Open J Biol Sci
Environmental toxicants and Infant Mortality in the USA
Ting Ji1, Chen Tian3, Xin-Zhong Ji4, Li Huang4, Yun-Cong Wang1, Han Liu3, Xiao-Dong Xie2*
2School of Basic Medical Science, Institute of Genetic, Lanzhou University, Lanzhou City, Gansu Province 730000, China
3The Secondary Hospital of Lanzhou University, Lanzhou City, Gansu Province 730000, China
4The Ningxia Hui Autonomous Region people’s Hospital, Yinchuan City, Ningxia Hui Autonomous Region 750000, China
Cite this as
Ji T, Tian C, Ji XZ, Huang L, Wang YC, et al. (2016) Correlation Analysis between mtDNA G10398A Polymorphism and Susceptibility of Patients with Type 2 Diabetes Mellitus in Northwest China. Open J Biol Sci 1(1): 014-017. DOI: 10.17352/ojbs.000003Introduction: The present work aimed to investigate the association between G10398A polymorphism in mtDNA with type 2 diabetes mellitus (T2DM) susceptibility among Chinese northwestern population.
Methods: Polymerase chain reaction followed by the restriction digestion of the amplicon to clarify the restriction fragment length polymorphism (PCR-RFLP) was used to score the G10398A variation of mtDNA NADH dehydrogenase3 (ND3) locus in a case-control study (n=422), including 209 patients with T2DM and 213 healthy controls.
Results: The genotypes of G10398A were AA, AG, GG and the frequencies of genotypes and allele distributions were not significantly different in T2DM patients compared to healthy controls (P>0.05, χ2 test). There were no significant differences between genotypes and clinic indicators (P>0.05, single factor analysis of variance).
Conclusions: The findings indicated that G10398A SNPs in ND3 locus of mtDNA were not in association with T2DM susceptibility in this population.
Abbreviations
mtDNA: mitochondrial DNA; DNA: Deoxyribonucleic Acid; T2DM: Type 2 Diabetes Mellitus; PCR-RFLP: Polymerase Chain Reaction-the Restriction Fragment Length Polymorphism; ND3: NADH Dehydrogenase3; ROS: Reactive Oxygen Species; FPG: Fasting Plasma Glucose; ALT: Alanine Amino Transferase; TC: Total Cholesterol; TG: Triglyceride; BUN: Burine Nitrogen; SNP: Single Nucleotide Polymorphism;
Introduction
Diabetes mellitus type 2 (T2DM) is a long term metabolic disorder that is characterized by hyperglycemia, insulin resistance and relative lack of insulin[1]. Common symptoms include increased thirst, frequent urination, and unexplained weight loss. Long-term complications from hyperglycemia include heart disease, strokes, diabetic retinopathy which can result in blindness, kidney failure, and poor blood flow in the limbs which may lead to amputations [2-4]. Mitochondrial gene (mtDNA) mutation type diabetes mellitus has gradually attracted people’s attention[5]. Human mitochondrial DNA (mtDNA) is a double stranded circular molecule with 16569bp, which has a high mutation rate, and has become a heated focus on various diseases due to the lack of protection and efficient DNA repair mechanism[5-9]. The mtDNA oxidative phosphorylation system encompasses five (I–V) multi subunit enzyme complexes. Complex I consists of seven mitochondrial genomic encoded subunits (ND1, ND2, ND3,ND4L, ND4, ND5 and ND6) and 36 nuclear genes arrayed within the mitochondrial inner membrane[10]. The ND3 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Position 10398 in the mtDNA is located in the gene encoding NADH dehydrogenase 3 (ND3). The G to A polymorphism in ND3 causes the substitution of alanine by threonine in amino acid 114 in the ND3 peptide. This amino acid substitution in the ND3 gene is hypothesized to affect protein function [11,12]. Darvishi et al. also reported that the G10398A polymorphism increased the risk of breast cancer in the Indian population [13]. In addition, mtDNA G10398A variant in African-American women with breast cancer provides resistance to apoptosis and promotes metastasis in mice [14]. Hui Xu also reported that Prognostic value of mitochondrial DNA content and G10398A polymorphism in non-small cell lung cancer [15]. At present, many investigations of association between T2DM and mtDNA from different races and territories found that frequencies of some mutations varied from areas and relevance with DM incidence, which meant that mutations may relate to DM morbidity or only be taken as natural polymorphism[16]. We aimed to test whether mitochondrial G10398A in ND3 locus, namely SNP (rs2853826), provided susceptibility to T2DM, from target population of Chinese northwestern T2DM patients, so as for new genetic clues of DM mechanism revealing.
Materials and Methods
Subjects
This study was approved by the Ethics Committee of Lanzhou University. After providing informed consent, 209 type 2 diabetic patients and 213 controls were recruited from the Hospital of Gansu Qingshui. All patients, who were Northwestern Chinese, were diagnosed according to the 1999 revised criteria for WHO; they did not have a family history of T2DM. The controls were age- and gender-matched unrelated healthy subjects recruited from individuals presenting for check-up in the hospital.
Genotyping analysis
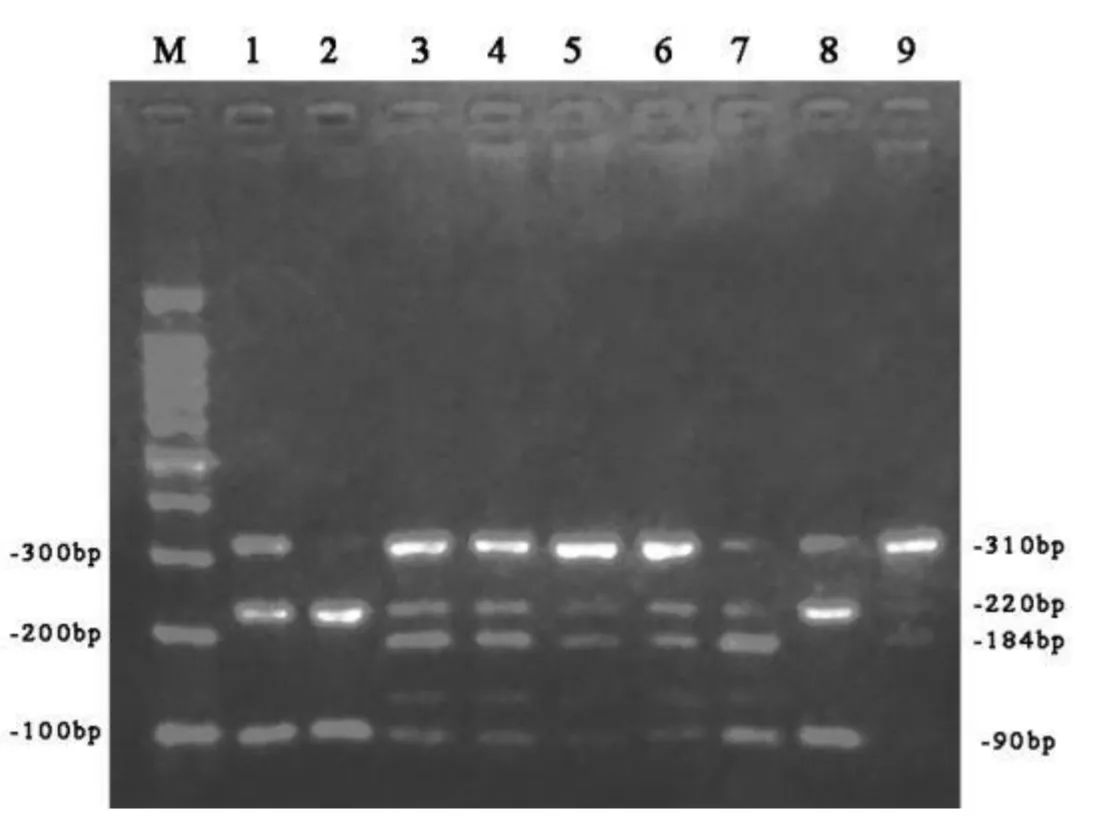
About 2 ml of venous blood was collected from each subject, using phenol chloroform method (Kunkel et al. 1977). The forward primer was 5´-tccttttacccctaccatgag-3´, and the reverse primer was 5´-tactatgcctagaaggaataat-3´. The PCR conditions were as follows. Initial denaturing was performed at 94 for 2min, and was followed by 30 cycles of denaturing at 94 for 30s, annealing at 54.3 for 30s, extension at 72for 45s, and final extension at 72 for 12 minutes. The PCR product was analyzed by electrophoresis in 2.0% agarose gel and visualized by ethidium bromide staining. The PCR product (310bp) was digested with DdeI (New England Biolabs, Beverly, MA) and separated on 2.5% agarose gel. The genotypic profiles were observed as three bands representing completely restricted fragments (sizes, 184bp, 90bp and 38bp) when G allele and two fragments 220bp and 90bp when an allele were present. Subsequent validation of the results was carried out through direct sequencing (Applied Biosystems, USA) to exclude any genotyping error or false positiveor negative results in PCR-RFLP.
Statistical analysis
Hardy–Weinberg equilibrium was assessed using χ2 test. Comparisons of genotype frequencies between T2DM patients and controls were performed using 2×2 contingency tables with χ2 analysis. Clinical characteristics were expressed as mean ± S.D. One-way analysis of variance was used to test for differences in means of phenotypic characteristics between genotypes. All P-values were two-tailed and P-values<0.05 were considered to be statistically significant. All statistical analyses were performed with SPSS for Windows (version 19.0IBM SPSS Statistics, USA).
Results
The genotypic profiles were observed as three bands representing completely restricted fragments (sizes, 184bp, 90bp and 38bp) when G allele and two fragments 220bp and 90bp when A allele were present (Figure 1).
In comparison with controls, cases had significantly higher levels of fasting plasma glucose (FPG), the plasma levels of total cholesterol(TC),triglyceride (TG), urea nitrogen (BUN)(P <0.05). However, there was no significance in age, the plasma levels of alanine transaminase (ALT) in both groups. This is consistent with diabetic features (Table 1).
The distributions of G10398A genotypes and alleles in theT2DM and control groups from a Chinese population are summarized in Table 2. The polymorphism (G10398A) fit well with the distributions expected under Hardy–Weinberg equilibrium for both groups. The genotype and allele distributions of the G10398A polymorphisms were not significantly different between theT2DM and control groups (Table 2). There is no association between the genotypes and clinical indicators T2DM group (Table 3).
Discussion
Type 2 diabetes mellitus (T2DM) affects a large population worldwide [17-19]. It was reported that the reactive oxygen species (ROS) overproduced by mitochondrion were associated with DM [20,21]. Mitochondrion is the main producer of ROS and its dysfunction causes superfluous ROS in insulin message pathway [22,23]. Meanwhile, the redundancy of ROS may accelerate cell apoptosis. It is widely accepted that high blood glucose and resultant oxidative stress mainly account for β cell functional injury, and elements, which affect ROS metabolism, can indirectly lead to the susceptibility to DM by influencing oxidative stress. Rare mtDNA mutation results in mitochondrion, β cell dysfunction and DM follows [24]. Bhat’s 2007 research demonstrated that mtDNAG10398A SNPs was missense mutation (amino acid change: Alanine → Threonine) and interfered β cell endocrine secretion by means of changing the conformation of the enzyme domain, decreasing the binding of transcription factors, preventing gene transcription and translation, debasing complexenzyme activity, less synthesis of ATP, excess of ROS [25-27]. This study suggested that G10398A mtDNA variance possibly played a role in providing susceptibility to T2DM in two North Indian populations.
We identified the genotypes of G10398A SNPs from Chinese northwestern population including 209 T2DM patients and 213 healthy controls. Frequencies of the genotypes and alleles were of no significant difference (P>0.05, χ2 test) and in no association with susceptibility to T2DM.In analyses of relationship between genotypes and clinic indicators, single transformation of genotypes has little influence on alternation of clinic indicators. Compared to the results of Bhat’s study in north India population, The probable reasons for the results were as followed: firstly, differentiated frequency distributions of mitochondrion gene mutations in various nations or populations; secondly, individual heterogeneity in mitochondrion gene mutation; additionally, diabetes is closely related to both the genetic and environmental elements, which have a close relationship with the individual life style and the environmental factors; moreover, the influence of sample size cannot be ignored.
Conclusion
To conclude, mtDNA G10398A variation has no association with T2MD in the present population group from the Chinese northwest. Therefore, further investigations are planned to include more SNPs or replicate in larger sample size to elucidate molecular mechanisms of predisposition caused by mtDNA mutation.
- Doria A, Patti ME, Kahn CR (2008) The emerging genetic architecture of type 2 diabetes. Cell Metab 8: 186-200 .
- Shalev A (1998) Insulin receptor substrate-2--a new candidate gene for NIDDM? Eur J Endocrinol 139: 263-264 .
- Takahashi Y, Kadowaki H, Momomura K, Fukushima Y, Orban T, et al. (1997) A homozygous kinase-defective mutation in the insulin receptor gene in a patient with leprechaunism. Diabetologia 40: 412-20 .
- Pasquel FJ, Umpierrez GE (2014) Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 37: 3124-3131 .
- Lynn, S., et al. (2003) Heteroplasmic ratio of the A3243G mitochondrial DNA mutation in single pancreatic beta cells. Diabetologia 46: 296-299 .
- Munakata K, Iwamoto K, Bundo M, Kato T (2005) Mitochondrial DNA 3243A>G mutation and increased expression of LARS2 gene in the brains of patients with bipolar disorder and schizophrenia. Biol Psychiatry 57: 525-532 .
- Zhao H, Young WY, Yan Q, Li R, Cao J, Wang Q, et al. (2005) Functional characterization of the mitochondrial 12S rRNA C1494T mutation associated with aminoglycoside-induced and non-syndromic hearing loss. Nucleic Acids Res 33: 1132-1139 .
- Czarnecka AM, Krawczyk T, Zdrozny M, Lubiński J, Arnold RS, et al. (2010) Mitochondrial NADH-dehydrogenase subunit 3 (ND3) polymorphism (A10398G) and sporadic breast cancer in Poland. Breast Cancer Res Treat 121: 511-518 .
- Yu Y, Lv F, Lin H, Qian G, Jiang YS, et al. (2015) Mitochondrial ND3 G10398A mutation: a biomarker for breast cancer. Genet Mol Res 14: 17426-17431 .
- Torroni A, Achilli A, Macaulay V, Richards M, Bandelt HJ (2006) Harvesting the fruit of the human mtDNA tree. Trends Genet 22: 339-345 .
- Pessin JE, Saltiel AR (2000) Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest 106: 165-169 .
- Mathews CE, Berdanier CD (1998) Noninsulin-dependent diabetes mellitus as a mitochondrial genomic disease. Proc Soc Exp Biol Med, 1998. 219: 97-108 .
- Darvishi K, Sharma S, Bhat AK, Rai E, Bamezai RN, et al. (2007) Mitochondrial DNA G10398A polymorphism imparts maternal Haplogroup N a risk for breast and esophageal cancer. Cancer Lett 249: 249-255 .
- Kulawiec M, Owens KM, Singh KK (2009) mtDNA G10398A variant in African-American women with breast cancer provides resistance to apoptosis and promotes metastasis in mice. J Hum Genet 54: 647-654 .
- Xu H, He W, Jiang HG, Zhao H, Peng XH, et al. (2013) Prognostic value of mitochondrial DNA content and G10398A polymorphism in non-small cell lung cancer. Oncol Rep 30: 3006-3012 .
- Jianpin Weng (2005) present condition of DM triggered by mtDNA mutation and suggestions about screening, diagnosis and treatment. Natl Med J China 2005: 1951-1956.
- Hu FB (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34: 1249-1257 .
- Permutt MA, Wasson J, Cox N (2005) Genetic epidemiology of diabetes. J Clin Invest 115: 1431-1439 .
- Lin Y, Sun Z (2010) Current views on type 2 diabetes. J Endocrinol 204: 1-11 .
- Sharma V, Sharma I, Singh VP, Verma S, Pandita A, et al. (2014) mtDNA G10398A variation provides risk to type 2 diabetes in population group from the Jammu region of India. Meta Gene 2: 269-273 .
- Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR (2009) An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer's disease. J Alzheimers Dis 16: 741-761 .
- Erol A (2007) Insulin resistance is an evolutionarily conserved physiological mechanism at the cellular level for protection against increased oxidative stress. Bioessays 29: 811-818 .
- Blein S, Berndt S, Joshi AD, Campa D, Ziegler RG, et al. (2014) Factors associated with oxidative stress and cancer risk in the Breast and Prostate Cancer Cohort Consortium. Free Radic Res 48: 380-386 .
- Mulder H, Ling C (2009) Mitochondrial dysfunction in pancreatic beta-cells in Type 2 diabetes. Mol Cell Endocrinol 297: 34-40 .
- Bhat A, Koul A, Sharma S, Rai E, Bukhari SI, et al. (2007) The possible role of 10398A and 16189C mtDNA variants in providing susceptibility to T2DM in two North Indian populations: a replicative study. Hum Genet 120: 821-826 .
- Oliver NA, Greenberg BD, Wallace DC (1983) Assignment of a polymorphic polypeptide to the human mitochondrial DNA unidentified reading frame 3 gene by a new peptide mapping strategy. J Biol Chem 258: 5834-5839 .
- Singh RK, Srivastava A, Kalaiarasan P, Manvati S, Chopra R, et al. (2014) mtDNA germ line variation mediated ROS generates retrograde signaling and induces pro-cancerous metabolic features. Sci Rep 4: 6571 .
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
