Biofilm Maturation and Resistance Quorum Sensing Connection
1Department of Biotechnology and Bioinformatics, JSS Academy of Higher Education and Research, Mysore, Karnataka, India
2Crescent School Of Pharmacy, B.S.Abdur Rahman Crescent Institute Of Science & Technology, Tamilnadu, India
Author and article information
Cite this as
Srinivas UB, Sivamani Y, Suresh JJ, Agarval P, Preethi N, Elayaperumal S. Biofilm Maturation and Resistance Quorum Sensing Connection. Open J Bac. 2025;9(1): 014-024. Available from: 10.17352/ojb.000029
Copyright License
© 2025 Srinivas UB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Abstract
Biofilm maturation is the stage in the biofilm lifecycle when bacterial cells in biofilm communicate with each other and grow. The development of biofilms is an essential survival strategy for numerous microbial species, encompassing a complicated, multi-stage process that begins with the initial attachment to a surface and progresses to the formation of a structured community. Growth of biofilm is associated with a higher level of mutations as well as with quorum-sensing-regulated mechanisms. Quorum sensing is a process of intercellular communication that allows bacteria to convey information regarding cell density, thereby facilitating adjustments in gene expression and the regulation of virulence factor expression in pathogenic bacteria. Biofilm cells experience significantly greater local cell densities compared to free-floating planktonic cell populations. The heightened quantities of metabolic byproducts, secondary metabolites, and other secreted or expelled microbial substances that biofilm cells experience are an evident result of this. Quorum sensing may coordinate induction to a biofilm lifestyle once the population density crosses a certain threshold level. Strong evidence obtained in multiple bacterial species which quorum sensing coincides with the activation of quorum sensing is formed biofilm formation and activates the maturation of the biofilm in a coordinated way. This chapter aims to address the issues of antibiotic resistance in biofilms by linking the processes of biofilm development and quorum sensing, providing valuable perspectives on potential new treatment approaches. There are several benefits provided by Ready-to-Eat (RTE) street vended foods, but data exists that pathogenic microorganisms may contaminate foods displayed for sale on the side of the road. However, there is a lack of data on the microbial characterization and antimicrobial resistance (AMR) trends of isolated pathogens from street food in Delhi. Considering Panipuri and noodles are the favorite RTE foods in India, the study aims to examine the occurrence including anti-microbial resistance patterns of common foodborne disease-causing microbes isolated from selected RTE foods. Sixty (60) RTE street-vended food samples from prevalent locations in Delhi, were analyzed by demonstrating mesophilic aerobic bacterial count, yeast and mold count, specified food-borne pathogens, and their AMR trend against clinically significant antibiotics. The mesophilic aerobic bacterial count varied from 1.0 x 102- 2.0 x 106 cfu/g whereas, yeast and mold 40 - 8.5 x 105 cfu/g. Among examined RTE samples, dominant organisms were 31 (51.7%) E. coli, 24 (40%) S. aureus followed by 7 (11.7%) P. aeruginosa, 7 (11.7%) V. cholerae and 5 (8.3%) Salmonella spp. All bacterial isolates showed substantial levels of antibiotic resistance in the antimicrobial susceptibility assays, notably against ciprofloxacin, tetracycline, gentamicin, and streptomycin (28.6 - 100%). The result showcased that the majority of RTE food samples were highly contaminated with one or more different pathogens possessing high resistance to existing antibiotics. Thus, a serious vigilance recommendation from the regulatory food authorities needs to come forward with monitoring the microbial risk associated with roadside food hawkers, and awareness among the individuals for food safety and safeguarding in the region.
Background on biofilms
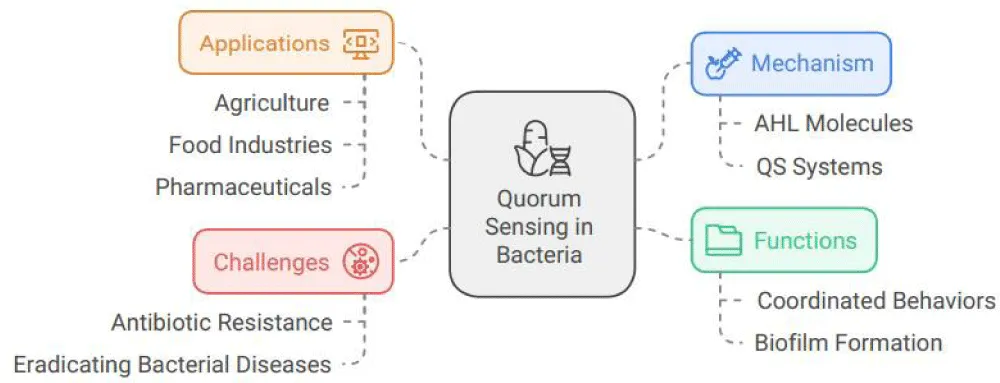
Over the past decade, our knowledge of bacterial biofilm development and quorum sensing has increased dramatically. The social lives of bacteria are fascinating and varied. The buildup of Acyl-homoserine Lactones (AHL) signaling molecules allows bacteria to interact and coordinate their actions, which is a unique phenomenon. When (AHL) builds up to a certain level, a reaction takes place. Their Quorum Sensing (QS) systems, which sense the density of other bacteria nearby, control their coordinated group behaviors. In short, bacterial bodies employ a biochemical message-exchanging system known as quorum sensing, which coordinates behavior changes in response to population density. The regulation of social interactions in some bacterial species is an important factor in the quorum-sensing activities in contexts such as agriculture, food industries, and even pharmaceuticals, where these organisms may, for instance, form biofilms or initiate virulence in nasty pathological symbioses. Understanding how quorum sensing functions in scenarios that resemble actual host environments is a current problem in the area. Currently available research indicates that it must form “biofilms” or vast colonies. It does this by employing a microbial trick called quorum sensing, in which the bacterium gathers and senses a critical number of cells via chemical signals before cooperating to exert virulence and pathogenesis, which can manifest in infectious illnesses in both people and animals. When they are working together, they initiate shared behaviors that are different from those observed in a single cell. They can do activities that get harder and harder, and many illnesses employ QS to initiate certain group behaviors. The bacteria are resistant to the majority of antibiotics, appear to be immune to several drugs and chemicals, and can easily survive in harsh settings thanks to quorum sensing along with biofilm synthesis. Remarkably, prior demonstration has shown quorum-sensing chemicals inhibiting the social tendency of a bacterial disease that is difficult to eradicate. Quorum sensing controls interactions among disease-causing and beneficial bacteria, higher organisms (growth promotion, symbiosis), and signal-producing organisms, as well as between various species found in the surrounding media.
Definitions of biofilms and their significance in various environments
A straightforward and general definition of biofilms is populations of bacteria adhered to a surface. Only twenty years ago, it was rediscovered that bacteria primarily adhere to surfaces in natural aquatic systems, which sparked a concentrated effort to investigate microbial biofilms. However, Henrici’s 1933 study was the first known observation we found about biofilms: “It is quite clear that water bacteria often grow on submerged surfaces rather than floating freely. Furthermore, Henrici’s thesis was written a few years before the microbial contamination of hulls of boats and ships in maritime surroundings was acknowledged as a significant issue [1].
Because bacteria, along with several advantage-taking microbes, may create biofilms within the body parts like tissues of the infected organism or medical bio compounds that are inserted, which can result in persistent infections, biofilms create trouble within both sapiens along animal pharmaceuticals. Microorganisms in pathogenic biofilms are naturally able to elude host immune responses and withstand antimicrobial treatments. Additionally, harmful germs that can spread and colonize new tissues may come from them. Among the notable illnesses linked to biofilms are mastitis in cattle, chronic ear infections in dogs, osteomyelitis, cystic fibrosis pneumonia, and periodontitis in humans. When recipients of indwelling medical devices are immunocompromised or in critical care, they are especially vulnerable to biofilm nosocomial infections. Animal illnesses linked to biofilms pose a major risk to human financial stability and livelihoods [2].
Overview of stages of biofilm
The process of biofilm production is not random. When germs engage with an outer layer, when bacteria reach the outer layer through sedimentation, liquid movement, or vigorous swimming, they first establish weak and transient attachments. The next stage is the characteristics of the bacterial cell exterior and the outer layer of the colonized influence irreversible attachment. Reversible attachment is followed by irreversible attachment if the physicochemical conditions are suitable (e.g., hydrophobicity or hydrophilicity of the surfaces and nature of the deposited chemicals on the substratum) Figure 1. A monolayer of single cells that are firmly attached to a surface is created during irreversible attachment. Microcolonies then grow until the mature biofilm is formed. Finally, if conditions alter, cells may break away from the biofilm and revert to a planktonic form [3].
Biofilm maturation
Genetic methods have recently started to shed light on the formation and growth of biofilms in Escherichia coli, Vibrio cholerae, Pseudomonas aeruginosa, and Pseudomonas fluorescens. In these conditions, it was demonstrated that the development of the cell exterior features, including flagella, pili, and other membrane linkers, was necessary for the creation of biofilms on abiotic surfaces. In a continuous flow environment, a P. aeruginosa mutant that was impotent to produce an acylated homoserine lactone quorum-sensing signal compound developed a uniform, less distinct biofilm. This suggested that for these bacteria to develop a complete biofilm, cell-to-cell contact is necessary.
When a mutation occurs in E. coli that prevents the manufacture of EPS colonic acid, a flat, tightly packed biofilm is created. Indicating that the creation or maintenance of biofilm architecture depends on EPS formation. Neither the molecular underpinnings of these variables’ role in biofilm formation nor the potential for other factors to contribute to the process of development has been thoroughly examined. Recent research has shown that factors expressed by plasmids enhance the capacity of some strains of Escherichia coli to form biofilms. Entero-aggregative E. coli (EAEC) may more easily form biofilms on abiotic surfaces and the intestinal mucosa when Aggregative Adherence Fimbriae (AAF/II) are present. Offered the first proof that the presence of conjugative plasmids causes some E. coli K 12 variants to form biofilms in continuous cultures. 29 distinct native self-transmissible plasmids through various groupings that were unsuitable were examined for their impact on biofilm synthesis. In the presence of depressed conjugative IncF plasmids, we present the evolution of the structure of E. coli K-12 biofilms, which grow in a chemostat environment. Fertility factor F pertains to the IncF antagonism category of bacterial plasmids, which is constrained by a controlled parasite-specific range in the family Enterobacteriaceae. The majority of genes associated with the combined transmission of IncF plasmids are located within a cohesive, unitary operon that is under the control of a single regulatory mechanism.
We outline the morphological transformation that takes place due to the presence of E. coli K-12 biofilms developed in a chemostat with the derepressed combined IncF plasmids. Inside Enterobacteriaceae, the fertility factor F is associated with the category of plasmids known as IncF, which is defined by a narrow range of hosts. Most of the genes that are responsible for the combined transfer of IncF plasmids (Tra genes) are plasmidically encrypted and organized into a single, regulation-free operon.
Description of the maturation process, including the structural and functional changes that occur
In order to assess the structure and development of a noninvasive observation, needed observe biofilms on solid-liquid interactions. A straightforward and efficient way to investigate these biofilm communities in a controlled and repeatable way is through continuous flow-cell cultures. The fact that the biofilm does not have to be moved over a liquid-air interface before being examined under a microscope is one important aspect of the flow-cells. Because it protects the adhering microorganisms in space from shearing stresses and maintains the cell configuration, the system trait is crucial. The strains of interest in this investigation were grown in a system of continuous flow cells with laminar flow and a borosilicate surface, irrigated with glucose minimum media. The arrangement of cells in surface communities is amenable to study using SCLM. To genetically were introduced into the bacteriophage l, attB’s chromosomal attachment site. In isogenic cultures containing the derepressed IncF plasmids R1drd19 or an imitative of plasmid F, pAR108, and a Gfpmut3* tagged variety of E. coli CSH26, SAR18, the biofilm g mark genes expressing fluorescent proteins in the model organism E. coli CSH23, the biofilm generation and architecture were investigated during 42 hours. 7.4of 104 CFU of SAR18, SAR[R1ded19], or SAR18[PAR108] were added to flow channels, and SCLM tracked the development of biofilms at different points in time. Single cells were uniformly dispersed throughout the borosilicate surface in every channel two hours following inoculation. Under laminar flow, the lone cells that managed to stay at the surface. The cells were discovered to be either affixed to the substrate across their lengthy face or via their pole during the initial stage of biofilm development. Hence, it can be concluded that derepressed IncF doesn’t speed up early cells\ surface adhesion events since the number of surface-associated cells in this system was the same across plasmids and those without the number of surface. SAR18 produced tiny, asymmetrical microcolonies with 4 to 14 cells close to one another and the substrate after 11 hours of development. The passage of cells injected with SAR18[r1drd19] and SAR[PAR108] also showed an enhancement in biomass on the exterior, even though huge cell clumps of equal measurements were barely visible at this point. Rather, the surface was more likely to contain a single or a pair of cells.
The variation in the formation of biofilms between strains with and without plasmids became increasingly apparent after twenty hours. Although the size of cell clusters increased somewhat, most SAR18 cells stayed directly attached to the surface. On the other hand, many of the sessile SAR [rdrs19] cells had developed a loose, irregularly shaped meshwork and were no longer in direct touch with the substratum. At this point, SAR [pAR108] showed an intermediate phenotype. Microcolony formations, which were 7–8 mm high and composed of loosely aggregated cells, punctured the substratum. During the remaining biofilm cultivation time, neither a change in architecture nor a notable increase in surface-associated biomass was seen in the biofilm generated by the strain SAR18, which lacks a plasmid. Conversely, the plasmid-carrying strain’s biofilms developed a significantly changed biofilm architecture and kept accumulating biomass. The SAR[r1drd18] cells’ loose meshwork became ten times thicker after 20 hours, from seven mm to seventy mm after thirty-six hours. After forty hours, EPS encapsulation may have contributed to the biofilm’s increased density and flow stability. Likewise, after forty hours, the unique microcolonies of SAR18[PAR108] gradually grew and reached a typical width of almost 100mm. as a result, the final biofilm structure of either plasmid containing strains showed all the traits of a fully grown, differentiation biofilm, including big, tulip shaped cell pillars divided by medicine filled channels that allowed swimming cells to freely migrate. The biofilms formed by the two strains did, however, differ noticeably from one another.
Cells on the surface proliferate to form microcolonies. Escherichia coli bacteria may swim in any direction on the laminar medium flow’s surface boundary layer and are continually released into the medium by growing biofilm (Tolker-Nielsen et al., 2000). Consequently, expect for the first four hours following inoculation, a sizable several floating bacteria at the base of each flow channel during the biofilm formation stage. Thus, plasmid promoted biomass increase in the biofilms produced by SAR18 [R1drd19] and SAR18 [pAR108]. Maybe due either to the growth of stationary cells or the attraction of moving planktonic cells, in the development of the three-dimensional plasmid-carrying strains developed biofilm was formed following the co-inoculation with 4.7 and 107 CFU of a CFB, a Yfp-tagged derivative of E. coli CSH24. Each bearing either the F plasmid derivative POX38km or R1drd19, in flow channels, SCLM observed the development of biofilms. There would be microcolonies that were distinctly cyan or yellow in hue if the primary origin of cell accession in the developing biofilms was surface cell multiplication. If the growth in cell mass was due to the attachment of motile bacteria, then the microcolonies would exhibit a population of cells, a mixture of cyan and yellow, luminous in color.
Mature biofilm formation does not involve cell-to-cell signaling mediated by AI-2
E. coli variants with a derepressed IncF plasmid exhibit a differentiation process during biofilm formation that is strikingly comparable to that which was formerly reported for Pseudomonas aeruginosa PAO1 (Sauer et al.,2002). The maturation process that results in the distinctive mushroom-shaped microcolonies and channel system in P. aeruginosa is believed to be significantly affected by the Las N acyl homoserine lactone (AHL) quorum-sensing system. It appears that E. coli don’t generate AHLs, in contrast to several other Enterobacteriaceae members. A second large class of bacterial cell-to-cell communication systems shares the autoinducer-2 (AI-2) quorum signaling molecule released by E. coli K-12 strains. The luxS gene’s product is necessary for the formation of AI-2. The capacity of R1drd19 and pAR108 to stimulate biofilm formation in E. coli DH5a, which is AI-2 defective due to a frameshift mutation in luxS, was investigated to assess the involvement of AI-2-mediated quorum sensing in biofilm maturation of derepressed IncF plasmid-harboring E. coli. Following the injection of 1 × 107 CFU of DH5a, DH5a [R1drd19], and DH5a[pAR108] into various floe channels, the development of biofilms was tracked using SCLM. The growth of biofilms was monitored by SCLM after 1 × 107 CFU of DH5a, DH5a [R1drd19], and DH5a [pAR108] were injected into different flow channels. In line with previous findings, E. coli DH5α was unable to produce a dense, recognizable biofilm under the conditions under investigation. Over the course of the 96-hour experiment, only the individual cells or baggy, tiny cell clumps stayed adhered to the exterior. The plasmids encouraged the formation of biofilms, and the resulting biofilm structure was similar to that of MG1655 and CSH26 from E. coli. These results show that the biofilm growing regulated by derepressed IncF plasmids does not need the manufacture of AI-2.
Discussion
The study’s most significant discovery is that when subjected to variants of E. coli K-12, one activity ̶ TraA pilus production ̶ may cause a bacterial exterior population to grow into what has been described as a really well-organized, fully grown biofilm. Previous attempts to create a strong, distinct E. coli have been utilized as a novel organism for several biofilm formation studies. In order to clarify the effects of conjugatively derepressed IncF plasmids on the biofilm formation and planning of E. coli K-12 variants, we have employed an accurately described system flow in this investigation. Our findings imply that adding a derepressed IncF plasmid does not enhance initial adherence to the glass surface. In fact, in contrast to the plasmid-inadequate variant, it took a plasmid to increase biomass at the substratum. It appears that derepressed IncF plasmids promote the development and differentiation of biofilms. E. coli variants lacking the plasmid generated large cell clumps divided by space channels, but they were unable to establish comparable structural characteristics. Significantly, the E. coli biofilm developed unique microcolonies at an early maturation those of other bacterial species due to the presence of the F plasmid variant. Under static settings, it was demonstrated that P. aeruginosa’s type Ⅳ pili mediated this early cell aggregation through twitching mobility.
It was demonstrated that the establishment and spread of early microcolonies in static cultures were caused by flagellar mobility of the Vibrio cholerae El Tor. Recent findings from flow chamber studies, however, indicate that the majority of Pseudomonas sp. Microcolonies that developed in flow conditions were the consequence of immobilized cell growth. In these circumstances, flagellar and twitching motility were not essential. The latter observations are supported by our current data. E. coli microcolonies are created via clonal development as opposed to free–swimming cells that adhere to the F plasmid. It appears that the flow regimes are reflected in the contradictory outcomes under flowing and static situations. Consequently, it could be challenging to make insightful comparisons between findings from various experimental configurations.
We discovered that the absence of curli, Ag43, type 1 fimbriae, or flagella did not affect the biofilm development caused by the F plasmid. It is currently unknown how flagella contribute to the development of E. coli biofilms under static conditions. It has been demonstrated that E. coli biofilms in rich and glucose-minimum media by using motility. Flagella, however, were not required for starting adherence and biofilm synthesis in an E. coli strain that produced more curli. Based on our findings, it can be concluded that under the experimental conditions employed, motility does not constitute the determining factor in the rate of cell attachment, which is in line with earlier findings in Pseudomonas sp.
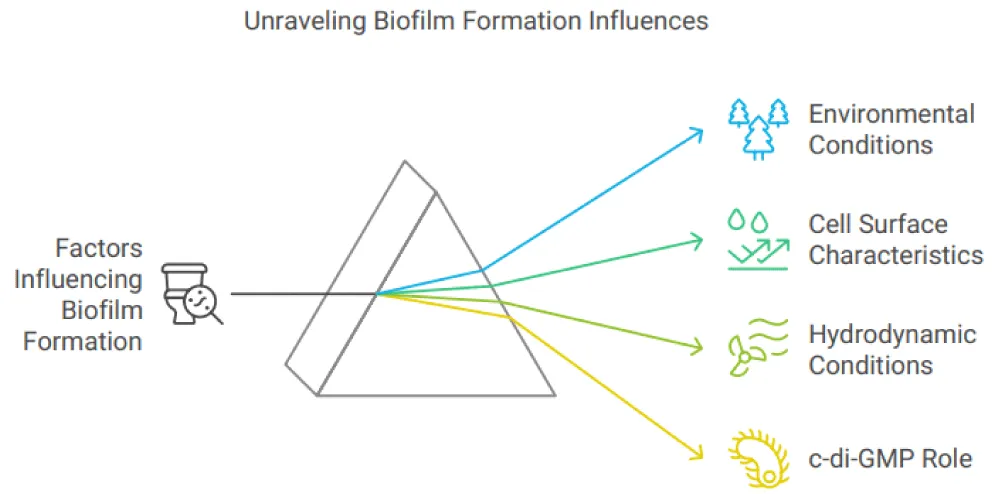
Factors influencing biofilm maturation
Numerous elements, including environmental elements like heat, pH, media accessibility, and hydrokinetic circumstances; cell surface characteristics like water hating, flagellation, and locomotion; and surface characteristics like water hating and coarseness, can affect the formation of biofilms. Certain characteristics of the cell exterior, especially the existence of external components like as flagella and fimbriae, as well as interactions that contribute to the synthesis of EPS and interactions between cells, like exterior-associated proteins or polysaccharides, may give one organism a ruthless edge in a mixed microbial community. Biofilm will stick to rough, hydrophobic surfaces that are coated in surface conditioning films more readily than hydrophilic bacteria, which, in contrast to hydrophobic germs, are less prone to adhering to surfaces. Environmental elements like as temperature, pH, and nutrient levels can also change the substratum’s physicochemical characteristics, including texture (rough or smooth), hydrophobicity, and charge. If flow velocity, water temperature, or nutrient content in aquatic settings don’t rise over threshold levels, the rate of microbial adhesion can be accelerated.
C-di-GMP, the bacterial 2nd messenger, is essential for the development of biofilm. It was the first to demonstrate that the chemical was an agonist of Glucanoacetobacter xylinus’s cellulose synthesis pathway. Since then, it has evolved into an essential chemical that controls the change from the mobile, planktonic lifestyle to the permanent, biofilm-associated one. Numerous bacteria, including but not limited to Vibrio cholerae, Salmonella enterica, and P. aeruginosa, have shown their function controlling the transition from a moving to a stationary state. Numerous bacteria synthesize c-di-GMO, which was recently shown to regulate a wide range of operations, including bacterial adherence and biofilm synthesis, EPS synthesis, bacterial mobility, and malignant control. Numerous several required bacterial processes, including cell cycle proliferation, type Ⅲ secretion, RNA regulation, along stress reaction, have also been demonstrated to be affected by cyclic-di-GMP. Within the cell, c-di-GMP levels, several phases of the biofilm formation process are controlled. For example, flagellar mobility is regulated by FleQ, a transcriptional regulator of flagellar gene expression, which in turn regulates reversible attachment, the first step in P. aeruginosa biofilm formation. However, a conformational shift that reduces the bacterial swimming motility happens when FleQ is bound by c-di-GMP. Additionally, it was demonstrated that during biofilm growth, the synthesis of extracellular polysaccharides was controlled by c-di-GMP, such as Pel and Psl in P. aeruginosa, as well as other biofilm matrix biopolymers. Additionally, biofilm dispersion can be controlled by cyclic Di-GMP.
Hydrodynamic conditions
Different hydrodynamic circumstances that biofilms encounter in various settings might impact the biofilm matrix. These factors affect the formation of biofilms by changing the way nutrients and oxygen are delivered and by applying shear pressures that might affect the cells’ capacity to stick to surfaces. It was also shown that fluid hydrodynamics affected the pace at which the bacterial cells, nourishment, and O2 were moved through the mass fluid to the biofilm. These variables may also affect the density and strength of the biofilm, which could have an impact on how nutrients and messages spread throughout the biofilm. Because the separation of the biofilm under hydrodynamic pressures diminishes viable biomass, which consequently affects EPS secretion, a dense and thinner biofilm may be the result of increased shear forces. Stronger adhesion and lower rates of detachment have been seen in cells cultivated under high shear conditions. Conditions of the Environment the concentration of the 2nd messenger c-di-GMP, the structure along with function of biofilms in aquatic habits like rivers a variety of environmental factors, including the biological component, can exert an influence (community composition, which includes bacteria, algae, and fungi), the chemical(Ph, nutrient availability, and toxicant effects,) the physical (temperature, light penetration, and ware current), the biological (the proportionate impact of producers and consumers, the depth of biomass, and the effects of herbivore), and the GMP, which regulates biofilm related elements like cell appendages, exterior proteins, EPS, and cell mobility. Most bacteria need a pH of about 7, while species-specific variables affect the ideal pH for polysaccharide release. The synthesis of exopolysaccharide is a crucial component of biofilm defense against environmental stresses such as PH. Hence, biofilm-associated bacterial cells exhibit greater resistance to PH changes than their planktonic counterparts. For example, the gel-like structure of a bacterial biofilm can impede the fast movement of ions and permit the establishment of a pH gradient within the extracellular matrix when the environment is highly acidic. Alkaline circumstances have Temperature can also affect how much bacterial temperature is have been seen to disrupt the formation of biofilms, resulting in poorly organized and overly thin biofilms, in addition to preventing adhesion for a few bacteria, including S. aureus and S. epidermis. Temperature can also affect how much bacterial biofilm is produced. The perfect degree for the growth of bacteria is linked to the enhancement in the intake of food. The presence and activity of various enzymes responsible for the regulation of several metabolic and biochemical processes in bacteria in nutrient metabolism is important. Therefore, the optimum temperature promotes bacterial growth and speeds up the production of biofilm; conversely, a temperature that deviates from the ideal might restrict bacterial growth by reducing response rates, which could influence the development of biofilms. Enzymes are not the only factor, as temperatures in the environment can also affect the physical properties of molecules present inside and outside cells. [4] (Figure 2).
Quorum sensing in biofilm
Quorum sensing, a mechanism by which individual diffusible molecules control a coordinated response, is how bacterial cells interact with one another. Quorum sensing has been reported between bacteria and higher organisms (inter-kingdom), among cells of similar variants (intraspecies), and across variants (interspecies). Given that one of the most difficult problems in evolutionary biology is to explain both cooperation and communication, it seems strange that it seems that bacteria frequently communicate using quorum sensing. Compared to intraspecies signaling, which can be explained by ideas like kin selection, communication across species is more challenging to explain from an evolutionary perspective. This probably involves other species using quorum-sensing molecules as manipulating molecules to “coerce” a response from another species or as “cues” to guide future behavior. Under these conditions, it would not be appropriate to characterize the use of quorum molecules as signaling. This paper seeks to connect the evolutionary theories that have been consciously developed about animal signals with the microbiological research on quorum sensing to ascertain if quorum sensing is, in fact, signaling or whether these chemicals are also used as cues or to force other cells [5] (Figure 3).
Definition of quorum sensing and its role in bacterial communication
Quorum-sensing autoinducers are chemical signal molecules that bacteria create and release; with the increase in the population, the external concentration of individuals also increases. Bacteria adapt their gene regulation and, thus, their activities in response to the presence of these autoinducers when the minimal stimulatory concentration of these autoinducers reaches a threshold level. These signal response systems are used by multicellular organisms like bacteria to coordinate specific actions throughout the whole population. To illustrate the similarities and differences between a few well-researched quorum-sensing systems, we show an overview of them below. Since bacteria depend on communication, we presume that their methods are similar. The differences in the systems are probably caused by the fact that each system was created especially to support life in the unique environment in which it resides.
The release of an outside-of-cells quorum-sensing signal, whose structure is unknown, requires the within-the-membrane protein AarA of Providencia stuartii (Rho: rhomboid protein). The extra membrane cleavage liberates and activates ligands that bind to the receptor for the growth factor on the skin, are depends on the serine protease Drosophila melanogaster Rho, which is comparable to AarA. RHO is required for the correct placement of the fly eye and the formation of wing veins. This suggests that some signaling pathways in eukaryotic species and bacteria have an evolutionary ancestry. Moreover, rho expression made up for the quorum sensing signaling deficit in a P. stuarti aarA mutant Rho/AarA homologous are almost similar in bacteria, archea, and eukaryotes, the three kingdoms of life the fact that five of the eight bacteria Aar/RHO orthologues that were studied preferentially split RHO substrates indicates a general The conservation of the substrates of RHO enzymes suggests that the mechanism of RHO bacterial homologues is generally conserved as well. These fascinating observations, however, demonstrate that bacterial and higher eukaryotic organisms have a similar mode of communication between cells, even if it is unknown if RHO or its homologous can promote any across-kingdom communication. Recent bioinformatics research suggests that the RHO\ AarA discovery is not an outlier, since prokaryotes and eukaryotes may share numerous signaling pathways. Plants and archaea lack homologs for the enzymes that vertebrates use to create cell-to-cell signaling molecules, but bacteria do. Phenyl ethanolamine N methyltransferase, glutamine decarboxylase, and histidine decarboxylase are the enzymes that catalyze the conversion of glutamate by Y γ-aminobutyric acid. Including industrial and clinical microbiology.
Ultimately, how we understand quorum sensing may have an impact on how we understand the evolution of higher creatures. Until recently, it was believed that quorum sensing only allowed for intraspecies communication, enabling bacterial clonal communities to simultaneously alter gene expression and count their cells. New research suggests that some autoinducers are either genus-specific or facilitate intergena contact, despite the fact that some seem to be highly species-specific. Additionally, there are increasing signs that cross-kingdom communication does occur. These findings support the idea that nature also contains eukaryotic, along with prokaryotic mechanisms that facilitate and obstruct bacterial enzymatic message transmission. Devices for detecting and relaying bacterial quorum-sensing signals are intricate and usually consist of many circuits organized in various configurations. Because certain organisms frequently inhabit complex chemical environments, some of which are speculated not to possess any information content, we theorize that different organizations of the quorum-sensing network evolved to meet the particular set of communication challenges that a particular species of bacteria faces. It seems that eukaryotes retain and employ some of these sophisticated methods for understanding intricate chemical vocabularies for comparable objectives [6].
Mechanism of quorum sensing
By generating, identifying, and reacting to tiny diffusible signal molecules known as autoinducers, bacteria in a community may transfer their messages to each other. Quorum sensing is an intercellular communication technique that was initially identified in the marine bioluminescent bacteria, i.e., Vibrio fischeri. V. fischeri has a symbiotic interaction with several marine species. The light that V. fischeri produces is used by the host in these partnerships for certain objectives, such as luring prey, evading predators, or locating a mate. The habitat in which V. fischeri lives is nutrient-rich in return for the light it supplies. Light from bioluminescence. Notably, marine bacteria have been observed to exhibit quorum-sensing-conveyed communal behavior or bioluminescence on a global scale.
With the help of a satellite sensor system, Miller et al. observed the significant bioluminescence emanating from a ‘milky sea’ found in the northwestern part of the Indian Ocean. The “milky sea” is a great example of a bioluminescence bloom mediated by quorum sensing, which is created by large populations of the marine bacteria V. harveyi coexisting with the microalga colonies on the sea’s surface. Recent studies have demonstrated that a sophisticated regulatory architecture ensures that the global scale of bioluminescence-mediated bacterial communal behavior is maintained. Comprising many quorum-sensing mechanisms. Over the ages, sailors have described enigmatic nighttime spectacles in which the sea surface emits a bright, consistent, and long-lasting radiance known as the “milky sea”. Numerous bacterial social behaviors and physiological processes, such as symbiosis, spore or fruiting body growth, bacteriocin synthesis, genetic competence, apoptosis, pathogenicity, and biofilm formation, are now understood to be regulated by a quorum-sensing system. Numerous procedures that are customized to meet the particular needs of various communities are governed by quorum sensing. Many bacteria have a crucial mechanism called quorum sensing that regulates social relationships and allows them to benefit from activities that would be hard for individual cells to carry out. There is mounting evidence that social interactions mediated by quorum sensing foster microbial connections and are considered to be important mechanisms controlling the pathogenicity of bacteria at the population level. These investigations have yielded significant understandings of the social biology of bacteria in bacterial illnesses and biofilms( Li & Tian,2012) [7].
Interplay between biofilm maturation and quorum sensing
Nearly every quorum-sensing mechanism that has been identified thus far has been investigated in the setting of planktonic cultures. Given that it streamlines the signaling process, this makes sense. It is commonly assumed that all bacteria in liquid cultures produce signal molecules at similar levels and have similar physiological properties. However, numerous external, internal, and nutritional factors can influence the synthesis, firmness, scattering, and effectiveness of signals to their receptors in a biofilm, making quorum sensing and signal transduction in biofilms even more complicated. Most of the time, it remains a mystery as to how the quorum-sensing signal molecules behave within a biofilm, and even more frequently does a quorum-sensing signal occurs there. Bacterial biofilms consist mainly of bacterial cells and an outside cell matrix composed of a mixture of released proteins, carbohydrates, DNA, RNA, and dead cells. AHL molecules should experience minimal obstacles to reach their destined receptors by the process of free diffusion within the biofilm area, since it is already established that they diffuse freely across the cell membrane. However, as tiny peptides most likely interact with charged molecules, external, internal, and physicochemical variables interior of biofilm are likely to have an impact on the signaling peptides synthesized by Gram-positive bacteria. There is still uncertainty regarding the possible impacts of signal peptides on signal peptides of diffusion-limited or non-specific binding to proteins, carbohydrates, nucleic acids, along cell wall constituents inside the biofilm. Furthermore, it costs a lot of money for Gram-positive bacteria to synthesize an active signal peptide. Keller and Surette state that P. aeruginosa requires just 8 ATP to make an AHL, but S. aureus requires 184 ATP to produce a signal peptide. Gram-positive bacteria have a far higher cost of manufacturing a signal peptide. Therefore, it is logical to believe that dietary or energy sources significantly affect Gram-positive biofilm activities and signal peptide-mediated quorum sensing. Theoretically, concentration, diffusion limitation, and receptor accessibility could all have an impact on the signal molecules utilized to estimate population density, and the purposeful or inadvertent creation of the same autoinducer by third parties, like AI-2. Some researchers have estimated the potential effect and probable processes by using mathematical models. The act of sensing a signal molecule that diffuses and interacts with the analogous sensing system after the activation of QS can be viewed as a process termed diffusion sensing (DS). This implies that QS is a single cell’s independent method of detecting mass-transfer limits. But there could be an evolutionary contradiction between the QS and DS notions. According to quorum sensing, bacteria sense their density to participate in social behavior; as a result, quorum sensing implies that sensing originated as a result of the advantages that the group enjoyed. However, as DS implies that sensing developed as a direct means of improving cell fitness, the emergence of autoinducer sensing does not indicate collective advantages. By bringing these disparate ideas together, Hense, et al. developed a novel idea for efficiency sensing (ES), which could help signaling cells expand in microcolonies or biofilms while avoiding some of the issues related to signaling in complex environments and upholding signal integrity. These authors propose, using a mathematical model, that density and geographical distribution should be quantified separately since the former may be more significant than the latter. A functional hypothesis known as efficiency sensing recognizes the potential for autoinducers to assess a mix of diffusion restriction, cell density, and autoinducer spatial distribution. As it contends that quorum sensing has benefited both individuals and communities, another unified developmental theory is ES. This fresh idea has, however, without any in situ difficulty and avoidance of autoinducer-sensing bacteria, articulated a characteristic mechanism of biofilm development and clonal cluster growth. Nonetheless, empirical validation is still required [7].
The significance of quorum sensing in the development of resistance within mature biofilms
Over the past 20 years, research on planktonic bacterial communities in chronic marine environments has demonstrated that exposure to environmental stresses may cause the bacterioplankton population to diversify into different morphotypes. Protozoan grazing is one of the most prevalent environmental stressors that bacteria face. It is thought that the protozoan predator’s selection for morphological differentiation, such as varying cell sizes and cell surface characteristics, has resulted in bacterial populations with morphologies that are resistant to grazing. Additionally, recent research has shown that protozoans affect the development of bacterial colonies known as biofilms and their three-dimensional structure. Increased stress tolerance is one of the primary characteristics of the biofilms that bacteria frequently form in their natural environments. It has been suggested that bacteria defend themselves against protozoan predation by developing biofilms and differentiating their cells.
The biofilm of wild-type S. marcescens is characterized by organized cell-chain filamentous structures with cross-linking of the chains within the biofilm to form an elaborate porous matrix, as opposed to the typical mushroom-tower structures that have been observed for several other Gram-negative bacteria under flow conditions. As we previously demonstrated, QS control contributes to the regulation of S. marcescens biofilm formation. The lengthy strands and other morphotypes unique to the biofilm of S. marcescens reflect the morphologies linked to the grazing resistance of planktonic bacterial communities. Therefore, we examined in this work whether resistance to grazing in S. marcescens is controlled by QS to produce filamentous biofilm by morphological growth. The management of toxicity, which is managed by QS, affects the resistance of Chromobacterium violaceum and Pseudomonas aeruginosa to grazing. Here, we demonstrate that the QS function of S. marcescens, the eating habit of the protozoan grazers, determines grazing resistance, and that the organism’s capacity to tolerate protozoan grazing is independent of its capacity to generate inhibitory or feeding deterrent compounds.
Example of specific bacteria
In comparison to the microcolony type biofilms, which are formed in static conditions around the amoeba A. polyphaga organism, which employs this amoeba for biofilm growth, biofilms developed in flow cells using S marcescens exhibit a great deal of structuring and cellular differentiation. To understand the influence of morphological differentiation during biofilm formation on opposition to grazing, we investigated the biofilm development under flow; this protozoan efficiently consumes the microcolony biofilms of P. aeruginosa and S. marcescens. Since the flabellate is a suspension, a feeder that is removed from the flow chamber by washing and does not easily colonize surfaces, Bodo saltans were not employed in these tests. Two kinds of tests were conducted: 1. initial colonization research, wherein A. polyphaga was exposed to undifferentiated biofilms that were one day old, and 2. Biofilms grazing resistance testing. When biofilms of S. marcescens had formed after three days, a polyphaga was introduced. The biofilms of S. marcescens samples in the initial colonisation studies exhibited a smooth film of cells overlaying the foundation without developed cells after being infected with A. polyphaga. After 97 hours, the natural type and quorum-sensing mutant biofilms were completely grazed, but the A. polyphaga free controls showed either regular wild type or quorum-sensing mutant biofilm. Additionally, similar amounts of A. polyphaga were found in the flow cells of the wild type and quorum mutant when the amoeba was added to the 1-day-old undifferentiated biofilms.
In the biofilm grazing resistance study, undisturbed three-day wild-type biofilms exhibited the presence of compact cell clumps. The elaborate architecture associated with mature biofilms resembles complex networks of cells; that is, the biofilm matrix was also populated by cell chains extending from the cell clumps. However, the quorum mutant controls day-old biofilms showed somewhat different traits, including filamentous cells in addition to cells that were confluently connected to the substrate. The quality of the amoeba colonizing the wild type and quorum-sensing strains of S. marcescens differed drastically from the observations of the initial colonization trials outlined above with respect to the introduction of A. polyphaga to 3-day-old differentiation biofilms. The QS mutant biofilm had around 11 times as many A. polyphaga (68.8 ± 24.5 mm−2) as the natural type (5.4 ± 6.7 mm−2). Following the addition of the amoeba, the QS mutant biofilm displayed a few tiny, specialized structures, such as filamentous cells, along with patches of cells covering the substratum. These results imply that the QS system, which regulates the development of the grazing-resistant filamentous biofilm, is essential for grazing resistance in S. marcescens during late biofilm development in flow systems. The biofilms produced with BHL from the quorum-sensing mutants showed a comparable decrease in A. polyphaga colonisation to that of the wild type, and they were shaped like the wild type [8].
Current strategies targeting biofilm maturation and quorum for treatment
Biofilms are collections of microorganisms where the cells are often encased in an extracellular polymeric substance (EPS) matrix that the organisms manufacture on their own and stick to one another and a surface. By forming biofilms, bacteria are able to greatly enhance their resistance to antibiotics and other antimicrobials. Biofilms are linked to up to 80% of bacterial illnesses in humans. By transforming microbial cells into their more susceptible planktonic phenotype, biofilm dispersal might enhance the antimicrobials’ therapeutic impact [9].
A global issue that necessitates the creation of other anti-infective tactics is the rise of germs that are resistant to multiple and pan-drugs. These include anti-virulence strategies, which are said to slow the emergence of resistance by focusing on pathogenicity without having a bacteriostatic or bactericidal impact. Since quorum-sensing systems in many pathogens regulate the production of virulence factors, quorum-quenching, or interference with QS, is frequently suggested as a tactic with a wide-ranging anti-virulence impact [10].
Numerous microbial genetic and molecular variables combine with complicated physical and biological traits to produce biofilm recalcitrance. The effectiveness of using antibiotics alone to treat biofilm infections is low since it frequently includes interactions between many species. By preventing biofilms from forming and bacteria from spreading inside biofilms that already exist, which leads the microbes associated with biofilms to enter their more susceptible, planktonic state, anti-biofilm agents have recently caught the attention of many researchers hoping to increase the efficacy of traditional antibiotic treatments. Planktonic bacteria can be released into the environment by either of the two methods for dispersing mature biofilms: active dispersion or passive dispersal. Passive dispersal in the physical dispersion is brought on by outside factors like brushing, mechanical assistance, or being torn away by the movement of interstitial fluid from the primary bulk. The uncontrolled propagation of microorganisms associated with biofilms that respond to external alterations is referred to as “active dispersal.”, including phagocyte challenge, harmful residues, bacteriophages, nutritional deficiencies, low oxygen levels, and antibiotic stress. Active dispersion is an essential stage of a biofilm’s life cycle that encourages bacterial survival and the spread of disease.
Conventional treatment methods
In contrast to the dozing heaps of aggregates of clonal cells, microbial biofilms are an active, self-made habitat with high variation, distribution, and organization. For the reason that the complex microenvironment inside the biofilm exhibits some attributes of cancer, these managerial approaches being developed clinically, for biofilm control mainly involve early and forceful irrigation and debridement techniques for physical clearance and the local administration of potent and prolonged anti-microbial. The mechanical removal methods have successfully removed biofilms from the clinical horizon, such as in the surgical removal of foreign material, wherein dead tissue, pus, or even dental plaque is removed from the biofilm-infected site. Biomaterials that are coated or loaded with a drug can be used to prevent the development of biofilm formation. To stop biofilms from forming, clinics have also used a range of antimicrobial metal or inorganic coatings. Laboratory studies indicate that in order to achieve statistically meaningful reductions in preexisting biofilms, prolonged incubation time with a large antibiotic dosage would be necessary. One crucial strategy to address this issue is in situ release. Compared to system treatment, greater localized antibiotic concentrations might last longer [9].
Targeting biofilm’s dormant cells
Although metabolically active cells are necessary for both antibiotic treatment and for biofilm active dispersion mechanisms, research indicates that persisters or dormant cells inside biofilms are crucial for drug tolerance. One method of treating biofilms without requiring microbial activity is the use of antimicrobial peptides (AMPs). Innate defense molecules against infections include AMPs in significant amounts. Of the more than 3000 peptides having antimicrobial qualities that have been found,2773 show antibacterial activity, according to data from the antimicrobial peptide database. A e of AMPs known as “anti-biofilm peptides” have demonstrated anti-biofilm ACTION in recent years. At doses far lower than those of antimicrobial, human peptide, the first identified anti-biofilm peptide, may inhibit and reduce P. Aeruginosa biofilms. One of the main benefits of AMPs is their widespread conversion, which makes them appealing as wide-working bactericidal drugs that might be effective against bacterial along fungal biofilms. An additional benefit of AMP is that it targets latent and fungal biofilms. When combined with antibiotics, artificial peptides that alter certain AMP sequences have been developed, demonstrated forbid efficacy, and can accelerate the disintegration of P. aeruginosa the disintegration of P. aeruginosa biofilms in invertebrate infection models. But microbiological proteases and their attachment to other host molecules or EPS matrix components may further diminish the effectiveness of AMPs. To overcome this, AMPs can be used with a strategy that targets the EMP matrix to improve their permeabilization and entry qualities once they are inside the biofilm. Although targeting resistant cells with AMPs is a potential approach, clinical development and commercialization are hindered by the high cost of AMP manufacturing and the challenge of sustaining action in a chemically and geographically varied milieu in vivo. Antibiotics like rifampin, which are used to treat infections brought on by slow-growing bacteria, offer an alternative to AMPs. Rifampin and Fosfomycin together can improve the effectiveness of treating foreign bodies. In vivo MRSA biofilm infections. According to recent research, not all of these peptides simply penetrate the EPS and kill the microorganisms to trigger biofilm dispersion. Some peptides break down biofilms at sub-MIC levels, as was previously shown for LL-37, indicating that they are preventing the EPS from forming or the bacteria from maintaining a biofilm. Only a few of the anti-biofilm peptides have been summarized because they have been the focus of a lot of studies. In addition to the previously stated, DNA cross-linking medication such as cisplatin [cis-diamminodichloroplatinum], which has demonstrated medical effectiveness towards bacterial infections, and the FDA-approved anti-cancerous Medication Mitomycin C (MMC), should be highlighted drugs that cross-link DNA merit more investigation as a unique therapeutic approach for infections that are challenging to treat since they mainly create intrastrand DNA crosslinks and eliminate persister cells using a method that does not require growth. Through passive transport and bioreductive activation, which results in DNA cross-linking on its own, the first broad-spectrum substance that can destroy persister cells in MMC. It also functions as a strong bactericide against a variety of bacterial insistent, such as mutualistic E. coli K-12 and viral E. coli, S. aureus, and P. aeruginosa species. Cisplatin was demonstrated to be more effective than MMC at eliminating P. aeruginosa insistent cells, a bit later than MMC. Besides, it is also rather efficient towards clinical strains of S. aureus and P. aeruginosa. Which cancer drugs also need to be noted is that they might have the inherent toxic effect, as antibiotic drugs do. For this reason, additional animal testing is necessary to determine the best treatment plans and dosages, as well as to determine whether using these medications in conjunction with traditional antibiotics enhances bacterial clearance.
Targeting quorum sensing
Bacteria modulate crucial development by being able to detect and react to changes in the environment. One of them is the mechanism of Quorum Sensing (QS), and another one is the cyclo-(di-GMP) signaling. Both systems are highly evolutionarily preserved and allow the bacteria to monitor the number of individuals and their surroundings at a particular moment. As mentioned earlier, c-di-GMP is an important dispersion signal that may regulate a range of adhesins, polysaccharides, and exo-enzymes that produce EPS. In populations with high cell densities, QS allows bacteria to limit the expression of specific genes, leading to more advantageous morphologies. QS is used by the opportunistic disease P. aeruginosa to coordinate the movement of swarms, exopolysaccharide production, pathogenicity, cell aggregation, and biofilm formation. Until an optimal amount is achieved, these microbes can proliferate inside an organism without inflicting any damage. After that, they turn hostile and enlarge until they are large enough to get past the symbiont defenses and create a biofilm that causes the host to get ill. For QS to happen, a signaling molecule has to attach to the appropriate transcriptional regulator. Cell-to-cell communication is therefore required as a result of certain targets’ downstream transcription, which causes pathogenic bacteria to produce several virulent determinants. To avoid QS by interfering with signaling, quorum quenching can be accomplished via (ⅰ) terminating the production of signaling molecules, (ⅱ) mimicking signaling molecules and binding to their receptors, (ⅲ) degrading signaling molecules, and (ⅳ) modifying signaling molecules. Using models of microorganisms both in vitro and in vivo, these substances—known as quorum-sensing inhibitors, or QSIs—have undergone extensive testing to determine how well they work on clinically relevant bacterial biofilms. Both bacteria retaining the violet stain used in Gram’s Method and losing the violet stain used in Gram’s Method, bacteria’s QS system is the target of QSIs. For example, the QS autoinducer AI-2 regulates the quantity and geographic distribution of biofilm cells in Helicobacter pylori, acting as a chemorepellent. Exogenous AI-2 caused biofilm dispersion and reduced the percentage of adhering cells in in vitro biofilms. Plants that contain oils and extracts from traditional medicine herbs have compounds that block QS and can inhibit biofilm formation. AHL acylase, AHL lactonases, and oxidoreductases are three of the quorum-quenching enzymes that can degrade QS signals. Meanwhile, it was demonstrated that AHL-lactonase produced by endophytic strain Bacillus cereus VT96 was capable of inhibiting the synthesis of AHL and biofilm drugs. Since QSIs do not endanger the bacteria’s DNA replication or cell division, they can prevent the creation of biofilms and the manufacture of virulence factors. As a result, it is unlikely that resistance would develop to these substances. As a result, these compounds are excellent adjunct medicines that could be used in conjunction with antibacterial medications to boost antibacterial treatment efficacy and lower the likelihood of developing resistance. However, the kind of biofilm and strain frequently determines lower bacterial loads. Numerous studies in this field have demonstrated that not all QS system has a beneficial result on biofilm synthesis and that the pathogenicity of QSIs is a significant regulating factor in biofilm growth. Therefore, few specific subsets of QSIs. The QS system can be viewed as a sort of system that the c-di-GMP pathway uses to interpret ecological data – concentration of nearby cells – according to a number of papers that go into great detail about the connection between QS and the c-di-GMP signal transduction pathway. Within the larger c-di-GMP circuit, QS integration allows bacteria to correlate the concentration of the community information with other features of the external in which bacteria reside [9].
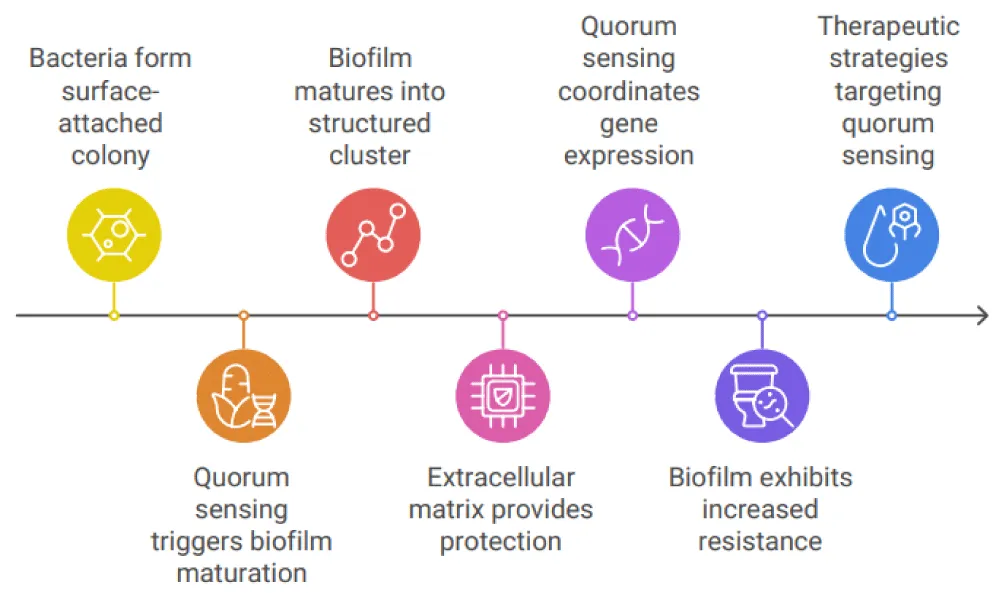
Conclusion
A key component of bacterial survival strategies is highlighted by the complex interplay between biofilm development and quorum sensing, particularly in persistent and challenging-to-treat illnesses. Bacteria go from a very basic, surface-attached colony to a highly structured and ordered cluster of cells enclosed in an extracellular matrix that they form as biofilms mature. This maturation process, which is partially fueled by quorum-sensing systems, improves the biofilm’s resistance to environmental shocks and increases its capacity to endure in harsh environments, including immune system clearance and antibiotic treatment. In order to facilitate the synchronized synthesis of the biofilm matrix, virulence factors, and resistance mechanisms, quorum sensing, a bacterial communication mechanism, enables the coordinated control of gene expression across the biofilm. These signals promote resistance against endogenous and external stressors by allowing bacteria to adjust to the shifting milieu inside the biofilm. In particular, quorum sensing controls the production of matrix constituents like proteins and polysaccharides, which create a barrier against immune cells and drugs. Additionally, it controls the expression of genes that result in antibiotic resistance, including those that generate enzymes that degrade antibiotics and efflux pumps. Another way to understand the variability within biofilms is to look at the relationship between quorum sensing and biofilm development. While less-resistant cells at the periphery may serve as sentinels or play other roles within the community, the more resistant, matrix-embedded cells within a biofilm are better protected from antimicrobial agents thanks to the coordinated activity triggered by quorum sensing. The difficulty of treating biofilm-associated illnesses, when bacteria at different phases of maturity show varied degrees of susceptibility, is highlighted by this variation in resistance levels. Crucially, this knowledge creates fresh opportunities for therapeutic action. It could be able to stop biofilms from forming and maturing or lessen their resistance to antibiotics by focusing on quorum-sensing pathways. Particularly in the case of chronic infections such as those brought on by Pseudomonas aeruginosa, Staphylococcus aureus, and other clinically significant pathogens, strategies that block quorum-sensing signals or disrupt the downstream pathways in charge of matrix production and resistance gene expression may improve the efficacy of current antimicrobial therapies. All things considered, the connection between quorum sensing along biofilm maturation highlights how crucial it is to take biofilm dynamics and bacterial communication into account when creating novel therapeutic approaches. Addressing the rising issue of antibiotic resistance and enhancing the treatment of biofilm-related illnesses, which still present major difficulties in clinical settings, requires a greater comprehension of these processes.
References
- O'Toole G, Kaplan HB, Kolter R. Biofilm Formation as Microbial Development. Annu Rev Microbiol. 2000;54(1):49-79. Available from: https://doi.org/10.1146/annurev.micro.54.1.49.
- Sentenac H, Loyau A, Leflaive J, Schmeller DS. The significance of biofilms to human, animal, plant, and ecosystem health. Funct Ecol. 2022;36(2):294-313. Available from: https://doi.org/10.1111/1365-2435.13947.
- Kierek‐Pearson K, Karatan E. Biofilm Development in Bacteria. 2005. p. 79-111. Available from: https://doi.org/10.1016/S0065-2164(05)57003-5.
- Alotaibi GF. Factors Influencing Bacterial Biofilm Formation and Development. Am J Biomed Sci & Res. 2021;12(6):617-626. Available from: https://doi.org/10.34297/AJBSR.2021.12.001820.
- Diggle SP, Gardner A, West SA, Griffin AS. Evolutionary theory of bacterial quorum sensing: when is a signal not a signal? Philos Trans R Soc Lond B Biol Sci. 2007;362(1483):1241-1249. Available from: https://doi.org/10.1098/rstb.2007.2049.
- Waters CM, Bassler BL. QUORUM SENSING: Cell-to-Cell Communication in Bacteria. Annu Rev Cell Dev Biol. 2005;21(1):319-346. Available from: https://doi.org/10.1146/annurev.cellbio.21.012704.131001.
- Li Y-H, Tian X. Quorum Sensing and Bacterial Social Interactions in Biofilms. Sensors. 2012;12(3):2519-2538. Available from: https://doi.org/10.3390/s120302519.
- Queck S, Weitere M, Moreno AM, Rice SA, Kjelleberg S. The role of quorum-sensing-mediated developmental traits in the resistance of Serratia marcescens biofilms against protozoan grazing. Environ Microbiol. 2006;8(6):1017-1025. Available from: https://doi.org/10.1111/j.1462-2920.2006.00993.x.
- Jiang Y, Geng M, Bai L. Targeting Biofilm Therapy: Current Research Strategies and Development Hurdles. Microorganisms. 2020;8(8):1222. Available from: https://doi.org/10.3390/microorganisms8081222.
- Piewngam P, Chiou J, Chatterjee P, Otto M. Alternative approaches to treat bacterial infections: targeting quorum-sensing. Expert Rev Anti Infect Ther. 2020;18(6):499-510. Available from: https://doi.org/10.1080/14787210.2020.1750951.
- Reisner A, Haagensen JAJ, Schembri MA, Zechner EL, Molin S. Development and maturation of Escherichia coli K‐12 biofilms. Mol Microbiol.. 2003;48(4):933-946. Available from: https://doi.org/10.1046/j.1365-2958.2003.03490.x.
- Subramani R, Jayaprakashvel M. Bacterial Quorum Sensing: Biofilm Formation, Survival Behaviour and Antibiotic Resistance. In: Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry. Springer Singapore; 2019;21-37. Available from: https://doi.org/10.1007/978-981-32-9409-7_3.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
