Comparative Study of Various Culture Media for Selecting the Enhancement of Mycelial Proliferation of Pleurotus ostreatus
1Scientist C, Analytical Science Division-Biology, Microbiology Department, Shriram Institute for Industrial Research, 19, University Road, Delhi-110007, India
2Scientist B, Analytical Science Division-Biology, Microbiology Department, Shriram Institute for Industrial Research, 19, University Road, Delhi-110007, India
3Assistant Director & Chief, Analytical Science Division-Biology, Food and Farm Department, Shriram Institute for Industrial Research, 19, University Road, Delhi-110007, India
4Deputy Director, Analytical Science Division-Biology, Toxicology Department, Shriram Institute for Industrial Research, 19, University Road, Delhi-110007, India
5Director, Shriram Institute for Industrial Research, 19, University Road, Delhi-110007, India
#These authors have equally contributed to the work
Author and article information
Cite this as
Dimri AG, Singh D, Nayak SK, Bhat B, Das M. Comparative Study of Various Culture Media for Selecting the Enhancement of Mycelial Proliferation of Pleurotus ostreatus. Open J Bac. 2025; 9(1): 025-029. Available from: 10.17352/ojb.000030
Copyright License
© 2025 Dimri AG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The cultivation of oyster mushroom (Pleurotus ostreatus) mycelium is a potential challenge in agro-biotechnological and food industries, due to the strain-selectivity, specificity, and productivity of growth pattern. The purpose of the study was to examine the effects of seven distinct culture media (PDA, WEA, CMA, CYGA, SDA, TSA, and PCA) on the mycelium growth of P. ostreatus. They were assessed in in-vitro aseptic conditions in a randomized design with five replicates. The radial mycelium development of P. ostreatus was monitored and documented every 48 hours for eight days in all seven media.
The findings of the experiment showed that after completion of the second day of incubation, there was an insignificant difference (p<0.05) in mycelial growth of P. ostreatus between various treatments used. The impact of various media on mycelial multiplication of the target oyster mushroom was significantly enhanced during the fourth day of inoculation. Additionally, several noticeable significant differences (p < 0.05) were found in potato dextrose agar, wheat extract agar, and Sabouraud dextrose agar in the form of increased and heavy mycelial growth, as 4.53, 4.24, and 4.26 centimeters, respectively, whereas comparatively the least mycelial growth was observed in CYGA, PCA, and CMA. After the eighth day of incubation, the data revealed that, according to the mycelial degree of growth with greater adaptive ability to grow on culture media, can be presented in the following order: PDA≈ WEA> SDA > TSA > CYGA > PCA > CMA. The capacity of the study is also to modify the culture technique for optimized growth of P. ostreatus for the method’s greatest advantage.
The Pleurotus genus of mushrooms is the second most widely grown in the world, after Agaricus bisporus, due to their versatility in adapting to a range of substrates from various origins [1]. Moreover, compared to other mushrooms, they need simpler substrates and are less susceptible to pathogens [2]. P. ostreatus (oyster mushroom) is regarded as a vital protein source and can represent a significant food source in underdeveloped nations afflicted by protein deficit. Their abundant supply of carbohydrates includes a variety of oligosaccharides, ergothioneine, and mono- and disaccharides that serve as prebiotics and are very helpful in preserving a balanced gut flora [3].
P. ostreatus active mycelial biomass fruiting bodies, and culture broth are also an ample source of bioactive substances exhibiting valuable therapeutic qualities as antimicrobial, anti-diabetic, anti-viral, immunomodulatory, prebiotic, hypocholesterolemic, antioxidant, and hypotensive properties [4,5]. Thus, researchers and producers are very much interested in preserving or enhancing these special properties in fresh oyster mushrooms since they are recognized as “functional foods” and valued for their inherent beneficial properties [6]. P. ostreatus over other edible mushrooms has many more advantages for cultivation as follows: has high-yield potential and high nutritional value and medicinal importance, grows fast under a wide range of pH (6–8) and temperatures (10 °C – 30 °C), demands a few environmental control, secretes a wide range of enzymes that are capable of degrading lingo-cellulosic biomass of substrates, does not need composting and colonize its substrates in a lesser time,. Moreover, P. ostreatus can be cultivated in general in cheap and simple ways; fruiting bodies of the mold are not often attacked by any diseases and pests, and their cultivation process needs only pasteurization, which is more economical and does not require any other sterilization method [7].
P. ostreatus has been one of the most widely used species in recent decades for the bioconversion of industrial wastes and the creation of bio-composite materials based on fungal mycelium in current environmentally friendly technologies [8]. As a result, P. ostreatus promotes sustainability through environmental restoration, food production, waste management, and economic benefits [9,10]. Enzymes produced by P. ostreatus have the capacity to degrade complex PAH (Polycyclic Aromatic Hydrocarbons) structures into simpler molecules that could serve as a nutritional source. The efficacy of fungal-mediated bioremediation is due to the fungus’s various processes, such as extracellular metabolite synthesis, enzymatic transformations, uptake, and intracellular metabolism. Additionally, the proliferation and colonization of fungi contribute to removing PAHs from polluted samples [11].
The culture medium used in the microbiological laboratories is a vital component in the success of fungal cultivation, as it contains essential nutrients such as cellulose, hemicellulose, and lignin for mycelial growth of Pleurotus sp. Most of these substrate materials need nitrogen and carbon components like wheat and rice bran in order to achieve an adequate C/N ratio [12]. Furthermore, the first screening step for evaluating the quality of spawning is the measurement of the mycelial mass growth rate on solid and liquid medium [9]. However, poor growth rates are typically one of the primary issues that have a detrimental impact on the ultimate cost of production, and many of the researchers are still very intrigued in studying P. ostreatus growth rates [13,14]. In this context, it is crucial to enhance the yield of the resultant mycelium and investigate its beneficial biological activity. Additionally, examining the mycelial growth pattern on various culture media may help to improve understanding and culture procedures related to this issue [15]. It is evident that not ample research has been conducted on the different types of mushrooms and the medium used for production [16]. The current investigation aims to determine the P. ostreatus mycelial colony growth suitability on various agar media for the enhancement of mushroom cultivation.
Materials and methods
Experimental design
The reference strain of P. ostreatus (DPRB 254) was procured from the Directorate of Mushroom Research, Indian Council of Agriculture Research (ICAR), Solan, Himachal Pradesh, India, and grown on reference media recommended by the supplier in aseptic laboratory conditions. After a week of incubation at 25 °C ± 1 °C in the BOD incubator, the completely developed white mycelium mat was harvested as a pure culture [17].
The experiment was executed in a completely randomized design with seven treatments as different growth media agar Corn Meal Agar (CMA: corn meal infusion from, 5g; agar 15g; distilled water 1000 mL); Potato Dextrose Agar (PDA: potatoes infusion from, 200g; dextrose 20g; agar 15g; distilled water 1000 mL); Chloramphenicol Yeast Glucose Agar (CYGA: yeast extract 5g; dextrose 20g; chloramphenicol 0.10g; agar 15g; distilled water 1000 mL); Tryptone Soya Agar (TSA: tryptone 15g; sodium chloride 5g; agar 15g; distilled water 1000 mL)); Sabouraud Dextrose Agar (SDA: peptone & tryptone 10g; dextrose 40g; agar 15g; distilled water 1000 mL); Wheat Extract Agar (WEA: wheat grain 32g; dextrose 5g; agar 15g; distilled water 1000 mL) and Potato Carrot Agar (PCA: carrot infusion from, 200g; potatoes infusion from, 250g; agar 15g; distilled water 1000 mL) used as standard check, each treatment performed in quintupled (n= 5) [18].
All seven dehydrated media were prepared by dissolving the prescribed quantity in distilled water as recommended by the manufacturer’s instructions (HiMedia Laboratories Pvt. Ltd., Mumbai, India), according to the received certificate of analysis, and were steam sterilized at 121 °C (at 15 psi for 15 minutes). After sterilization of seven media, 20 ml - 25 ml of each medium was poured into 5 different Petri dishes in aseptic conditions inside a bio-safety cabinet type II and was allowed to solidify. A six millimeter circular disc mycelium mat was cut perpendicular and amended with the 6 mm stainless steel cork borer from the edges of actively growing P. ostreatus culture and transferred to the center of each solid media Petri dish. After that, parafilm was used to seal the plates and incubated at 25 ℃ ± 1 ℃, humidity not more than 70%, in an incubator for 8 days in a supine position in dark conditions [9]. The mycelial growth was observed after completion of 48 hours. To get the first data, the diameter of the developing mycelium was measured horizontally across the petri dish using a plastic clear geometry scale, which includes mycelial density and viability. The data was recorded at an interval of two days for 8 days in a row [19].
Statistical analysis
All experiments were performed in quintuplets (n = 5) to ensure the precision of the results. Data analysis was performed with SPSS software. The difference between samples was estimated using a one-way analysis of variance (ANOVA) with Duncan’s multiple range tests (DMRT) to compare the mean significant differences between mean ± SD of seven treatments at p ≤ 0.05 significance level.
Results
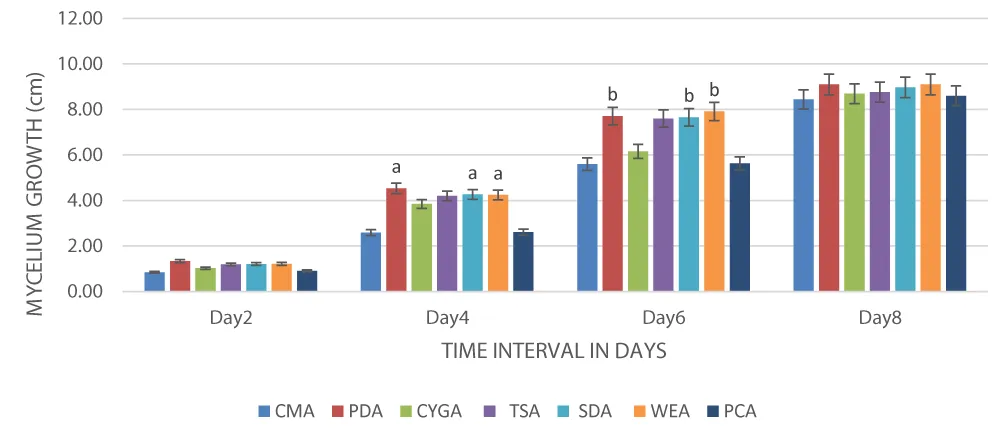
In vitro comparison of seven types of treatments in the form of culture media was used for the determination of linear mycelial growth of P. ostreatus. Results revealed, after 2nd day of incubation between various media, multiplication of mycelia is represented as growth depicted highest on potato dextrose agar (1.34 cm), trailed by wheat extract agar (1.22 cm) and sabouraud dextrose agar (1.21 cm), whereas lowest on corn meal agar (0.85 cm) and followed by potato carrot agar (0.91 cm). A varied mycelial proliferation pattern was observed in the day-to-day results of the present work. After completion of day 4, the highest growth was found on potato dextrose agar (4.53 cm), followed by Sabouraud dextrose agar (4.26 cm), wheat extract agar (4.24 cm), tryptone soya agar (4.20 cm), and the lowest on corn meal agar (2.59 cm), followed by potato carrot agar (2.61 cm). After completion of day 6 incubation, the target fungal growth was found uppermost on wheat extract agar (7.91 cm), then potato dextrose agar (7.71 cm), and least on corn meal agar (5.60 cm). On day 8, data showed that mycelial development was more adaptable to growing on solid culture media, with a higher degree of proliferation, can be presented in the following order: wheat extract agar (9.10 cm) ≈ potato dextrose agar (9.10 cm) > sabouraud dextrose agar (8.97 cm) > tryptone soya agar (8.76 cm) > chloramphenicol glucose yeast extract agar (8.70 cm) > potato carrot agar (8.60 cm) > corn meal agar (8.44 cm) (Table 1). The variance analysis showed the mycelial growth on different solid culture media, and the results exposed that Wheat extract agar supported the maximum mycelial growth of P. ostreatus and was found significantly superior over the rest of the media tested.
Figure 1 illustrates the sigmoid growth curve of P. ostreatus in various culture media on day two of incubation. There were slight notable variations among various media used. However, following the fourth day of incubation, the major impact of various media on mycelial growth intensified. Furthermore, there were notable variations among the media exhibiting the greatest mycelial development (potato dextrose agar, wheat extract agar, and sabouraud dextrose agar) and those media that showed the least mycelial growth (chloramphenicol yeast glucose agar, potato carrot and corn meal agar) after completion of the eighth day of incubation.
Discussion
P. ostreatus is the focus of extensive research due to its distinctive features, which open up broad prospects for its application in various sectors of modern industry related to biotechnology [20].
The most popular standard technique for subculturing of microorganisms is the streaking method. The present investigation phase challenges by adopting this technique for sub-culturing of Pleurotus ostreatus. The mycelial growth rate was not achieved as designed; thus, a modification in culture technique was done, which included cutting a circular disc of mycelium mat from the edges of actively growing P. ostreatus culture and inoculating the centre of the culture media Petri dish. The modified culture technique (circular disc) proved more effective than the traditional streaking method. The streaking is done with the inoculation loop, while streaking the mycelium of fungus only adheres to the small part of the loop’s diameter, whereas in the case of the modified culture technique (circular disc), it contains the media with a larger part of the mycelial mass of P. ostreatus which enhances the luxuriant growth at the time of sub culturing of fungus. The outcome unequivocally demonstrates increased mycelial proliferation of P. ostreatus.
The influence of temperature conditions on the mycelial growth of P. ostreatus plays a significant role. Earlier studies suggest that the optimal temperature for mycelium growth of oyster mushroom species was obtained at 25 ± 1 °C [21]. Hence, all our experiments were performed at 25 ± 1 °C. The present comparative studies of P. ostreatus on seven treatments as culture media revealed that the fungi showed various mycelial proliferation patterns depending upon the constituents present in the culture media. Differences in mycelial growth of P. ostreatus strains may be related to their availability of appropriate nutrients, primarily sources of carbon, nitrogen, vitamins, and macro and micro elements. This research investigation allowed us to develop beneficial and alternative solid agar media for better yield of P. ostreatus.
Our results are in accordance with the findings of earlier researchers, who stated that potato dextrose agar was suitable and the most economical medium for culture of Pleurotus species to achieve high-level mycelium biomass, exopolysaccharides, and mycelium protein [12,22]. This result is also supported by other investigators such as Poudel, et al. and Maurya, et al. [23]. It is shown that, in contrast to the other media studied, P. ostreatus grows notably fast on potato dextrose agar medium [21,24].
Carbohydrates are a major source of carbon and play an essential role in cell structure and storage. In this context, the present study was designed to evaluate the suitability of various carbon sources for mycelium growth of the oyster mushroom [25]. The media WEA and PDA, containing higher amounts of glucose and sucrose than other sources among different media used, possess higher growth potential and other nutrients like protein, fat, and vitamin E that increase the bioactivity of the fungus [9]. This may perhaps be one of the reasons for higher mycelial growth.
This finding supported earlier research showing that fungus may quickly catabolize glucose for simple cellular energy generation [26].
The three main nutritional components for the growth of Pleurotus sp. are cellulose, hemicellulose, and lignin. To get the optimal C/N ratio, most of these substrate materials need to have nitrogen as well as carbon sources like wheat and rice bran [10].
Nitrogen is an important component of the cell wall of all fungi and is required for the formation of the nitrogenous bases of DNA and proteins. In this investigation, both organic nitrogen and inorganic nitrogen sources were used to evaluate their effects on the mycelium growth of oyster mushrooms. The media types and nutrient concentrations can greatly influence growth kinetics, in agreement with the findings of another study [24].
Conclusion
A popular mushroom, P. ostreatus, may be grown on different types of media and substrates. New information about the traits of P. ostreatus strains and their capacity to adapt and grow on a variety of nutrient-solid media has been updated in this article, along with a novel modified culture technique. The most popular culture medium for mycelial growth is potato dextrose agar; nonetheless, wheat extract agar and Sabouraud dextrose agar are also appropriate for luxuriant mycelial growth. These media are also economically feasible and available locally, and can be used as a substitute for potato dextrose agar. At the same time, attention should be paid to regulating the culture media, incubation temperature, and culture technique so that fungi can develop under ideal conditions to promote mycelial proliferation and, ultimately, oyster mushroom yield.
Author’s contributions
Amita Gaurav Dimri and Dushyant Singh designed the study, methodology, execution of work, data analysis and interpretation, and drafting of the manuscript. S. K. Nayak and Binu Bhat edited and provided a thorough reading of the manuscript. Mukul Das conceived the idea for the preparation and critical review of the manuscript. All authors read and approve the final manuscript.
Acknowledgment
The authors sincerely appreciate the management of the Shriram Institute for Industrial Research for providing the infrastructure and assistance for the research work involved in the preparation of this Article, for which the manuscript is assigned the number SRI-MS#20250408-02.
- Melanouri EM, Dedousi M, Diamantopoulou P. Cultivating P. ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part II: Effect on productivity and quality of carposomes. Carb Res Conv. 2022;5:52–60. Available from: http://dx.doi.org/10.1016/j.crcon.2021.12.004
- Muswati C, Simango K, Tapfumaneyi L, Mutetwa M, Ngezimana W. The effects of different substrate combinations on the growth and yield of Oyster Mushroom (P. ostreatus). Int J Agro. 2021;10:1–10. Available from: https://doi.org/10.1155/2021/9962285
- Effiong ME, Umeokwochi CP, Afolabi IS, Chinedu SN. Assessing the nutritional quality of Pleurotus ostreatus (oyster mushroom). Front Nutr. 2024;10:1279208. Available from: https://doi.org/10.3389/fnut.2023.1279208
- Quinonez-Martínez M, Pena-Aviles K, Martinez Ruiz NR, Garza-Ocanas F, Najera-Medellin JA, Olivas Sanchez MP. Production of P. ostreatus cultivated in substrates made from two invasive weeds. Agroci. 2022;56(3). Available from: https://agrociencia-colpos.org/index.php/agrociencia/article/view/2796
- Gafforov Y, Yamac M, Inci S, Rapior S, Yarasheva M, Raseta M. Ethnobiology of Uzbekistan. Springer. 2023.
- Melanouri EM, Diamantis I, Dedousi M, Dalaka E, Antonopoulou P, Papanikolaou S, et al. Pleurotus ostreatus: Nutritional enhancement and antioxidant activity improvement through cultivation on spent mushroom substrate and roots of leafy vegetables. Ferment. 2025;11:20. Available from: https://doi.org/10.3390/fermentation11010020
- Lesa KN, Khandaker MU, Faruque MR, Sharma R, Islam F, Mitra S, et al. Nutritional value, medicinal importance, and health-promoting effects of dietary mushroom (Pleurotus ostreatus). J Food Qual. 2022;9:Article ID 2454180. Available from: https://onlinelibrary.wiley.com/doi/10.1155/2022/2454180
- Sydor M, Cofta G, Doczekalska B, Bonenberg A. Fungi in mycelium-based composites: usage and recommendations. Materials. 2022;15(8):6283. Available from: https://doi.org/10.3390/ma15186283
- Krupodorova TA, Barshteyn VYu, Bisco NA, Ivanova TS. Some macronutrient content in mycelia and culture broth of medicinal mushrooms cultivated on amaranth flour. Int J Med Mush. 2012;14:285–93. Available from: https://doi.org/10.1615/intjmedmushr.v14.i3.50
- Barshteyn V, Krupodorova T. Utilization of agro-industrial waste by higher mushrooms: modern view and trends. J Microbiol Biotechnol Food Sci. 2016;5:563–77. Available from: https://www.researchgate.net/publication/303702219_Utilization_of_agro-industrial_waste_by_higher_mushrooms_modern_view_and_trends
- Efenudu FO. Bioremediation of PAH's using Pleurotus ostreatus. Am J Environ Stud. 2024;7(1):1–15. Available from: https://www.researchgate.net/publication/377145173_Bioremediation_of_PAH's_using_Pleurotus_ostreatus
- Poudel S, Bhusal P, Poudel S. Evaluation of different culture media on the mycelial proliferation of Pleurotus ostreatus in vitro. J Pl Phy Path. 2023;11:4. https://www.scitechnol.com/peer-review/evaluation-of-different-culture-media-on-the-mycelial-proliferation-of-empleurotus-ostreatus-in-vitroem-Ookd.php?article_id=22801
- Sabri MA, Shatha AS, Rukaibaa AC. Utilization of agricultural and animal wastes in the growth of novel Iraqi strains of edible mushrooms Pleurotus ostreatus and brown Agaricus bisporus. Pl Arch. 2019;19:1188–93.
- Pham VL, Pham NDH, Nguyen HLN, Nguyen TMD, Nguyen TMT, Nguyen MT, et al. The relationship between mycelial growth and fruit bodies of oyster mushrooms (Pleurotus spp.) collected from southern Vietnam. Int J Agric Technol. 2023;19:203–14. Available from: http://www.ijat-aatsea.com/pdf/v19_n1_2023_January/16_IJAT_19(1)_2023_Pham,%20V.%20L.(34).pdf
- Devi KS, Behera B, Mishra D, Maiti TK. Immune augmentation and Dalton's lymphoma tumor inhibition by glucans/glycans isolated from the mycelia and fruit body of P. ostreatus. Int Immunopharmacol. 2015;25:207–17. Available from: https://doi.org/10.1016/j.intimp.2015.01.026
- Patel H, Gupte A, Gupte S. Biodegradation of fluoranthene by basidiomycetes fungal isolate P. ostreatus HP-1. Appl Biochem Biotechnol. 2009;157:367–76. Available from: https://doi.org/10.1007/s12010-008-8286-0
- Yang YR, Guo YX, Wang QY, Hu BY, Tian SY, Yang QZ, et al. Impacts of composting duration on physicochemical properties and microbial communities during short-term composting for the substrate for oyster mushrooms. Sci Total Environ. 2022;847:157673. Available from: https://doi.org/10.1016/j.scitotenv.2022.157673
- Sun Y, Liu J. Purification, structure, and immunobiological activity of a water-soluble polysaccharide from the fruiting body of Pleurotus ostreatus. Bioresour Technol. 2009;100:983–6. Available from: https://doi.org/10.1016/j.biortech.2008.06.036
- Tesfay T, Godifey T, Mesfin R, Kalayu G. Evaluation of waste paper for cultivation of oyster mushroom (P. ostreatus) with some added supplementary materials. AMB Express. 2020;10:15. Available from: https://doi.org/10.1186/s13568-020-0945-8
- El-Ramady H, Neama A, Zakaria F, Khandsuren B, Xhensila L, Toros G, et al. Green biotechnology of oyster mushroom (Pleurotus ostreatus L.): a sustainable strategy for myco-remediation and bio-fermentation. Sustain. 2022;14:3667. Available from: https://doi.org/10.3390/su14063667
- Lenka KC, Padhan B, Pradhan N, Mantry T, Sahu R, Venkatlaxmi S. The effect of growth conditions on mycelial run of oyster mushrooms spp. (Pleurotus spp.): implication for agricultural practices. Bhar Kris Anus Pat. 2022;37:137–43. Available from: https://arccjournals.com/journal/bhartiya-krishi-anusandhan-patrika/BKAP470
- Mihai RA, Melo Heras EJ, Florescu LI, Catana RD. The edible gray oyster fungi P. ostreatus (Jacq. ex Fr.) P. Kumm is a potent waste consumer, a biofriendly species with antioxidant activity depending on the growth substrate. J Fungi. 2022;8:274. Available from: https://doi.org/10.3390/jof8030274
- Maurya AK, Pant H, John V. Effect of media and substrates for spawn production of Dhingri mushroom (Pleurotus ostreatus). J Nat Res Develop. 2019;14:88–92. Available from: http://dx.doi.org/10.13140/RG.2.2.21808.79362
- Hoa HT, Wang C. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology. 2015;43:14–23. Available from: https://doi.org/10.5941/myco.2015.43.1.14
- Krupodorova T, Barshteyn V, Tsygankova V, Sevindik M, Blume Y. Strain-specific features of P. ostreatus growth in vitro and some of its biological activities. BMC Biotechnol. 2024;24:9. Available from: https://doi.org/10.1186/s12896-024-00834-9
- Bakratsas G, Polydera A, Nilson O, Chatzikonstantinou AV, Xiros C, Katapodis P, et al. Mycoprotein production by submerged fermentation of the edible mushroom P. ostreatus in a batch stirred tank bioreactor using agro-industrial hydrolysate. Foods. 2023;12:2295. Available from: https://doi.org/10.3390/foods12122295
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
