Open Journal of Bacteriology
Microbial Landscape of Ready-to-Eat Street Vended Food: Evaluation of Quality, Safety and Antimicrobial Resistance Pattern
Dushyant Singh1*, Amita Gaurav Dimri2#, Sumant Kumar Nayak3, Binu Bhat4 and Mukul Das5
1Scientist B. Analytical Science Division-Biology, Microbiology Department,
Shriram Institute for Industrial Research, 19, University Road, Delhi-110007, India
2Scientist C, Analytical Science Division-Biology, Microbiology Department, Shriram Institute for
Industrial Research, 19, University Road, Delhi-110007, India
3Assistant Director & Chief, Analytical Science Division-Biology, Food and Farm Department, Shriram
Institute for Industrial Research, 19, University Road, Delhi-110007, India
4Deputy Director, Analytical Science Division-Biology, Toxicology Department, Shriram Institute for
Industrial Research, 19, University Road, Delhi-110007, India
5Director, Shriram Institute for Industrial Research, 19, University Road, Delhi-110007, India
#Equally Contributed for the Manuscript
Cite this as
Singh D, Dimri AG, Nayak SK, Bhat B, Das M (2025) Microbial Landscape of Ready-to-Eat Street Vended Food: Evaluation of Quality, Safety and Antimicrobial Resistance Pattern. Open J Bac 9(1): 001-013. DOI: 10.17352/ojb.000028Copyright License
© 2025 Singh D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.There are several benefits provided by Ready-to-Eat (RTE) street vended foods, but data exists that pathogenic microorganisms may contaminate foods displayed for sale on the side of the road. However, there is a lack of data on the microbial characterization and antimicrobial resistance (AMR) trends of isolated pathogens from street food in Delhi. Considering Panipuri and noodles are the favorite RTE foods in India, the study aims to examine the occurrence including anti-microbial resistance patterns of common foodborne disease-causing microbes isolated from selected RTE foods. Sixty (60) RTE street-vended food samples from prevalent locations in Delhi, were analyzed by demonstrating mesophilic aerobic bacterial count, yeast and mold count, specified food-borne pathogens, and their AMR trend against clinically significant antibiotics. The mesophilic aerobic bacterial count varied from 1.0 x 102- 2.0 x 106 cfu/g whereas, yeast and mold 40 - 8.5 x 105 cfu/g. Among examined RTE samples, dominant organisms were 31 (51.7%) E. coli, 24 (40%) S. aureus followed by 7 (11.7%) P. aeruginosa, 7 (11.7%) V. cholerae and 5 (8.3%) Salmonella spp. All bacterial isolates showed substantial levels of antibiotic resistance in the antimicrobial susceptibility assays, notably against ciprofloxacin, tetracycline, gentamicin, and streptomycin (28.6 - 100%). The result showcased that the majority of RTE food samples were highly contaminated with one or more different pathogens possessing high resistance to existing antibiotics. Thus, a serious vigilance recommendation from the regulatory food authorities needs to come forward with monitoring the microbial risk associated with roadside food hawkers, and awareness among the individuals for food safety and safeguarding in the region.
Introduction
Street foods are defined as an array of Ready-to-Eat (RTE) meals and drinks made and sold by vendors in crowded places [1]. Meals, snacks, and beverages sold by food vendors on the street are extensively consumed by people around the globe in developing nations, and a considerable proportion of customers suffer from illnesses such as antimicrobial resistance, diarrhea dysentery, enteric fever, and other gastrointestinal disorders [2]. Microorganism contamination of RTE street-vended food has become known as a serious public health problem worldwide [3]. In recent years, foodborne infections have been among the most prevalent worldwide public health challenges, and their consequences for human health and economic growth are becoming more broadly recognized [1].
Studies on RTE street-vended foods have emphasized them as a foremost risk to public health [4,5]. Epidemiological data indicate that food sold on the street causes 600 million of the world’s population to suffer from foodborne diseases annually, with 56 million fatalities each year [6,7]. Since street food vendors are found in many nations and have been linked to as many as 70% of identified outbreaks, street food safety must continue to be a top priority [8,9]. Bacterial contamination and foodborne intoxications account for almost 60% of the burden of foodborne infection [10,11]. Salmonella species, Escherichia coli, Staphylococcus aureus, Shigella species, Bacillus cereus, and Clostridium perfringens are a few of the often found foodborne pathogens in RTE food products sold on the street [12]. Unhygienic practices significantly contribute to the entry of pathogenic bacteria into food including contamination of raw materials from surfaces and food utensils, unhygienic surface conditions of the working environment, and improper storage or refrigeration [13].
Antibiotics have made significant contributions to the treatment of bacterial diseases, and foodborne infections [14,15] and certain organisms have evolved Antimicrobial Resistance (AMR) mechanisms to defeat the medications and sustain in different environmental conditions [16]. AMR is a spontaneous phenomenon that happens when genetic modification causes bacteria to become resistant to antimicrobial agents [17,18]. Consequently, treating bacterial infection becomes challenging or impossible, raising the possibility of severe signs and symptoms, disability, as well as death in both humans and animals [19]. The “One Health approach” must be comprehended and collaboratively implemented by the scientific community and the general public [20].
An emerging worldwide public health concern is the absence of novel medications for efficient treatments against Multidrug-Resistant (MDR) pathogens and their development [21]. Overuse of antibiotics will raise healthcare expenses, mortality, morbidity, and environmental damage; neglecting the AMR issue would accelerate the onset of the “post-antibiotic era” [22,23].
Even though people of all ages are susceptible to the burden of street foodborne illnesses such as cholera, typhoid fever, food poisoning, and diarrhoea, but school-age group and those residing in the below-poverty sub-regions of many countries bear 40% of the burden due to foodborne illnesses alone [24]. Additionally, traveler’s diarrhoea brought by eating food from street vendors is a foremost nuisance for tourists arriving in underdeveloped nations and might be a significant barrier to travel [25].
Due to the inadequate assessment program in developing realms such as India, there is limited methodical information available regarding the occurrence of food-related pathogens in RTE street-vended products such as panipuri and noodles. Moreover, the multiple drug resistance of foodborne pathogens was rarely investigated within both RTE foods. To date, there is no study conducted on antibiotic resistance trends and sensitivity patterns in pathogenic organisms isolated from RTE pani puri and noodles from street vendors in Delhi, India. Therefore, the current cross-sectional study aimed to evaluate the microbiological quality, degree of foodborne pathogens, and dominance of AMR bacteria present in the most common street-vended food of India namely pani puri and noodles.
Materials and methods
Study design, study area and period
Community-based cross-sectional research was conducted at fifteen North Delhi India vending sites, from February - April 2024. North Delhi- known as the administrative district of the National Capital Territory (NCT) of Delhi in India, the Yamuna River, and the district of central Delhi on the East, and by the district of North West Delhi to the westbound North Delhi (Figure 1). A high number of vendors and customers embrace a dense population of students and teenagers. In the city, there are many well-known shops, cafeterias, restaurants, hotels, and beside them are various street vended foods.
Sample collection and transportation
A total of sixty (60) samples were collected from various locations in North Delhi, which contains fifteen (15) noodles and fifteen (15) Panipuri test samples. Further, each Panipuri sample was segregated into three parts (khatta pani, mashed potato, and puri) on the basis of their components. All samples were aseptically collected in sterilized broad-mouthed PET jars as per the sampling guidelines of FSSAI (Food Safety Standard Authority of India, 2021) and followed by transportation in an ice-box with ice packs to the microbiology laboratory of Shriram Institute for Industrial Research, Delhi. To maintain the temperature condition of 2-6 °C during transportation and storage, a calibrated digital temperature monitoring device was used, to prevent microbial growth formation and could get the exact count by maintaining aseptic conditions. Samples after collection were encoded from RTE-1 to RTE-60.where RTE-1 is Khatta Pani, RTE-2 mashed potato, RTE-3 puri and RTE-4 noodles for one location. A similar test food item sequence was repeated for the rest of the fourteen locations.
The food composition of RTE food items used in the study
Microbial analysis
For all microbiological quality tests, ISO 6887-1:2017 [26] and ISO 7218:2024 [27] were strictly followed. All sixty (60) samples were tested for the enumeration of mesophilic aerobic bacteria as well as yeasts, and molds. They were also tested for the presence of intestinal pathogenic Escherichia coli, enteric pathogen Salmonella spp., coagulase-positive Staphylococcus aureus, potentially enteropathogenic Vibrio cholerae, and opportunistic pathogen Pseudomonas aeruginosa in the specified quantity of sample as prescribed in the standard protocol.
The microbiological tests we selected were more pertinent for the microbiological quality evaluation of the ready-to-eat street food samples evaluated here, considering our experience and available resources.
Evaluation of the food hygiene indicators
Enumeration of mesophilic aerobic bacteria: To determine the bacterial load was executed in accordance with ISO 4833-1:2013 [28]. The total mesophilic aerobic bacterial count was performed by serial dilution method of initial suspension up to subsequent decimal dilutions, followed by pour plate technique using melted plate count agar media (HiMedia Laboratories, India), then incubated the petri dishes at 30 °C ± 1 °C up to 72 h. After completion of incubation, the colonies were enumerated by using a Quebec colony counter on the surface of petri dishes with a range between 10 to 300 colonies. Averages were calculated to estimate the number of cfu/g.
Enumeration of yeasts and molds: Quantification of yeast and molds was obtained in accordance with ISO 21527-1:2008 [29]. After spreading 0.1 ml of the initial suspension and successive decimal dilutions on Dichloran Rose Bengal Chloramphenicol Agar (HiMedia Laboratories, India) and incubating in an upright position for 3 to 5 days at 25 °C in a BOD incubator, the number of yeasts and molds was counted under Quebec colony counter and express the results in cfu/g of sample.
Evaluation of food safety indicators
Escherichia coli: A well-homogenised sample of 25 grams was added to 225 mL of nutrient broth medium (1:10 dilution) to create a tenfold dilution of the sample for pre-enrichment, and the flask was incubated at 37 0C for 24 h. Subcultured on selective agar plates as MacConkey agar and Eosin methylene blue agar (HiMedia Laboratories, India) by using streak plate technique then kept in a BOD incubator at 37 0C for 24 h. Selective agar dishes were observed for distinctive colonies once the incubation period was completed, such as pink or red colonies on MacConkey agar and green metallic-sheen colonies on Eosin methylene blue agar dishes. Five characteristic isolated colonies from each plate were streaked on a nutrient agar slant tube then Gram staining was performed for morphological and vital biochemical confirmation. Reference positive control used as Escherichia coli (ATCC 8739) [30].
Salmonella spp: Salmonella species were examined according to the microbiological food standard—Horizontal method for detection of Salmonella spp. ISO 6579–1:2017. Briefly, the non-selective pre-enrichment was done by adding 25 g of proper homogenized sample into 225 mL buffered peptone water medium (HiMedia Laboratories, India). The conical flask was incubated for 24 h at 37 °C. Specified test inoculum of pre-enriched sample inoculated in Müller-Kauffman Tetrathionate broth and Rappaport Vassiliadis Soya (HiMedia Laboratories, India) broth incubated at 37 °C and 42 °C for 18 to 20 h respectively. Selective isolations were performed by streaking onto Brilliant green agar and Xylose Lysine Deoxycholate (HiMedia Laboratories, India) agar. After getting selective isolated characteristic colonies on both selective agar dishes. Sub-culture isolated five suspected colonies onto nutrient agar and identification was done using morphological, biochemical, and serological tests, where positive control was used as Salmonella enterica (ATCC 14028) and Salmonella typhimurium (ATCC 23564) [31].
Staphylococcus aureus: To detect Staphylococcus aureus, prepare selective pre-enrichment as initial suspension, a 25-gram well-homogenized test sample was diluted with 225 ml of cooked meat medium containing 10% salt and kept in BOD incubator for 24 h at 37 0C. After completion of the incubation period, a loopful inoculum was streaked on Baird Parker Agar and incubated for 30 h at 37 0C. Five isolated presumptive colonies from each sample were subcultured on a nutrient agar slant and further biochemical characterization was performed by Gram staining and coagulase test. Staphylococcus epidermidis (ATCC 12228) and Staphylococcus aureus (ATCC 6538) were used as standard reference material [32].
Pseudomonas aeruginosa: Pseudomonas aeruginosa was isolated under ISO 16266-1: 2021. Initially, 25 g of the test sample was added in 225 ml of Asparine Proline Broth (HiMedia Laboratories, India), and after incubation a loopful of inoculum was streaked on Skim Milk Agar (HiMedia Laboratories, India), incubated for 48 h at 42°C. Briefly, five suspected fluorescent colonies were picked, and key biochemical confirmation included Gram staining, oxidase, catalase, growth test at 37 and 42 °C, oxidative fermentation, nitrate reduction, gelatin liquefaction, casein hydrolysis, production of fluorescence under UV illuminator and pigment production. Pseudomonas aeruginosa (ATCC 9027) was used as standard reference material [33].
Vibrio cholera: Vibrio cholerae was detected in accordance with the standard—horizontal method for determination of Vibrio spp. ISO 21872–1:2017. Primary selective enrichment was prepared by adding 25 g of homogenized test portion in 225 ml alkaline saline peptone water (ASPW, HiMedia Laboratories, India), followed by incubation at 41.5 °C for 6 h. Secondary selective enrichment was accomplished by transferring 1 ml of culture obtained above in 10 ml ASPW medium, incubated at 41.5 °C for 18 h then primary isolation was done by streaked on thiosulphate citrate bile and sucrose agar (TCBSA, HiMedia Laboratories, India) and chromogenic agar (HiMedia Laboratories, India)., incubated for 24 h at 37°C Five characteristic typical colonies from each plate were sub cultured and confirmed by means of appropriate biochemical test in accordance with standard reference protocol [34].
Antimicrobial Resistance (AMR)
Using Mueller-Hinton Agar (MHA) and the Modified Kirby Bauer Disc Diffusion technique, isolates were tested for antimicrobial resistance testing. It was carried out in compliance with the recommendations established by the Clinical and Laboratory Standards Institute (CLSI) [35,36]. A homogeneous suspension was obtained by carefully mixing three to five bacterial colonies in a tube containing five millilitres of normal saline (0.85%). The bacterial suspension’s turbidity was measured against 0.5 McFarland turbidity standards. A sterile cotton swab was then used to swab the prepared bacterial suspension across the whole surface of Muller Hinton Agar (HiMedia Laboratories, India). Discs antibiotics were placed on the surface of the medium using sterile forceps within fifteen minutes and incubated at 37 °C for 24 h. The zone of inhibition around each disk was measured in millimeters (mm) with the help of a zone reader and the diameter of inhibition zones was evaluated in accordance with regulatory guidelines as sensitive, resistant, and intermediate. The antibiotic discs used in the study are: ciprofloxacin (5 μg), meropenem (10 μg) tetracycline (30 μg), gentamicin (10 μg), streptomycin (10 μg) and imipenem (10 μg).
Operational definitions
Street food: Food that is ready-to-eat and is offered for sale by a vendor on a street or other public setting, such as a market.
Ready-to-eat foods: Foods that are consumed directly without any further processing.
Street food vendors: Persons who sell meals to the general public on the street without a permanent building but with a temporary structure or transportable facility
Statistical analysis
All statistical analyses were assessed using version 8 of Graph Pad Prism. To compare related proportions, Fisher’s exact test was applied. Significant was defined as a probability value < 10 %. The criteria for significance at a 95% level of assurance are determined by the p-value. Differences at p ≤ 0.05 were regarded as statistically significant using ANOVA [37].
Results
In the present study, a sum of 60 RTE street vended food samples were obtained from various public places of the national capital territory of India. Out of 60 samples, 15 samples of Panipuri were split into three dissimilar segments (the liquid khatta pani, puri, and solid mashed potato), thus compromising 45 samples of Panipuri in the study, rest 15 were noodle samples. Street vendors share the space with a number of other street vendors and set up their food booths, carts, or temporary structures on the side of the road each day. They prepare/cook these foodstuffs completely or partially at home and store them at room temperature, which promotes the growth of heterotrophic organisms including food-borne pathogens.
In context to microbiological results as mesophilic aerobic bacterial count (MABC) it was found that out of a total of 60 RTE street vended food samples 31 (51.66%) were satisfactory, 8 (13.33%) marginal and 21 (35%) unsatisfactory. Among, 45 Panipuri samples, (Table 1) the percent level of MABC unsatisfactory was highest in mashed potato 86.7% whereas, the lowest was exhibited in Puri 6.7% samples. In addition, 15 samples of noodles showed 20% lies in unsatisfactory and 66.7% satisfactory criteria, respectively.
Overall, the quality of RTE street-vended food samples is based on the pre-established microbiological process hygiene and food safety criteria by the ICMSF (International Commission on Microbiological Specifications for Foods). The sample was acquired from levels of marginally unsatisfactory (≥ 105, cfu/g), satisfactory (< 104, cfu/g), and marginal (< 105, cfu/g).
In terms of yeast and mold, only 23.3% of the RTE Street vended food samples have satisfactory levels below less than 10 cfu per g. On the other hand, the majority of samples (76.7%) were above the unsatisfactory level, indicating a significant prevalence of yeast and molds (Table 2). Due to inadequate handling and serving procedures, RTE meals sold on the street are commonly associated with gastrointestinal diseases.
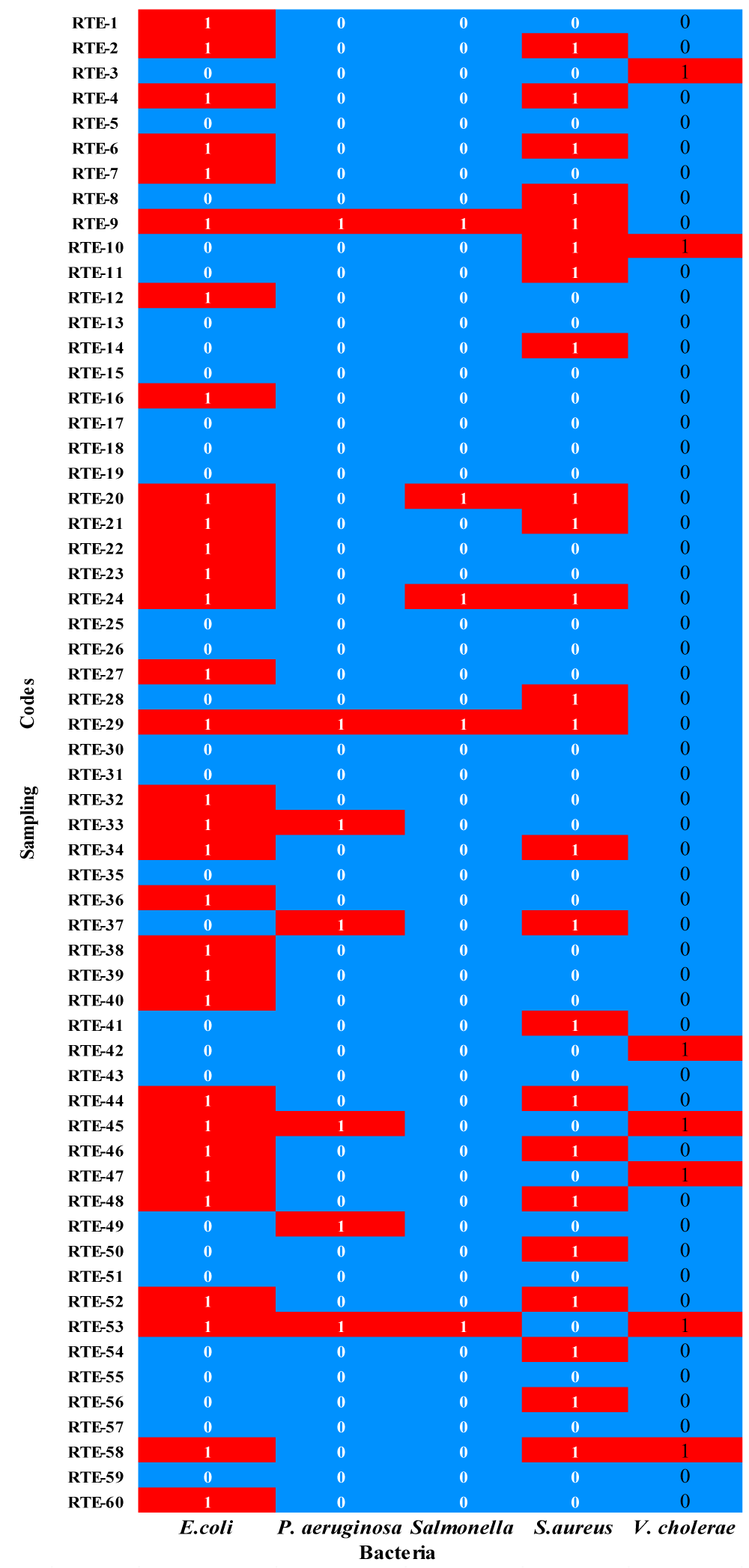
Figure 2 shows the Heatmap of the distribution of specific bacteria in RTE street vended food samples from various locations of Delhi and illustrates the presence or absence of five pathogenic bacteria across 60 sampling codes (RTE-1 to RTE- 60). The level of unsatisfactory in RTE Street vended foods was related to the presence of several microbial isolates which affected the food safety criteria. The descending trend of isolated foodborne pathogens in percent value exhibited 31 (51.7%) E. coli, 24 (40%) S. aureus, 7 (11.7%) P. aeruginosa, 7 (11.7%) Vibrio cholerae and 5 (8.3%) Salmonella spp. The major occurrence of these isolates may be due to unhygienic handling of foods, poor personal hygiene of the vendors, and poorly cleaned dishes, thus indicating waterborne contamination.
The percent existence of food-borne pathogens in Panipuri sample was 16 out of 45 (35.6%), whereas all samples of noodles were contaminated. E. coli and S. aureus were found to be the major contaminants in both categories of analyzed RTE samples.
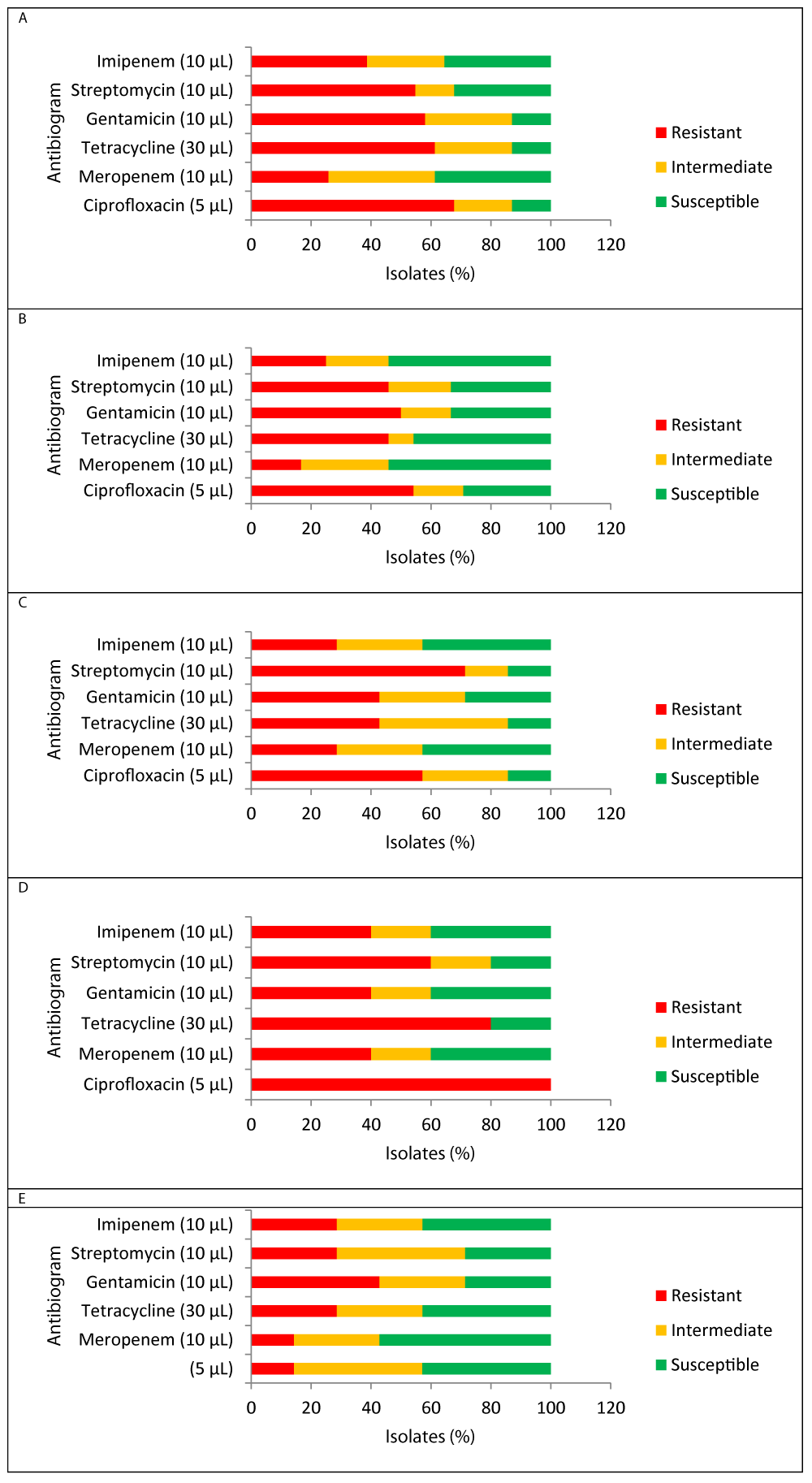
Prevalence of antibiogram for E. coli, S. aureus, P. aeruginosa, Salmonella, and V. cholerae isolates against six antibiotics tested are compiled in Table 3 to Table 4 respectively.
The antimicrobial resistance trend of E. coli against challenged antibiotics is exhibited in Table 3 and Figure 3A. The E. coli isolates from panipuri and noodles samples that demonstrated the highest level of antibiotic resistance were Ciprofloxacin (67.7%) followed by Tetracycline (61.3%) and Gentamicin (58.1%), respectively. Meropenem (38.7%) showed the isolates’ maximum antibiotic susceptibility then Imipenem (35.5%), and streptomycin (32.3%), respectively. All tested antibiotics were ineffective against one isolate of E. coli from noodles (RTE-44). Out of six clinically significant antibiotics ≥ 50 percent of isolated E.coli showed multiple drug resistance.
S. aureus isolates showed the greatest resistance to ciprofloxacin, gentamicin, streptomycin, and tetracycline, respectively. The sensitivity of S. aureus was mostly determined by Meropenem and Imipenem (54.2%). The puri sample had the lowest MDR to streptomycin, whereas the noodle sample had the highest at 66.7%. Overall, 39.6% of S. aureus isolates were found antibiotics resistant, concurrently (Table 5, Figure 3B).
P. aeruginosa showed extreme resistance to streptomycin (71.4%), trailed by ciprofloxacin (57.1%), tetracycline, and gentamicin (42.9 %) (Table 6, Figure 3C). Furthermore, P. aeruginosa isolated from only Khatta Pani samples exhibited resistance to all 6 antibiotics (Table 7,8).
The isolates of Salmonella spp. exhibited the highest level of antibiotic resistance to ciprofloxacin (100 %) > tetracycline (80 %) > streptomycin (60 %). Meropenem, imipenem, and gentamicin (40%) were relatively efficient against five isolates of Salmonella spp. (Table 6, Figure 3D). Noteworthy, isolated Salmonella spp. showed an anti-microbial resistant average trend (60%) to all examined antibiotics (Table 8).
Three out of seven isolates characterized as sucrose-positive V. cholerae were gentamicin resistant. Furthermore, two isolates were tetracycline, streptomycin, and imipenem resistant (Table 4, Figure 3E). Three isolates were found to be multi-drug resistant 42.9% (Table 6).
Discussion
The present cross-sectional analysis of RTE street food from markets in fifteen different locations showed frequent burden of food-borne pathogens and occurrence of antimicrobial resistance against various clinically significant antibiotics. The chosen food products in our study are among the most commonly consumed in Delhi, India but the contamination extent of food safety hazards has been petitely studied, and they are created in environments where antibiotics are potentially overused, resulting in the risk of exposing the consumers to AMR. It is pertinent to state that these two types of food items are being consumed in the majority of cities in India.
Foodborne illnesses are thought to be significantly influenced by the chemical and microbiological contamination of street vended foods [38]. The primary risk factors linked to RTE street-vended meals are identified as inadequate infrastructure, poor environmental cleanliness, and improper food handling [39]. Studies evaluating the hygienic conditions and methods as well as the microbiological quality of RTE street vendors’ food sold in mobile establishments are notably lacking [40].
In the present study, there was a significant correlation between food category and microbiological quality. An almost identical ratio between unsatisfactory and satisfactory samples was obtained. On the flip side, marginal samples were found to decline as compared to the microbiological quality of RTE foods (Table 3). Similar to the finding of this study, Dieni et al have also reported the compromised microbiology quality of food items from Burkina Faso [41]. There was a noticeable marginal or unsatisfactory group in 53.3% of the Panipuri (n = 45) and 33.3% of the noodles (n = 15). The product categories with the highest percentage of marginal or unacceptable products were either Panipuri’s khatta pani or mashed potato, (Fisher’s exact test: p > 0.05). The results from this study are in contrast to the microbiological quality reported in other recent studies of ready-to-eat foods tested at different global locations where a much lower proportion of results were interpreted as of unsatisfactory/potentially injurious microbiological quality [25].
Similar to a prior study conducted in Kalaburagi, India, the mesophilic aerobic bacterial count was determined in 96 (60%) samples out of a total of 160 samples [42]. Numerous variables, such as environmental conditions favour bacterial proliferation and the primary concern of sanitary measures to prevent food contamination. Some studies provide information on the contamination of RTE meals by bacteria or resistant bacterial species to educate public health and food safety interventions, highlighting the significance of RTE foods in the establishment or re-emergence of microbial hazards to health [43].
Yeast and mold serve as indicators of food hygiene quality [25] and their presence in food samples not only compromises quality but also poses substantial health risks. Molds have the potential to release secondary metabolites such as mycotoxins, detrimental substances with adverse effects on the health of humans. However, the present study showed notable concerns emerged as yeast and mold exceeded the reference standard in 76.7 % of samples (Unsatisfactory > 102 cfu/g) indicating a potential risk to food hygiene safety [44-47]. In addition, 73.3% of Panipuri (n = 45) fall in marginal or unsatisfactory level. Although it was 86.7% in the case of the noodle sample (n = 15), this was not significantly different (p > 0.05) compared to similar research findings [41].
In this study, out of 60 RTE Street vended food samples, 31 (51.7%) E. coli, 7 (11.7%) P. aeruginosa, 24 (40%) S. aureus, 5 (8.3%) Salmonella and 7 (11.7%) Vibrio cholerae were observed. These results were consistent with the findings from a study conducted by Yadav et al. in Janakpurdham, Dhanusha. [48]. Food poisoning may result from the contamination of street foods with these microorganisms kept at room temperature. Therefore, to prevent foodborne diseases and protect public health, it is crucial to apply food hygiene and safety measures that include but are not limited to, good practices, proper handling, regular monitoring, effective surveillance, setting and enforcement of regulations, and creating awareness [49].
In the present study, E. coli and S. aureus were identified as pre-dominant isolated organisms followed by P. aeruginosa, Salmonella spp., and V. cholerae, which suggest the potential pathogens accountable for endemic and epidemic disease outbreaks. Further, studies from Bangladesh, India, and Ethiopia support the two predominance bacteria prevalence in street foods [25,50,51].
The conditions in which panipuri is prepared and vended are the cause of the bacterial contamination. Usually, vending locations lack running water, so people wash their hands and dishes in buckets and occasionally without soap. Lack of food preparation techniques and inadequate hand washing by food workers may be the cause of the occurrence of E. coli, Staphylococcus, Klebsiella, and Salmonella spp. Vehicular transmission, sewage, unsanitary surroundings, and poor personal hygiene can all contribute to the incidence of P. aeruginosa as substantial contamination from S. aureus has been suggested due to poor handling of food [52].
Food handlers are a significant risk factor for foodborne illness, and an exploratory study conducted in several Indian states, including Gujarat, Rajasthan, and Karnataka, found that all street food samples tested exhibited unsatisfactory microbiological quality. The study also identified these foods as possible carriers of clinically relevant virulence Staphylococcus aureus and/or E. coli [53-55].
Although antibiotics have greatly aided in the development of essential medicines, treatment of bacterial diseases, and prevention of foodborne infections, many organisms evolved antimicrobial resistance mechanisms that overcome the drugs and persist in various environments. The World Health Organisation (WHO) and national healthcare agencies have identified a list of AMR “priority pathogens” as a result of the worldwide increase in AMR [56]. The complexity and diversity of AMR in different bacteria that may be associated with the food we frequently eat at several RTE street food places were demonstrated by the various investigations.
Addressing this, E. coli has shown a remarkable ability to become resistant to a broad range of antibiotics. Numerous investigations have reported this resistance in various E. coli strains.
Studies have shown that multiple antibiotic classes, such as aminoglycosides, tetracyclines, phenols, β-lactams, and sulfonamides observed resistance against E.coli through food, water, and hand rinse [57].
The current study findings showed, 67.7% (21/31) of the isolated E.coli were found to be ciprofloxacin resistant, however, more than 38.7 % (12/31) responded better to meropenem. The proportion of isolates of multiple drug-resistant (MDR) E. coli was 51.2% (16/31). The results are consistent with other studies that were carried out to evaluate AMR [58-60]. According to Méndez-Moreno et al. [61], E. coli strains obtained from faeces were susceptible to meropenem.
More than 85 % of MDR isolates were resistant to three or more antimicrobial classes [62].
Among the 24 S. aureus isolates assessed in the present study, a comparatively high level of resistance was observed to ciprofloxacin (54.2%), gentamicin (50%), streptomycin and tetracycline (45.8%). Conversely, isolated S. aureus exhibited 54.2% susceptibility to meropenem, imipenem, and 45.8% against tetracycline. The results are in line with other research on RTE foods offered by street vendors [63]. Likewise, 41.7% (10/24) isolated S. aureus were found to be MDR. The increased prevalence of methicillin-resistant S. aureus (MRSA) strains is the most epidemiologically significant phenomenon linked to antibiotic resistance in S. aureus. Multidrug-resistant strains of S. aureus are a major concern in both the hospital and food production environments because of their high survival rate (even for several months) [64].
Due to the chronic prevalence of Salmonella spp. infections, antibiotic resistance in this genus of bacteria was unavoidable. The highest potential of resistance among the five isolates of Salmonella was found against ciprofloxacin (100 %) and tetracycline (80%), which is consistent with the findings of other researchers in India [65]. Among the isolates, 60% (3/5) contained MDR Salmonella spp.
According to reports, Pseudomonas species which is resistant to antibiotics may potentially be found in the food chain. The present work also highlighted the isolation of P. aeruginosa from RTE street vended food which presented an MDR of 42.9%, with resistance to streptomycin (71.4%) and ciprofloxacin (57.1%). The susceptibility rate of P. aeruginosa to meropenem and imipenem was 42.9%. Variations in resistance between Pseudomonas species have also been reported in other studies [66].
A steady rise in seawater temperatures brought on by climate change is the primary cause of an upsurge in Vibrio infections and their categorisation as emerging foodborne pathogens [67]. Recently, the recurrent emergence of antimicrobial-resistant V. cholerae has contributed to treatment failures [68]. Regarding the results of the present study, V. cholerae discovered the highest resistance of 42.9% against gentamicin followed by 28.6% for tetracycline with 57.1% susceptibility to meropenem. The results are consistent with several research findings showing the development of antibiotic resistance, including tetracycline [69]. The present study results show that 28.6% of the isolated V. cholerae unveiled MDR, which was in agreement with the data of a similar study [70].
The complexity of AMR and the requirement for all-encompassing, cooperative strategies at the international level to address this complicated issue should be emphasised. Urgent and concerted actions are needed to reduce the risks associated with AMR across various circumstances, from legislation to raising public knowledge of responsible antibiotic use.
Conclusion
The pathogens and microbial load identified in the present investigation are a matter of human health concern because RTE food consumption has been increasing gradually in parallel with customers’ shifting food choices, convenience, and cost.
It is essential to remember that even if the food items seem edible upon initial inspection, they may frequently contain pathogenic microbes that cannot be seen via the naked eye leading to product spoilage. Additionally, this study found that contaminated RTE street foods showed the presence of antimicrobial resistance microbes collected from street food serving vendors poses potential health risks to consumers.
Antimicrobial resistance is affecting the global population, resulting in health and financial losses. The ‘One Health’ concept is supported by the WHO, under which suitable approaches can be developed and implemented to control AMR. Prioritising food safety should be the main goal of efforts to combat AMR and contamination. This can be done by increasing consumer and business owner awareness, promoting food safety policies, putting in place national surveillance systems, and investing in infrastructure, qualified resource education, and training. Together, in order to achieve objective 3 (excellent health and well-being) of the UN sustainable development goals in the nation, it is also necessary to make sure that premium raw materials are obtained to ensure customer safety. According to the study insights and its potential extensions, street food vendors should undergo ongoing training and standardisation of food safety procedures, with supervision from the local governments that control these food service enterprises.
The manuscript has been identified with Number SRI- MS#20241223-01 by the Shriram Institute for Industrial Research.
The authors extend sincere thanks to the management of the Shriram Institute for Industrial Research for providing financial support and infrastructure facilities for bringing the manuscript to its present form.
- Auwal MR, Faruk MO, Emon M, Ali MS. Isolation and identification of antimicrobial multidrug resistant bacteria from street food. Med Sci Forum. 2023;24. Available from: https://doi.org/10.3390/ECA2023-16394
- Khan FI, Saha ML. Bacteria laden street food (Chatpati) and their multiple antibiotic resistance index. Bangladesh J Bot. 2018;44(4):599–604. Available from: https://doi.org/10.3329/bjb.v44i4.38596
- Ahidjo BA, Loe MWC, Ng YL, Mok CK, Chu JJH. Current perspective of antiviral strategies against COVID-19. ACS Infect Dis. 2020;6(7):1624–1634. Available from: https://doi.org/10.1021/acsinfecdis.0c00236
- Lee H, Yoon Y. Etiological agents implicated in foodborne illness worldwide. Food Sci Anim Resour. 2021;41(1):1–7. Available from: https://doi.org/10.5851/kosfa.2020.e75
- Bizuye A, Tewelde S, Agimas A, Asfaw M, Tadele E, Mesfin E. Bacteriological quality of street vending potato chips in Gondar Town, North West Ethiopia. Int J Bacteriol Virol Immunol. 2014;1(2):014-019. Available from: https://www.researchgate.net/publication/282706216_Bacteriological_Quality_of_Street_Vending_Potato_Chips_In_Gondar_Town_North_West_Ethiopia
- Alemu G, Mama M, Siraj M. Bacterial contamination of vegetables sold in Arba Minch Town, Southern Ethiopia. BMC Res Notes. 2018;11(1):775. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-018-3889-1
- Buted DR, Ylagan A. Street food preparation practices. Asia Pac J Educ Arts Sci. 2014;1(2):53–60. Available from: https://www.semanticscholar.org/paper/Street-Food-Preparation-Practices-Buted-Ylagan/fb7c49c73d1a99a9aa500fcde1523ccb051a913b
- Nemo R, Bacha K, Ketema T. Microbiological quality and safety of some street-vended foods in Jimma Town, southwestern Ethiopia. Afr J Microbiol Res. 2017;11(14):574–585. Available from: http://dx.doi.org/10.5897/AJMR2014.7326
- Birgen BJ, Njue LG, Kaindi DM, Ogutu FO, Owade JO. Determinants of microbial contamination of street-vended chicken products sold in Nairobi County, Kenya. Int J Food Sci. 2020;1. Article Id: 2746492: 1-8. Available from: https://doi.org/10.1155/2020/2746492
- World Health Organization. WHO estimates of the global burden of foodborne diseases: Executive summary. World Health Organization; 2015. Available from: https://iris.who.int/bitstream/handle/10665/199350/9789241565165_eng.pdf
- Buted DR, Ylagan AP. Street food preparation practices. Asia Pac J Educ Arts Sci. 2014;1(2):53–60. Available from: https://asiapjournals.org/street-food-preparation-practices/
- Salamandane A, Silva AC, Brito L, Malfeito-Ferreira M. Microbiological assessment of street foods at the point of sale in Maputo (Mozambique). Food Qual Saf. 2021;5:1–9. Available from: http://dx.doi.org/10.1093/fqsafe/fyaa030
- Schlundt J. New directions in foodborne disease prevention. Int J Food Microbiol. 2002;78(1-2):3–17. Available from: https://doi.org/10.1016/s0168-1605(02)00234-9
- Hutchings MI, Truman AW, Wilkinson B. Antibiotics: Past, present and future. Curr Opin Microbiol. 2019;51:72–80. Available from: https://doi.org/10.1016/j.mib.2019.10.008
- Muteeb G, Rehman MT, Shahwan M, Aatif M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals. 2023;16(11):1615. Available from: https://doi.org/10.3390/ph16111615
- Urban-Chmiel R, Marek A, Stepie´n-Pýsniak D, Wieczorek K, Dec M, Nowaczek A, Osek J. Antibiotic resistance in bacteria—A review. Antibiotics. 2022;11(8):1079. Available from: https://doi.org/10.3390/antibiotics11081079
- Duarte AC, Rodrigues S, Afonso A, Nogueira A, Coutinho P. Antibiotic resistance in drinking water: old and new strategies to remove antibiotics, resistant bacteria, and resistance genes. Pharmaceuticals. 2022;15(4):393. Available from: https://doi.org/10.3390/ph15040393
- Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens. 2021;10(10):1310. Available from: https://doi.org/10.3390/pathogens10101310
- Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial resistance: a growing serious threat for global public health. Healthcare. 2023;11(13):1946. Available from: https://doi.org/10.3390/healthcare11131946
- Chandra P, Mk U, Ke V, Mukhopadhyay C, U DA, V R. Antimicrobial resistance and the post-antibiotic era: better late than never effort. Expert Opin Drug Saf. 2021;20(11):1375-90. Available from: https://doi.org/10.1080/14740338.2021.1928633
- Rybak JM, Barker KS, Muñoz JF, Parker JE, Ahmad S, Mokaddas E, et al. In vivo emergence of high-level resistance during treatment reveals the first identified mechanism of amphotericin B resistance in Candida auris. Clin Microbiol Infect. 2022;28(6):838-43. Available from: https://doi.org/10.1016/j.cmi.2021.11.024
- Kraemer SA, Ramachandran A, Perron GG. Antibiotic pollution in the environment: from microbial ecology to public policy. Microorganisms. 2019;7(6):180. Available from: https://doi.org/10.3390/microorganisms7060180
- Miller EA, Ponder JB, Willette M, Johnson TJ, VanderWaal KL. Merging metagenomics and spatial epidemiology to understand the distribution of antimicrobial resistance genes from Enterobacteriaceae in wild owls. Appl Environ Microbiol. 2020;86(20):e00571-20. Available from: https://doi.org/10.1128/AEM.00571-20
- World Health Organization. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007-2015. World Health Organization; 2015. Available from: https://iris.who.int/bitstream/handle/10665/199350/?sequence=1
- Derbew G, Sahle S, Endris M. Bacteriological assessment of some street-vended foods in Gondar, Ethiopia. Int J Food Saf. 2013;15(6):33-38. Available from: https://www.researchgate.net/publication/258553377_Bacteriological_Assessment_of_Some_Street_Vended_Foods_in_Gondar_Ethiopia
- International Organization for Standardization (ISO). ISO 6887-1: 2017- Microbiology of the food chain — Preparation of test samples, initial suspension and decimal dilutions for microbiological examination — Part 1: General rules for the preparation of the initial suspension and decimal dilutions. Available from: https://www.iso.org/standard/63335.html
- International Organization for Standardization (ISO). ISO 7218: 2024: Microbiology of the food chain — General requirements and guidance for microbiological examinations. 2024. Edition 4. Available from: https://www.iso.org/standard/79508.html
- International Organization for Standardization (ISO). ISO 4833-1:2013/ Amd 1: 2022- Microbiology of the food chain — Horizontal method for the enumeration of microorganisms: Colony count at 30 °C by the pour plate technique. Available from: https://www.iso.org/standard/53728.html
- International Organization for Standardization (ISO). ISO 21527-1: 2008- Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of yeasts and moulds. Part 1: Colony count technique in products with water activity greater than 0. 95. Available from: https://www.iso.org/standard/38275.html#:~:text=ISO%2021527%2D1%3A2008%20specifies,by%20means%20of%20the%20colony
- Indian Standards (IS). IS 5887-1: 2022- Methods for Detection of Bacteria Responsible for Food Poisoning - Isolation, Identification and Enumeration of Escherichia coli.
- International Organization for Standardization (ISO). ISO 6579: 2017/ Amd 1: 2020- Microbiology of the food chain — Horizontal method for the detection, enumeration and serotyping of Salmonella Part 1: Detection of Salmonella spp. Available from: https://www.iso.org/standard/56712.html
- Indian Standards (IS). IS 5887-2: 2022- Methods for detection of bacteria responsible for food poisoning: Part 2 Isolation, identification and enumeration of Staphylococcus aureus and faecal streptococci.
- International Organization for Standardization (ISO). ISO 16266-1: 2021- Water quality — Detection and enumeration of Pseudomonas aeruginosa — Method by membrane filtration.
- International Organization for Standardization (ISO). ISO 21872-1: 2017/ Amd 1: 2023- Microbiology of the food chain — Horizontal method for the determination of Vibrio spp. Detection of potentially enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 34th ed. CLSI supplement M100; 2024.
- Clinical and Laboratory Standards Institute (CLSI). Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious organisms. 3rd ed. CLSI supplement M45; 2018.
- Jørgensen F, Sadler-Reeves L, Shore J, Aird H, Elviss N, Fox A, et al. An assessment of the microbiological quality of lightly cooked food (including sous-vide) at the point of consumption in England. Epidemiol Infect. 2017;145(7):1500-1509. Available from: https://doi.org/10.1017/s0950268817000048
- World Health Organization (WHO). Basic steps to improve safety of street-vended food. International food safety authorities network INFOSAN. International Food Safety Authorities Network (INFOSAN); 2010. Available from: https://www.who.int/groups/fao-who-international-food-safety-authorities-network-infosan/about
- European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union one health 2021 zoonoses report. EFSA J. 2022;20(12):e07666. Available from: https://doi.org/10.2903/j.efsa.2022.7666
- Abrahale K, Sousa S, Albuquerque G, Padrão P, Lunet N. Street food research worldwide: A scoping review. J Hum Nutr Diet. 2019;32(2):152-74. Available from: https://doi.org/10.1111/jhn.12604
- Dieni I, Bagre TS, Tapsoba F, Zongo O, Barro N. Identification of molds and the influence of physicochemical factors in attiéké from Burkina Faso. Afr J Food Sci. 2024;18(8):118-123. Available from: https://doi.org/10.5897/AJFS2024.2319
- Sreeshyam KS, Anandi B, Waghmare AS, Umar N, Goudgoan DB, Kumar A, et al. A cross-sectional study to determine bacteriological contamination and its risk factors among panipuri vendors in Kalaburagi city. Int J Acad Med Pharm. 2024;6(4):347-53. Available from: https://academicmed.org/Uploads/Volume6Issue4/70.%20[3595.%20JAMP_Pavan%20S.%20Kalasker]%20347-353.pdf
- Kadariya J, Smith TC, Thapaliya D. Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. Biomed Res Int. 2014;2014(1):827965. Available from: https://doi.org/10.1155/2014/827965
- Food Standards Australia New Zealand. Compendium of Microbiological Criteria for Food 2022. Available from: https://www.foodstandards.gov.au/sites/default/files/publications/Documents/Compendium_revised%20Dec%202022.pdf
- New South Wales Food Authority. Microbiological quality guide for ready-to-eat foods: A guide to interpreting microbiological results. NSW/FA/CP028/0906. 2009;32:08-17. Available from: https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/scienceandtechnical/microbiological_quality_guide_for_RTE_food.pdf
- Centre for Food Safety, Food and Environmental Hygiene Department. Microbiological guidelines for food for ready-to-eat food in general and specific food items. 2014. Available from: https://www.cfs.gov.hk/english/food_leg/files/food_leg_Microbiological_Guidelines_for_Food_e.pdf
- Radomir K, Rockliff S. Microbiological quality of ready-to-eat food. Act Health Protection Service. 2014.
- Yadav NP, Yadav RK, Prakash S. Bacterial contamination of street-vended food pani puri available in Janakpurdham, Dhanusha. Tribhuvan Univ J Microbiol. 2019;6(1):70-75. Available from: http://dx.doi.org/10.3126/tujm.v6i0.26587
- Makinde OM, Ayeni KI, Sulyok M, Krska R, Adeleke RA, Ezekiel CN. Microbiological safety of ready-to-eat foods in low- and middle-income countries: A comprehensive 10-year (2009 to 2018) review. Compr Rev Food Sci Food Saf. 2020;19(2):703-732. Available from: https://doi.org/10.1111/1541-4337.12533
- Tabashsum Z, Khalil I, Nazimuddin M, Mollah A, Inatsu Y, Bari ML. Prevalence of foodborne pathogens and spoilage microorganisms and their drug-resistant status in different street foods of Dhaka city. Agric Food Anal Bacteriol J. 2013;3(4):281–92. Available from: https://www.researchgate.net/publication/259708756_Prevalence_of_Foodborne_Pathogens_and_Spoilage_Microorganisms_and_Their_Drug_Resistant_Status_in_Different_Street_Foods_of_Dhaka_city
- Sharma I, Mazumdar J. Assessment of bacteriological quality of ready-to-eat food vended in streets of Silchar city, Assam, India. Indian J Med Microbiol. 2014;32(2):169-171. Available from: https://doi.org/10.4103/0255-0857.129809
- Balali GI, Yar DD, Afua Dela VG, Adjei-Kusi P. Microbial contamination, an increasing threat to the consumption of fresh fruits and vegetables in today’s world. Int J Microbiol. 2020;2020(1):3029295. Available from: https://doi.org/10.1155/2020/3029295
- Mehta HD, Saradava DA, Mehta DN. Bacteriological analysis and hygiene of street food panipuri: A case study of Morbi city-Gujarat, India. Ind J Pure Appl Biosci. 2020;8(4):313-317. Available from: https://pdfs.semanticscholar.org/2123/78f9e953e2f4eb49f29c63a5e24527214dc5.pdf
- Saxena G, Agarwal M. Microbial quality assessment of street-vended Golgappa and Bhelpuri sold in Jaipur city of Rajasthan. Int J Food Nutr Sci. 2013;2(1):71-77. Available from: https://www.researchgate.net/publication/353572794_Microbial_Quality_assessment_of_street-vended_gol_gappa_and_bhelpuri_sold_in_Jaipur_city_of_Rajasthan_INTERNATIONAL_JOURNAL_OF_FOOD_AND_NUTRITIONAL_SCIENCES_MICROBIAL_QUALITY_ASSESSMENT_OF_STREET-VEND
- Raghupathi C, Kharate A, Antony N, Awati B, Jamadar D, Adeppa J, et al. Microbial assessment of street-vended panipuri from different zones of Bidar, Karnataka. J Exp Zoo India. 2022;25(2):1807-1813. Available from: https://connectjournals.com/file_html_pdf/3604002H_1807A.pdf
- Fusaro C, Miranda-Madera V, Serrano-Silva N, Bernal JE, Ríos-Montes K, González-Jiménez FE, et al. Antibiotic-resistant bacteria isolated from street foods: A systematic review. Antibiotics. 2024;13(6):481. Available from: https://doi.org/10.3390/antibiotics13060481
- Johura FT, Tasnim J, Barman I, Biswas SR, Jubyda FT, Sultana M, et al. Colistin-resistant Escherichia coli carrying mcr-1 in food, water, hand rinse, and healthy human gut in Bangladesh. Gut Pathog. 2020;12(5):1-8. Available from: https://doi.org/10.1186/s13099-020-0345-2
- Ortega-Paredes D, De Janon S, Villavicencio F, Ruales KJ, De La Torre K, Villacís JE, et al. Broiler farms and carcasses are an important reservoir of multi-drug-resistant Escherichia coli in Ecuador. Front Vet Sci. 2020;7:547843. Available from: https://doi.org/10.3389/fvets.2020.547843
- Mohamed SA, Ardiyati T, Rifa'i M. Detection of class 1 integron-associated gene cassettes and tetracycline resistance genes in Escherichia coli isolated from ready-to-eat vegetables. Ann Med Surg. 2020;55:327-31. Available from: https://doi.org/10.1016/j.amsu.2020.04.044
- Sivakumar M, Abass G, Vivekanandhan R, Anukampa, Singh DK, Bhilegaonkar K, et al. Extended-spectrum beta-lactamase (ESBL) producing and multidrug-resistant Escherichia coli in street foods: A public health concern. J Food Sci Technol. 2021;58:1247-61. Available from: https://doi.org/10.1007/s13197-020-04634-9
- Méndez-Moreno E, Caporal-Hernandez L, Mendez-Pfeiffer PA, Enciso-Martinez Y, De la Rosa López R, Valencia D, et al. Characterization of diarrheagenic Escherichia coli strains isolated from healthy donors, including a triple hybrid strain. Antibiotics. 2022 Jun 21;11(7):833. Available from: https://doi.org/10.3390/antibiotics11070833
- Samy AA, Mansour AS, Khalaf DD, Khairy EA. Development of multidrug-resistant Escherichia coli in some Egyptian veterinary farms. Vet World. 2022;15(2):488-495. Available from: https://doi.org/10.14202/vetworld.2022.488-495
- Eromo T, Tassew H, Daka D, Kibru G. Bacteriological quality of street foods and antimicrobial resistance of isolates in Hawassa, Ethiopia. Ethiop J Health Sci. 2016;26(6):533-542. Available from: https://doi.org/10.4314/ejhs.v26i6.5
- Sergelidis D, Angelidis AS. Methicillin-resistant Staphylococcus aureus: A controversial foodborne pathogen. Lett Appl Microbiol. 2017;64(6):409-18. Available from: https://doi.org/10.1111/lam.12735
- Jacob JJ, Solaimalai D, Rachel T, Pragasam AK, Sugumar S, Jeslin P, et al. A secular trend in invasive non-typhoidal Salmonella in South India, 2000–2020: Identification challenges and antibiogram. Indian J Med Microbiol. 2022;40(4):536-40. Available from: https://doi.org/10.1016/j.ijmmb.2022.07.015
- Heir E, Moen B, Åsli AW, Sunde M, Langsrud S. Antibiotic resistance and phylogeny of Pseudomonas spp. isolated over three decades from chicken meat in the Norwegian food chain. Microorganisms. 2021;9(2):207. Available from: https://doi.org/10.3390/microorganisms9020207
- Dutta D, Kaushik A, Kumar D, Bag S. Foodborne pathogenic Vibrios: Antimicrobial resistance. Front Microbiol. 2021;12:638331. Available from: https://doi.org/10.3389/fmicb.2021.638331
- Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J. Cholera. Lancet. 2017;390(10101):1539-1549. Available from: https://doi.org/10.1016/S0140-6736(17)30559-7
- Qiao J, Zhang Q, Alali WQ, Wang J, Meng L, Xiao Y, et al. Characterization of extended-spectrum β-lactamases (ESBLs)-producing Salmonella in retail raw chicken carcasses. Int J Food Microbiol. 2017;248:72-81. Available from: https://doi.org/10.1016/j.ijfoodmicro.2017.02.016
- Yang Y, Xie J, Li H, Tan S, Chen Y, Yu H. Prevalence, antibiotic susceptibility and diversity of Vibrio parahaemolyticus isolates in seafood from South China. Front Microbiol. 2017;8:2566. Available from: https://doi.org/10.3389/fmicb.2017.02566

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
