwt.2012.08.003
- Kailasapathy K. Microencapsulation for gastrointestinal delivery of probiotic bacteria. In: Kwak HS, editor. Nano-and microencapsulation for foods. Hoboken (NJ): Wiley; 2014. p. 167–205. Available from: https://researchers.westernsydney.edu.au/en/publications/microencapsulation-for-gastrointestinal-delivery-of-probiotic-bac
- Yoon KY, Woodamns EE, Hang YD. Probiotication of tomato juice by LAB. J Microbiol. 2004;42:315–318. Available from: https://pubmed.ncbi.nlm.nih.gov/15650688/
- Luckow T, Delahunty C. Which juice is "healthier"? A consumer study of probiotic nondairy juice drinks. Food Qual Prefer. 2004;15(7-8 SPEC.ISS.):751–759. Available from: https://doi.org/10.1016/j.foodqual.2003.12.007
- Tuorila H, Cardello AV. Consumer responses to an off-flavor in juice in the presence of specific health claims. Food Qual Prefer. 2002;13:561–569. Available from: http://dx.doi.org/10.1016/S0950-3293(01)00076-3
- Sheehan VM, Ross P, Fitzgerald GF. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innovative Food Sci Emerg Technol. 2007;8:279–284. Available from: http://dx.doi.org/10.1016/j.ifset.2007.01.007
- Shah NP. Functional foods from probiotics and prebiotics. Food Technol. 2001;55:46–53. Available from: https://www.scirp.org/reference/referencespapers?referenceid=2029365
- Prado FC, Lindner JDD, Inaba J, Thomaz-Soccol V, Brar SK, Soccol CR. Development and evaluation of a fermented coconut water beverage with potential health benefits. J Funct Foods. 2015;12:489–497. Available from: https://doi.org/10.1016/j.jff.2014.12.020
- Charalampopoulos D, Wang R, Pandiella SS, Webb C. Application of cereals and cereal components in functional foods: a review. Int J Food Microbiol. 2002;79:131–141. Available from: https://doi.org/10.1016/s0168-1605(02)00187-3
- Clemens R, Pressman P. Heyday in grain land. Food Technol. 2006;60(11):18.
- Jideani IA, Jideani VA. Developments on the cereal grains Digitaria exilis (acha) and Digitaria iburua (iburu). J Food Sci Technol. 2011;48:251–259. Available from: https://doi.org/10.1007/s13197-010-0208-9
- Kalui CM, Mathara JM, Kutima PM. Probiotic potential of spontaneously fermented cereal based foods—a review. Afr J Biotechnol. 2010;9:2490–2498. Available from: https://www.ajol.info/index.php/ajb/article/view/79704
- Waters DM, Mauch A, Coffey A, Arendt EK, Zannini E. Lactic acid bacteria as a cell factory for the delivery of functional biomolecules and ingredients in cereal based beverages: a review. Crit Rev Food Sci Nutr. 2015;55:503–520. Available from: https://doi.org/10.1080/10408398.2012.660251
- Muthukumarasamy P, Holley RA. Microbiological and sensory quality of dry fermented sausages containing alginate-microencapsulated Lactobacillus reuteri. Int J Food Microbiol. 2006;111:164–169. Available from: https://doi.org/10.1016/j.ijfoodmicro.2006.04.036
- Rivera-Espinoza Y, Gallardo-Navarro Y. Nondairy probiotic products. Food Microbiol. 2010;27:1–11. Available from: https://doi.org/10.1016/j.fm.2008.06.008
- Salama MS, Musafija-Jeknic T, Sandine WE, Giovannoni SJ. An ecological study of LAB: isolation of new strains of Lactococcus including Lactococcus lactis subsp. cremoris. J Dairy Sci. 1995;78:1004–1017. Available from: https://doi.org/10.3168/jds.S0022-0302(95)76716-9
- Ulrich A, Müller T. Heterogeneity of plant-associated streptococci as characterized by phenotypic features and restriction analysis of PCR-amplified 16S rRNA. J Appl Microbiol. 1999;84:293–303. Available from: https://doi.org/10.1046/j.1365-2672.1998.00343.x
- Klijn N, Weerkamp AH, de Vos WM. Detection and characterization of lactose-utilizing Lactococcus spp. in natural ecosystems. Appl Environ Microbiol. 1995;61:788–792. Available from: https://doi.org/10.1128/aem.61.2.788-792.1995
- Bauer S, Tholen A, Overmann J, Brune A. Characterization of abundance and diversity of lactic acid bacteria in the hindgut of wood and soil-feeding termite by molecular and culture-dependent techniques. Arch Microbiol. 2000;173:126–137. Available from: https://link.springer.com/article/10.1007/s002039900120
- Nomura M, Kobayashi M, Narita T, Kimoto-Nira H, Okamoto T. Phenotypic and molecular characterization of Lactococcus lactis from milk and plants. J Appl Microbiol. 2006;101:396–405. Available from: https://doi.org/10.1111/j.1365-2672.2006.02949.x
- Saxelin M, Korpela R, Mayra-Makinen A. Introduction: classifying functional dairy products. In: Mattila-Sandholm T, Saarela M, editors. Functional dairy products. New York: CRC Press; 2003. p. 1–15. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/20033063724
- Ritter P, Kohler C, Von Ah U. Evaluation of the passage of Lactobacillus gasseri K7 and bifidobacteria from the stomach to intestines using a single reactor model. BMC Microbiol. 2009;9:1–9. Available from: https://doi.org/10.1186/1471-2180-9-87
- Figueroa-González I, Quijano G, Ramírez G, Cruz-Guerrero A. Probiotics and prebiotics. Perspectives and challenges. J Sci Food Agric. 2011;91:1341–1348. Available from: https://doi.org/10.1002/jsfa.4367
- Ouwehand AC, Salminen S. The health effects of cultured milk products with viable and non viable bacteria. Int Dairy J. 1998;8:749–758. Available from: https://doi.org/10.1016/S0958-6946(98)00114-9
- Céspedes M, Cárdenas P, Staffolani M, Ciappini MC, Vinderola G. Performance in nondairy drinks of probiotic Lactobacillus casei strains usually employed in dairy products. J Food Sci. 2013;78:M756–M762. Available from: https://doi.org/10.1111/1750-3841.12092
- Morelli L. In vitro assessment of probiotic bacteria: from survival to functionality. Int Dairy J. 2007;17:1278–1283. Available from: https://doi.org/10.1016/j.idairyj.2007.01.015
- Vinderola G, Céspedes M, Mateolli D, Cárdenas P, Lescano M, Aimaretti N, et al. Changes in gastric resistance of Lactobacillus casei in flavored commercial fermented milks during refrigerated storage. Int J Dairy Technol. 2011;64:269–275. Available from: https://doi.org/10.1111/j.1471-0307.2010.00659.x
- Bunning VK, Crawford RG, Tierney JT, Peeler JT. Thermotolerance of Listeria monocytogenes and Salmonella typhimurium after sublethal heat shock. Appl Environ Microbiol. 1990;56:3216–3219. Available from: https://doi.org/10.1128/aem.56.10.3216-3219.1990
- Saarela M, Virkajarvi I, Nohynek L, Vaari A, Matto J. Fibers as carriers for Lactobacillus rhamnosus during freeze-drying and storage in apple juice and chocolate-coated breakfast cereals. Int J Food Microbiol. 2006;112:171–178. Available from: https://doi.org/10.1016/j.ijfoodmicro.2006.05.019
- Bhat A, Irorere V, Bartlett T, Hill D, Kedia G, Charalampopoulos D, et al. Improving survival of probiotic bacteria using bacterial poly-γ-glutamic acid. Int J Food Microbiol. 2015;196:24–31. Available from: https://doi.org/10.1016/j.ijfoodmicro.2014.11.031
- Shori AB. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and nondairy beverages. Food Biosci. 2016. Available from: http://dx.doi.org/10.1016/j.fbio.2015.11.001
- Iravani S, Korbekandi H, Mirmohammadi SV. Technology and potential applications of probiotic encapsulation in fermented milk products. J Food Sci Technol. 2015;52:4679–4696. Available from: https://doi.org/10.1007/s13197-014-1516-2
- Perricone M, Bevilacqua A, Altieri C, Sinigaglia M, Corbo MR. Challenges for the production of probiotic fruit juices. Beverages. 2015;1:95–103. Available from: https://doi.org/10.3390/beverages1020095
- Maleki D, Azizi A, Vaghef E, Balkani S, Homayouni A. Methods of increasing probiotic survival in food and gastrointestinal conditions. Prensa Med Argent. 2015;101:1–9. Available from: https://www.scholarsliterature.com/article_pdf/4/scientific_4_59_07022019052620.pdf
- González-Ferrero C, Irache J, González-Navarro C. Soybean protein-based microparticles for oral delivery of probiotics with improved stability during storage and gut resistance. Food Chem. 2018;239:879–888. Available from: https://doi.org/10.1016/j.foodchem.2017.07.022
- El-Salam MHA, El-Shibiny S. Preparation and properties of milk proteins-based encapsulated probiotics: a review. Dairy Sci Technol. 2015;95:393–412. Available from: https://hal.science/hal-01297180/document
- Guiné RPF, Barroca MJ, Coldea TE, Bartkiene E, Anjos O. Apple fermented products: An overview of technology, properties and health effects. Processes. 2021;9(2):1–25. Available from: https://www.mdpi.com/2227-9717/9/2/223#
- Tiwari C, Bakshi M, Vichitra A. A rapid two-step protocol of in vitro propagation of an important medicinal herb Centella asiatica Linn. Afr J Biotechnol. 2013;12:1084–1090. Available from: https://doi.org/10.5897/AJB2012.2945
- Granato D, Branco GF, Nazzaro F, Cruz AG, Faria JAF. Functional food and nondairy probiotic food development: trends, concepts, and products. Comp Rev Food Sci Food Saf. 2010;9:292–302. Available from: https://doi.org/10.1111/j.1541-4337.2010.00110.x
- Matilla-Sandholm T, Myllarinen P, Crittenden R, Mogensen G, Fonden R, Saarela M. Technological challenges for future probiotic foods. Int Dairy J. 2002;12:173–182. Available from: https://cris.vtt.fi/en/publications/technological-challenges-for-future-probiotic-foods
- Vinderola G, Burns P, Reinheimer J. Probiotics in nondairy products. In: Vegetarian and Plant-Based Diets in Health and Disease Prevention. Amsterdam (Netherlands): Elsevier. 2017;809–835. Available from: https://doi.org/10.1016/B978-0-12-803968-7.00044-7
- Lee YK, Salminen S. The coming of age of probiotics. Trends Food Sci Technol. 1995;6(7):241–245. Available from: https://doi.org/10.1016/S0924-2244(00)89085-8
- Wochner KF, Becker-Algeri TA, Colla E, Badiale-Furlong E, Drunkler DA. The action of probiotic microorganisms on chemical contaminants in milk. Crit Rev Microbiol. 2018;44(1):112–123. Available from: https://doi.org/10.1080/1040841X.2017.1329275
- Aspri M, Papademas P, Tsaltas D. Review on nondairy probiotics and their use in nondairy based products. Fermentation. 2020;6(1):1–20. Available from: https://doi.org/10.3390/fermentation6010030
- Ramon Estevez JN, Arroyave Manco JC, Garcia-Arboleda W, Echeverry S, Pino I, Acevedo A, et al. Microencapsulated Probiotics in Feed for Beef Cattle are Better Alternative to Monensin Sodium. Int J Probiotics Prebiotics. 2023;18(1):30–37. Available from: https://www.nchpjournals.com/ManuscriptIjpp?id=2521
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
