Open Journal of Bacteriology
A simple and cost-effective method for reducing microbial load in Salacia chinensis and Picrorhiza kurroa using glutaraldehyde
Shankar Mane, Umesh Chandra Dash, Shital Palghadmal, Nisha Mehta and Sriram Padmanabhan*
Cite this as
Mane S, Dash UC, Palghadmal S, Mehta N, Padmanabhan S (2024) A simple and cost-effective method for reducing microbial load in Salacia chinensis and Picrorhiza kurroa using glutaraldehyde. Open J Bac 8(1): 006-013. DOI: 10.17352/ojb.000027Copyright License
© 2024 Mane S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Microorganisms like fungi, bacteria, and viruses are naturally present in different parts of a plant (root, bark, and leaf) and these become inhabitants of the plant. The establishment of modern Hazard Analysis and Critical Control Point (HACCP) approaches has therefore been made to evaluate the microbial load in medicinal plants. During the manufacturing of herbal extracts as drugs, the quality of the herbal extracts is dictated by the level of presence of microbes. In order to make the herbal extracts HACCP compliant as far as microbial load is concerned; we carried out studies with Glutaraldehyde (GA) as an antimicrobial agent in the manufacturing process in two randomly selected herbs of Salacia chinensis and Picrorhiza kurroa. The outcome of our analysis revealed that 0.5% of GA treatment to the pulverized raw material of Salacia chinensis and Picrorhiza kurroa is effective for reduction of > 99.99% microbial load with minimal effect on the percentage yield of extracts (10% - 20%) and their bioactive molecules Salacinol and Picroside I&II as seen by LC-MS and HPLC analysis, respectively. Furthermore, in this article, we demonstrate the use of sodium bisulfite for the removal of the residual GA in the extract to make the process eco-friendly and environment-safe.
Abbreviations
SC: Salacia chinensis; PK: Picrorhiza kurroa; GA: Glutaraldehyde (PubChem CID-3485); HACCP: Hazard Analysis and Critical Control Point; SB: Sodium Bisulfite (PubChem CID-23665763); HPLC: High-Performance Liquid Chromatography; LC-MS: Liquid Chromatography-Mass Spectrometry; DNPH: 2,4-Dinitrophenylhydrazine (PubChem CID-3772977); SCDA: Soya Casein Digest Agar; ESI: Electron Spray Ionization; CID: Collision-Induced Dissociation Energy
Introduction
In recent times, the use of herbal medicines has gained momentum due to the resistance of microbes to overly-used antibiotics and their side effects [1,2] The herbal plant parts or raw materials that are used for manufacturing herbal medicines often get contaminated with airborne bacterial and fungal pathogens while handling and processing, and thus manufacturing units need to check for such kinds of contaminations [3-6]. Consumption of such herbal medicines is lethal to human health [7]. The microbial contamination in the herbal extracts ranges from 103 to 106 cfu/gm as reported previously [8]. Thus it is essential to combat the microbial load from the raw material prior to further process of manufacturing and should be an integral part of the process while manufacturing of herbal medicines or supplements [9-11].
Salacia chinensis (SC), a widely distributed plant in Asian territories is used as a herbal medicine for various diseases such as menstrual disorders, antitussive, sedative agent, anti-cancer, hepatic diseases, skin diseases, diabetes, hepatitis, and arthritis [12-15]. Salacinol, a polyphenol present in the root bark of SC is one of the active compounds used as a traditional medicine to treat diabetes and is known to exhibit strong inhibitory activities on several α-glucosidases, according to the Indian system of medicine (ISM) [16,17]. Because of its various medicinal applications, SC was used in the present study [18]. Picrorhiza kurroa (PK), is a perennial herb found in the Himalayan province of Nepal; known to have therapeutic uses against jaundice, leukoderma, fever, anti-inflammatory, and urinary-related diseases and used since ancient times as per the ISM [19,20] The key glycoside ‘Kutkin’, which is a combination of Picroside-I and II, has a significant hepatoprotective effect [21]. As it is a commonly used plant extract for various ailments, it was selected for this study.
Glutaraldehyde (GA) is a water-soluble organic compound consisting of a di-aldehyde that functions as a broad-spectrum disinfectant with properties like low corrosion, low toxicity, high efficiency, and high stability [22-24]. GA is widely used for cold sterilization of medical equipment, as an aseptic agent, and for processing aids in the food industry [24-26] GA is shown to possess bactericidal activity against a range of pathogenic bacteria [23]. Based on these applications, GA was selected in the present study for the elimination of microbial contamination from Pulverized Raw Material (PRM) prepared using plant parts [27].
In the current study, Pulverized Raw Materials (PRM) of SC and PK were prepared from their plant parts like roots and rhizomes respectively by using plain water as an extracting solvent. However, these plant parts harbor high levels of microbial contamination as mentioned earlier which may be one of the constituents present in the final end product, posing a threat to human health. Hence, a potential novel thought process was conceptualized to decrease the microbial load in the PRM by treatment with GA. To determine the effectiveness of GA treatment, percentage yield was estimated and the quality of the extract post-treatment was determined using HPLC analysis. The predominant bacteria contaminating SC and PK PRM was isolated and the Minimum Inhibitory Concentration (MIC) of GA was determined against these bacterial isolates to understand the efficiency of GA as an antimicrobial agent. The bacterial load in the PRM was determined pre and post-GA treatment by percentage inhibition assay using the agar plate method and inhibition was also corroborated using the Soleris® system. To validate that the GA treatment does not affect the biological active components in the extract; the bioactive contents in both the extracts were measured before and after GA treatment. To ensure the elimination of residual GA in the final end product, Sodium Bisulfite (SB) was used to remove or make GA inactive as reported earlier [28]. Reduction in GA content by SB was demonstrated through HPLC analysis, thus providing a safe and cost-effective method.
Materials and methods
Soleris® Instrument, Lansing, USA, was used for determining the inhibition of bacteria by GA from SC and PK PRM. A rotary evaporator (Buchi, USA) was used to concentrate and get the extract in dried form. LC-MS/MS (Agilent Technologies 6460 triple quadrupole system, U.S) was used for estimation of bioactive Salacinol from SC extract, HPLC system (Waters 2998 Photodiode Array Detector; e2695 Separations Module; Chromeleon™ Chromatography Data System, USA) was used to determine bioactive from PK and to determine residual GA pre and post-treatment with SB, Microplate Reader (Multiskan Skyhigh, ThermoFisher Scientific, USA) was used to measure Optical Density (OD) of MIC assay.
Chemicals and reagents
2,4-Dinitrophenylhydrazine (DNPH) was procured from Sigma Aldrich (USA), Glutaraldehyde solution (AR Grade, 50%) from Tokyo Chemical Industry Co. Ltd. (Japan), ortho-Phosphoric acid (85%) from Aladdin (Shanghai, China), Soyabean Casein Digest Broth (SCDB) and Soyabean casein digest agar (SCDA) from TM Media (Delhi, India), Picroside I, and Picroside II standard compounds from Natural remedies (Bangalore, India), Sodium Bisulfite A.R Grade from Rankem, India and NF-TVC vials specific for Soleris® Instrument were procured from Neogen, Lansing, USA. All chemicals and solvents used were of analytical and HPLC grade.
Source of plant material, collection, pulverization, and extraction
The commercial plant parts of SC and PK were collected from the production unit of SAVA Healthcare, Malur, and Karnataka, India. The SC roots and PK rhizomes were dried and pulverized to a semi-powdered form. About 50 g of this pulverized powder was added to 250 mL (1:5 ratios) of the extraction solvent (water) and then soaked with continuous stirring for 3 h with the following 3 consecutive extractions. The filtrates were pooled, and concentrated with a rotary evaporator to obtain dry powder (extract) which was stored and used for further analysis. In another set, 0.5% GA was used to treat the raw material of SC and PK by soaking for 1 h at room temperature which was then filtered. Further, the same extraction procedure as mentioned above was followed for both plants with 3 consecutive extractions. The PRM without and with GA treated were stored separately for further use [29-31].
Bacterial cultures and their maintenance
Predominant bacterial contaminant from the PRM of SC and PK was isolated using the streak plate method to obtain a pure culture. Culture isolates from SC and PK PRM were termed SC and PK isolates, respectively. The isolated cultures were characterized based on their morphology and gram character. They were maintained on SCDA plates and slants at 2-8°C until further use in the study. Freshly grown bacterial culture 6-24 h in SCDB was used for all the microbial assays.
MIC of GA
MIC was performed by the micro broth dilution method. A stock solution of GA (16%, v/v in water) was prepared. A pre-inoculum of SC and PK isolate was set up in SCDB medium at 37 °C for 6 h. 100 µL of sterile medium was added to all the wells in a 96-well plate. To the 1st wells in a row A1, B1, and C1, 100 µL of the GA stock solution was added. The GA stock was serially diluted by performing two-fold dilutions from the 1st to the 10th well performed in triplicate. 100 µL inoculum bacterial isolate was added to all wells, except for medium control wells in the 12th column. The plates were incubated at 37 °C overnight on a shaker at 100 rpm [31,32].
Percentage inhibition of microbial load by GA from SC and PK using agar plate method
About 0.5 gm of GA-treated and untreated PRM of SC and PK were weighed and dissolved in 4.5 mL sterile saline to form a homogenous solution using a vortex mixer. For the microbial analysis of PRM, serial 10-fold dilutions of 10-1, 10-2, 10-3, 10-4, and 10-5 were prepared using sterile water respectively. About 0.1 mL suspension from each dilution was spread on the SCDA medium. Medium control and diluent control (0.1mL sterile water used for performing dilutions) were kept as controls. The plates were incubated at 37 °C for 24 h. The percentage inhibition of bacteria in SC and PK PRM by glutaraldehyde was determined based on the log reduction of bacteria [33,34].
Inhibition of bacteria in SC and PK PRM by GA using Soleris® instrument
The elimination of microbial load from the PRM was validated by the use of the Soleris® instrument. Soleris® is a photodiode-based optical detection system with a temperature-controlled incubator, which enables the detection of the growth of microorganisms in selected growth media, with supplements [35]. SC and PK PRM were prepared as mentioned for percentage inhibition of microbial load by GA and 1ml stock of each dilution from 10-1 to 10-5 were aseptically added to the NF-TVC vials and results were obtained in the form of graphs generated from the instrument-specific software.
Estimation of bioactive components from SC and PK extract
LC-MS/MS analysis for estimation of Salacinol from SC extract: The SC extract was analyzed to identify and estimate the bioactive component Salacinol by LC-MS/MS as described earlier [36]. The detection was carried out by using a positive-mode Electron Spray Ionization (ESI) probe mode. The sample flow rate was set at 0.3 mL/min, and the column temperature was maintained at 30 °C. Depending on the kind of parent molecule ion, the mass distribution was set between 50 to 1000 m/z, and the Collision-Induced Dissociation energy (CID) was controlled within the range of 1 to 30. The MS parameters of Salacinol were optimized to confirm the optimal ionization, and ion transfer conditions, which attained the optimal signal of both the precursor and fragment ions. The source parameters were identical for all injections [37].
HPLC analysis of bioactives from PK extract: The bioactive molecule in PK extract was estimated by HPLC analysis. Briefly, 100 mg of dried PK extract was dissolved in 100 mL of methanol. Then, chromatographic fractionation was performed by using a BDS Hypersil C-18 column (150 x 4.6 mm x 5 m or equivalent). The Chromeleon software (version 7.2) was used to control the instrument and analyze the spectral data. About 20 μL of prepared PK PRM and the standards (Picroside I and II) were injected. The flow rate was set to 1 mL/min and the detection of the eluted peaks was done at 255 nm [38].
Estimation of GA concentration in the extracts post 0.5% GA treatment
Preparation of glutaraldehyde standard and DNPH solution: The standard 50% glutaraldehyde solution (GA; 250 μL) was diluted using 100 mL of acetonitrile. Then to 50 μL of this GA solution, the DNPH solution (1, 2, 4 mL) was added. Acetonitrile (q.s. 5 mL) was then added to it, and the samples were shaken vigorously to mix well. For the preparation of the DNPH solution, 50 mg of DNPH; and 0.25 mL of 85% phosphoric acid were added and the volume was adjusted to 50 mL using acetonitrile. The sample was mixed properly until it dissolved [28]. For the HPLC analysis, 20 μL of each solution was injected.
SC and PK extract preparation and HPLC analysis to estimate residual GA: Herbal extracts of SC and PK were processed for HPLC analysis to estimate the residual glutaraldehyde concentration. Briefly, 100 mg of dried extract (SC and PK) was dissolved in 100 mL of methanol and mixed using a vortex mixer with sonication for 15 mins. After sonication, each of the samples was filtered using a 0.45-micron membrane filter (Himedia, India). The chromatographical fraction was done using the BDS Hypersil C-18 column (150 x 4.6 mm x 5µm or equivalent). The instrument data analysis was performed by Chromeleon software (version 7.2). About 20 μL of the sample extract and the standards were injected. The flow rate was set to 1 mL/min and detection was performed at 255 nm. Injections were performed in triplicate [28].
Estimation of residual GA from extracts after sodium bisulfite treatment using HPLC
Herbal extracts (GA treated; 10 mg) of SC and PK were dissolved in acetonitrile and then 2.2-fold (as per test sample in weight) sodium bisulfite was added to the reaction mixture. Further, the solution was mixed vigorously for 15 minutes followed by sonication. Then the same DNPH derivatization method was followed for HPLC analysis as mentioned above [28].
Results
Effect of GA treatment on the percentage yield of the extracts
The results indicate that there was minimal reduction in the yield of SC and PK extracts of 10% - 20% due to GA
Treatment as presented (Table 1) It can be seen that glutaraldehyde does not affect the extract yield to a great extent as only a 10% - 20% loss in yield is obtained. The process can be optimized further to reduce the losses during glutaraldehyde treatment and increase recovery.
Type of bacteria isolated from the extracts
The SC and PK PRM before treatment with GA were tested to determine the microbial load present. The predominant bacteria present in the sample were isolated and termed SC isolate and PK isolate respectively. The SC isolate showed a circular, cream-colored opaque colony with an irregular margin on the SCDA medium and was aerobic gram-positive bacilli. The PK isolate showed a circular, white-colored opaque colony with an irregular margin on the SCDA medium and was aerobic gram-positive bacilli. Both the cultures were found to be non-pathogenic.
Percentage inhibition of microbial load from the extract by GA treatment
In the current study, in order to reduce the microbial load, we have used the GA as an antimicrobial agent during the extraction of SC and PK. We found that 0.5% glutaraldehyde effectively eliminated the microbial load present in SC and PK reflecting > 99.99% bacterial reduction in the treated extracts. (Table 2) [33]. The results indicate that GA is an efficient antimicrobial agent capable of inhibiting > 99.99% of bacteria from the extract, thus treatment with an antimicrobial agent like GA can be incorporated in the process for making plant extracts contamination-free and safe for use.
MIC of GA against SC and PK isolates
To determine whether GA is capable of inhibiting bacterial isolates, an MIC was performed as a preliminary assay to determine the efficiency of GA as an antimicrobial agent and to determine the minimum effective concentration of GA at which it shows inhibition for use in the treatment of the extracts. Based on the MIC concentrations as shown here (Table 3), the concentration of GA for the treatment of extracts was determined to be 0.5%.
Inhibition of bacteria in SC and PK extract by GA using Soleris® instrument
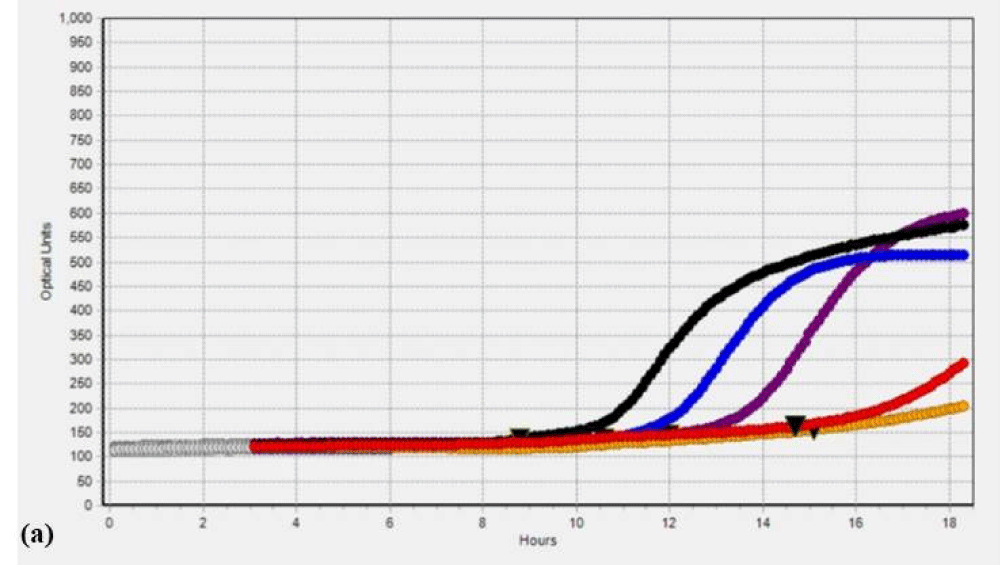
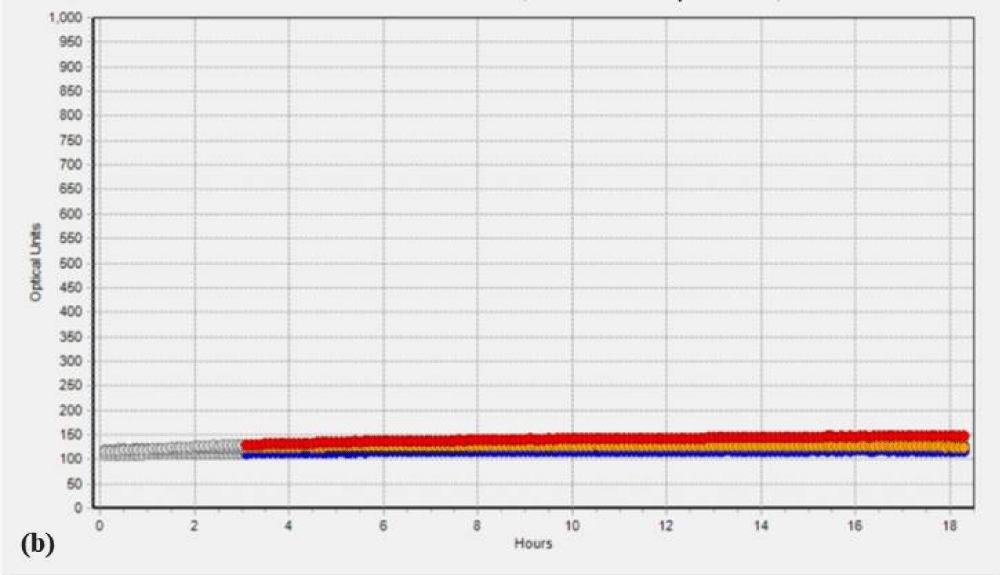
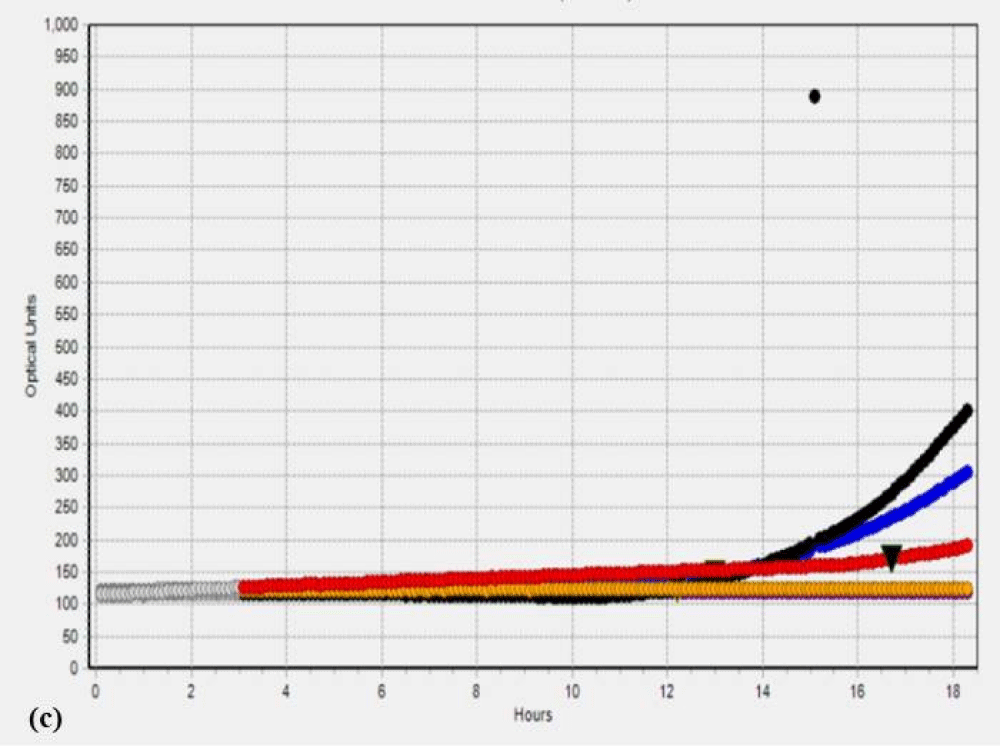
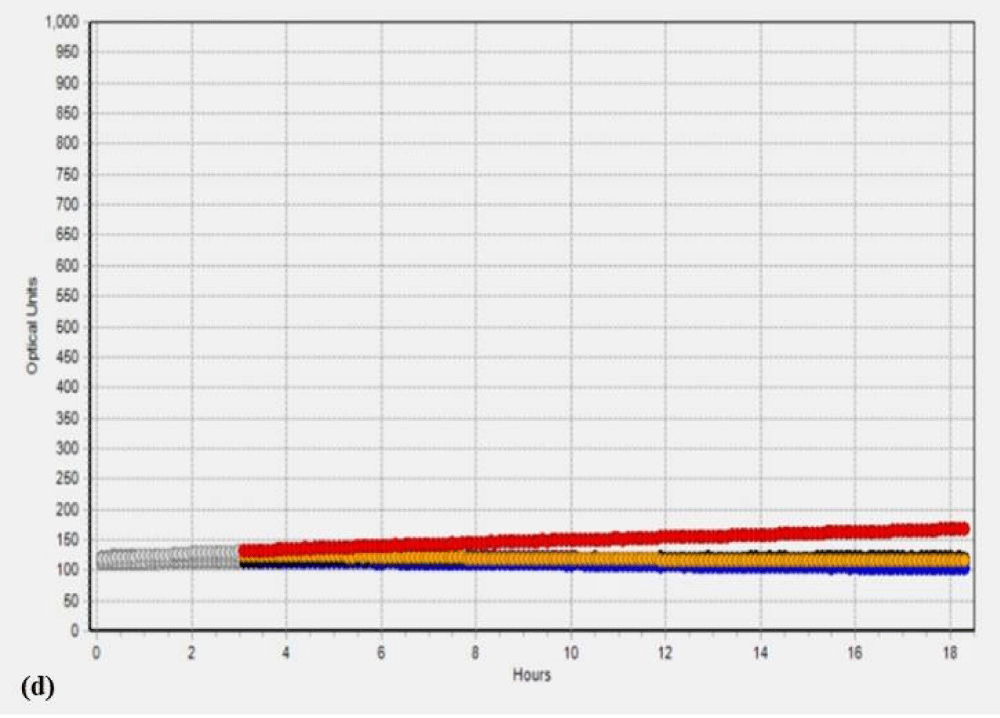
Additionally, to validate the microbial data, we analyzed the SC and PK extracts on the Soleris® system. Soleris® system is a sensitive instrument that can detect the growth of bacteria. The outcome of the Soleris® graph clearly indicates that glutaraldehyde treatment is effective in the removal of microbial load from the extract as depicted in Figure 1 Each of the colored lines in Figure 1a,1b, black, blue, purple, red and orange represents extract dilution as 10-1, 10-2, 10-3, 10-4 and 10-5 of the SC extract. The curved lines trending upwards in the untreated extract Figure 1a indicate bacterial growth measured in terms of optical density based on its detection time, the highest microbial load is observed in the lower dilution of 10-1 and the least microbial load in the dilution of 10-5 and flattened lines in the Figure 1b indicates the absence or no detectable live cells for GA treated extracts. The instrument is sensitive to the detection of live cells in the vials. Each of the colored lines in Figures 1c,1d, black, blue, red, orange, and purple represents extract dilution as 10-1, 10-2, 10-3, 10-4, and 10-5 of the PK extract and interpretation is same as mentioned above for SC extract.
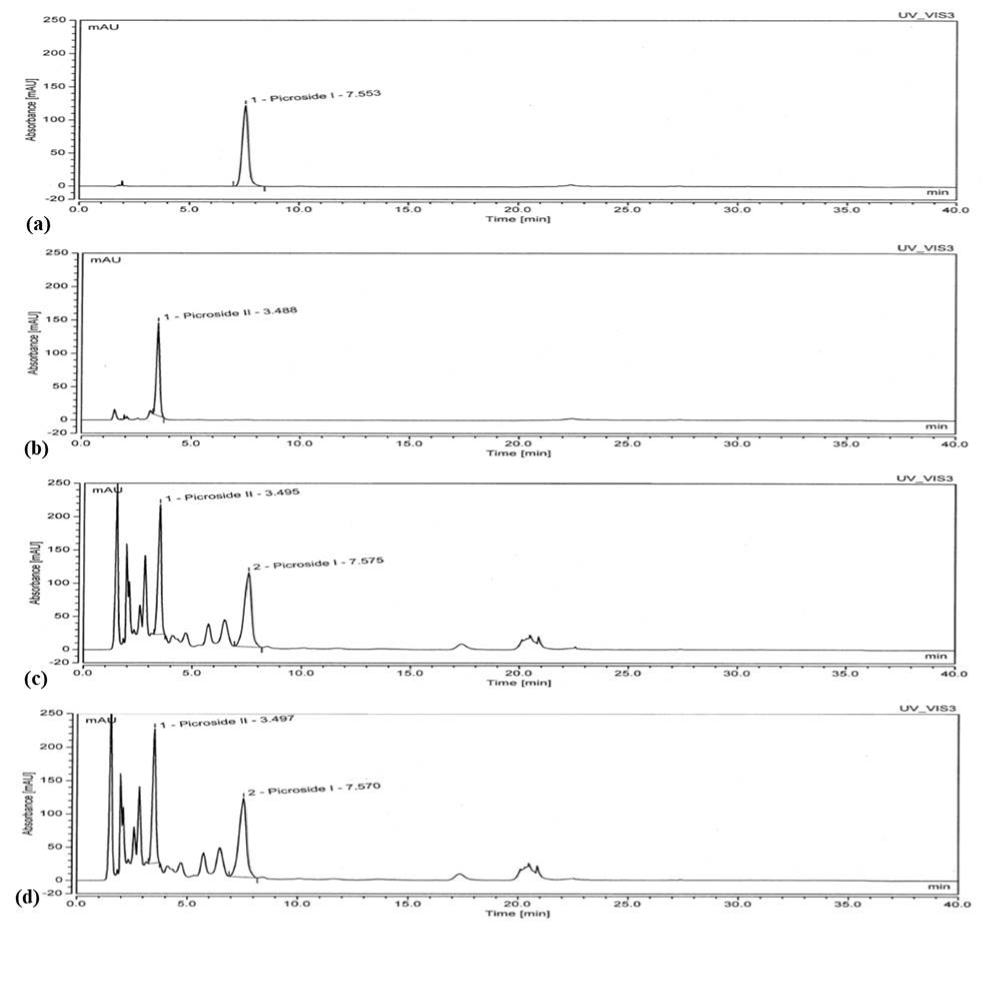
Estimation of bioactive’s – Salacinol in SC extract by LC-MS/MS and Picroside I and II in PK extract by HPLC analysis
In the current study, we performed LC-MS/MS analysis to quantify the Salacinol content of SC extract with water as solvent and water containing GA. Salacinol content was determined to be 0.489 and 0.456 % in the extract with and without GA, respectively [36,37]. The outcome of the analysis clearly indicated that the GA treatment did not affect the Salacinol content (Table 4). As per HPLC analysis, bioactive’s from PK extract, Picroside-I, and Picroside-II showed retention times of 7.57 and 3.49 min, respectively, similar to the standards (Figure 2 and Table 4). It can be clearly seen that the content of both Picroside-I and Picroside-II did not vary after the treatment with 0.5% GA.
Estimation of residual glutaraldehyde in SC and PK extract post-treatment with SB
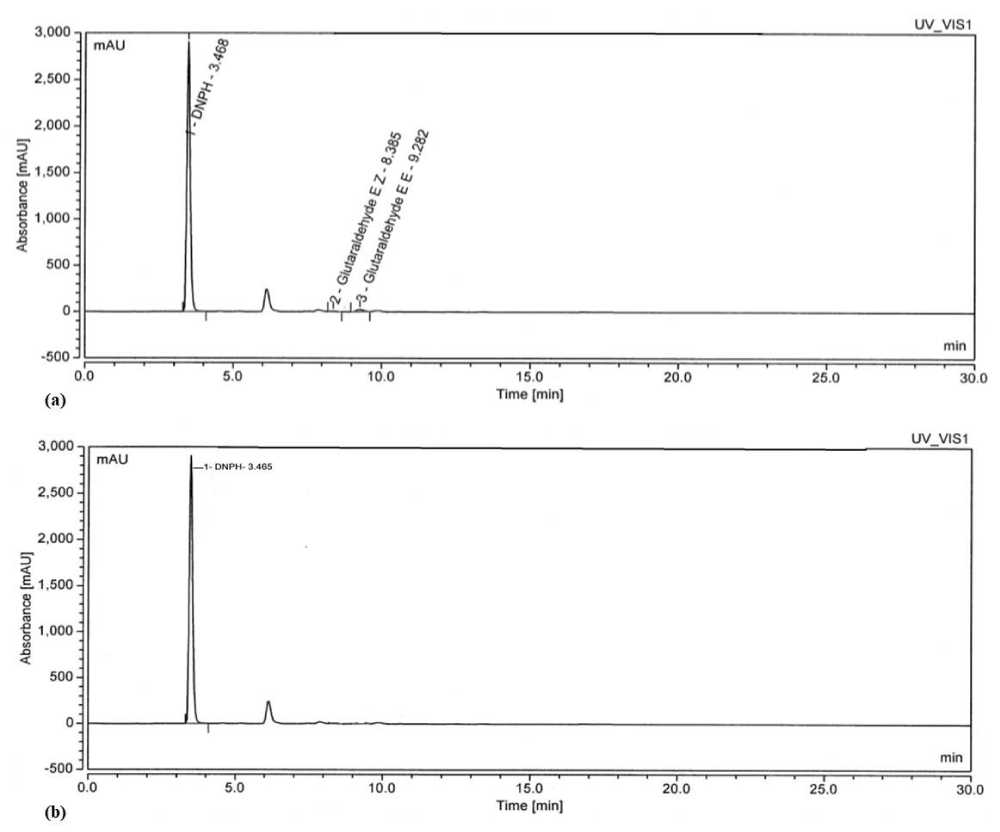
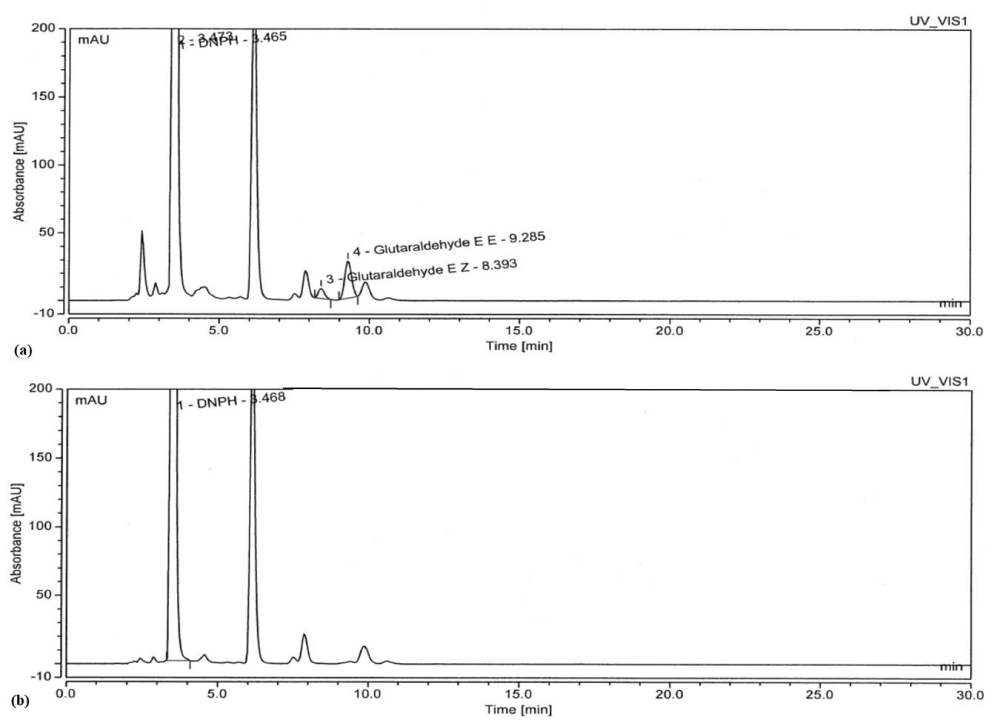
The residual GA in the extracts of SC and PK was estimated using the standard HPLC method; it was observed that trace quantities of GA were absorbed by the SC and PK extracts during the extraction process which can be seen in the chromatogram (Figures 3,4). The area and percentage of the chromatogram clearly depicts that some amount of GA was present in the extracts; however, it was not significantly high. Further to make the extracts free of residual GA, we added SB and interestingly the concentration of GA decreased significantly [28]. In order to validate the removal of GA by SB, solutions of 0.5% glutaraldehyde were reacted with a 2.2-fold SB, and the subsequent reaction mixture was analyzed by using HPLC. The chromatogram in Figure 3 and 4 reflects the size of the GA–DNPH peak expected at a low concentration of GA remaining in the solution (< 100 ppm). Based on the chromatogram peak of SC and PK shown in Figure 3 and 4, no detectable GA is observed after the reaction with a 2.2-fold of SB for 15 min, signifying that SB completely inactivated the GA with the reduction of 98.45 % and 99.96 %, respectively [28].
HPLC analysis provides proof, that residual GA can be removed from the extract by using SB thereby, making the extract eco-friendly, safe, and compliant with the regulatory requirements.
Discussion
The plant parts used as raw materials for the production of pharmaceutical applications are often infested with fungi that produce mycotoxins and bacteria that produce aflatoxin making it unsafe for use [39]. Due to the collection of plant material from different geographical locations variation is observed in raw material with respect to quality as well as contamination. Thus a standard method needs to be established for the evaluation of the quality of raw material to maintain the quality of pharmaceutical products and increase its efficiency [40,41]. To maintain quality and manufacture standardized herbal products good manufacturing practices need to be followed stringently as reported previously [42,43]. The current study was focused on the development of a safe, simple, and cost-effective method for the removal of microbial contaminants from the PRM or herbal extracts without impacting the natural bioactive agents present in the PRM or extract. The microbial count inherited in the SC and PK PRM was found in the range of 104 and 103 cfu/g as presented in the results, respectively. The bacteria predominantly present in the SC and PK PRM were isolated for use in the study. The isolated bacterial strains from SC and PK PRM, termed SC isolate and PK isolate respectively were used for determining the MIC of the antimicrobial agent to be used for the study. Various cold sterilization methods like gamma irradiation or antimicrobial agents like GA are often used for sterilization to reduce the microbial load in herbal raw materials. Methods like gamma irradiation can be used but it is not effective in the elimination of microbial cells like Staphylococcus epidermidis and Escherichia coli as reported in a study [44]. GA on the other hand is proven to be effective in the control of biofilms produced by micro-organisms such as Bacillus cereus and Pseudomonas fluorescens [45]. Thus, to eliminate the microbial contamination from the PRM, Glutaraldehyde was selected as an antimicrobial agent due to its applications in various allied fields. As reported earlier, GA is effective as disinfection at a concentration of 0.5% as reported earlier [30], and depending on the MIC of GA obtained for the isolates in our hands, GA at 0.5% final concentration was used for treatment of the PRM to ensure complete removal of microbial contamination (> 99.99%) as proved by microbial analysis using agar method as well as Soleris® system.
To determine that the GA treatment method did not affect the bioactives from the PRM and the extract; the bioactives namely Salacinol from SC and Picroside I and II from PK were estimated before and after the GA treatment. The analysis result of the PRM or extracts showed that before and after GA treatment content of the bioactives is equivalent, proving the GA treatment method is safe for use in the manufacturing processes. The extracts after GA treatment will be used for manufacturing medicines, health supplements, or food additives, the end route being human consumption. To ensure the safety of these extracts for human consumption, the extracts after the GA treatment were analyzed using HPLC to determine any residual GA in the extracts. HPLC analysis showed the presence of traces of Glutaraldehyde in the extracts as it may be absorbed by the extracts to a small extent during treatment. Efforts were made to remove this residual GA using SB as demonstrated previously [28]. It is purported that GA is inactivated using SB by forming a complex with it. HPLC analysis after inactivation of the residual GA in the extract by SB demonstrates the complete inactivation of GA thereby, ascertaining the safety of the extracts for use and making it compliant with HACCP regulations.
A simple, cost-effective, and safe method as unveiled in this study can be implemented as a routine for the exclusion of micro-organisms in the PRM or herbal extracts. However, the proportion of GA required for treatment of the PRM or extracts may vary depending on the inherent nature of the plant material or extract. With regulatory requirements like HACCP in place, the raw materials for making products for human consumption should meet compliance. Also if products are prepared from raw materials like herbal extracts people are always cautious about the content and safety before consumption. If such methods are instigated upon preparation of extracts then consumers are ensured that the products are safe for consumption.
Conclusion
Microbial contamination in the plant raw materials deteriorates the quality of extracts used for manufacturing Ayurveda medicines. Antimicrobial agents like GA can be employed for the removal of such microbial contamination from plant raw materials as well as extracts. To the best of our knowledge, this is the first report on the use of GA in reducing microbial load on plant-based raw materials. It is an economically viable and safe solution as GA does not disrupt the inherent nature and bioactive contents of the plant material. The use of GA may be further explored to make the products HACCP compliant and improve the quality of Ayurveda medicines making them safe for consumption.
The authors are grateful to Mr. Vinod R Jadhav, Chairman, SHL, and Mr. Dinesh Kapoor, MD, SHL for their constant support and encouragement.
Credit authorship contribution statement
Shankar Mane: Arrived at the concept of the use of glutaraldehyde in reducing the microbial load of herbal extracts and carried out all the MIC experiments.
Umesh Chandra Dash: Performed the extraction, estimation of residual glutaraldehyde by HPLC, and data generation.
Shital Palghadmal: Estimation of bioactive molecules by HPLC, and HPLC data interpretation.
Nisha Mehta: Preliminary drafting of the manuscript, formatting the figures as per the journal’s requirement, data analysis, and interpretation
Sriram Padmanabhan: Designed Study, Project administration, Resources, Supervision, Writing- review, and Editing
Declaration of competing interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
- Kunene NF, Hastings JW, von Holy A. Bacterial populations associated with a sorghum-based fermented weaning cereal. Int J Food Microbiol. 1999 Aug 1;49(1-2):75-83. doi: 10.1016/s0168-1605(99)00062-8. PMID: 10477073.
- Wilson C, Dettenkofer M, Jonas D, Daschner FD. Pathogen growth in herbal teas used in clinical settings: a possible source of nosocomial infection? Am J Infect Control. 2004 Apr;32(2):117-9. doi: 10.1016/j.ajic.2003.09.004. PMID: 15057205.
- Abualhasan M, Jaradat N, Sawaftah Z, Mohsen H, Najjar D, Zareer W. Evaluation of Heavy Metals and Microbiological Contamination of Selected Herbals from Palestine. Open Life Sci. 2019 Nov 15;14:448-453. doi: 10.1515/biol-2019-0050. PMID: 33817180; PMCID: PMC7874764.
- Alharbi SF, Althbah AI, Mohammed AH, Alrasheed MA, Ismail M, Allemailem KS, Alnuqaydan AM, Baabdullah AM, Alkhalifah A. Microbial and heavy metal contamination in herbal medicine: a prospective study in the central region of Saudi Arabia. BMC Complement Med Ther. 2024 Jan 2;24(1):2. doi: 10.1186/s12906-023-04307-y. PMID: 38166914; PMCID: PMC10759756.
- Halt M. Moulds and mycotoxins in herb tea and medicinal plants. Eur J Epidemiol. 1998 Apr;14(3):269-74. doi: 10.1023/a:1007498613538. PMID: 9663520.
- Kosalec I, Cvek J, Tomić S. Contaminants of medicinal herbs and herbal products. Arh Hig Rada Toksikol. 2009 Dec;60(4):485-501. doi: 10.2478/10004-1254-60-2009-2005. PMID: 20061249.
- Başaran N, Paslı D, Başaran AA. Unpredictable adverse effects of herbal products. Food Chem Toxicol. 2022 Jan;159:112762. doi: 10.1016/j.fct.2021.112762. Epub 2021 Dec 9. PMID: 34896186.
- Bhosale RD, Padmanabhan S. Evaluation of Microbial Load of Herbal Raw Materials: a Necessary Quality Control Measure to Ensure Safety of Finished Herbal Preparations. Adv Biotechnol Microbiol. 2021;16(2). doi: 10.19080/AIBM.2021.16.555934.
- Qin L, Jiang JY, Zhang L, Dou XW, Ouyang Z, Wan L, Yang MH. Occurrence and analysis of mycotoxins in domestic Chinese herbal medicines. Mycology. 2020 Feb 20;11(2):126-146. doi: 10.1080/21501203.2020.1727578. PMID: 32923021; PMCID: PMC7448902.
- Rates SM. Plants as source of drugs. Toxicon. 2001 May;39(5):603-13. doi: 10.1016/s0041-0101(00)00154-9. PMID: 11072038.
- Sen T, Samanta SK. Medicinal plants, human health and biodiversity: a broad review. Adv Biochem Eng Biotechnol. 2015;147:59-110. doi: 10.1007/10_2014_273. PMID: 25001990.
- Jeykodi S, Deshpande J, Juturu V. Salacia Extract Improves Postprandial Glucose and Insulin Response: A Randomized Double-Blind, Placebo Controlled, Crossover Study in Healthy Volunteers. J Diabetes Res. 2016;2016:7971831. doi: 10.1155/2016/7971831. Epub 2016 Oct 10. PMID: 27803937; PMCID: PMC5075619.
- Salehi B, Ata A, V Anil Kumar N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, Abdulmajid Ayatollahi S, Tsouh Fokou PV, Kobarfard F, Amiruddin Zakaria Z, Iriti M, Taheri Y, Martorell M, Sureda A, Setzer WN, Durazzo A, Lucarini M, Santini A, Capasso R, Ostrander EA; Atta-ur-Rahman; Choudhary MI, Cho WC, Sharifi-Rad J. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules. 2019 Sep 30;9(10):551. doi: 10.3390/biom9100551. PMID: 31575072; PMCID: PMC6843349.
- Erten F, Orhan C, Tuzcu M, Er B, Defo Deeh PB, Sahin N, Özercan IH, Juturu V, Sahin K. Salacia chinensis exerts its antidiabetic effect by modulating glucose-regulated proteins and transcription factors in high-fat diet fed-streptozotocin-induced type 2 diabetic rats. J Food Biochem. 2020 Dec;44(12):e13513. doi: 10.1111/jfbc.13513. Epub 2020 Oct 5. PMID: 33020991.
- Li Y, Huang TH, Yamahara J. Salacia root, a unique Ayurvedic medicine, meets multiple targets in diabetes and obesity. Life Sci. 2008 May 23;82(21-22):1045-9. doi: 10.1016/j.lfs.2008.03.005. Epub 2008 Mar 28. PMID: 18433791.
- Morikawa T, Ninomiya K, Tanabe G, Matsuda H, Yoshikawa M, Muraoka O. A review of antidiabetic active thiosugar sulfoniums, salacinol and neokotalanol, from plants of the genus Salacia. J Nat Med. 2021 Jun;75(3):449-466. doi: 10.1007/s11418-021-01522-0. Epub 2021 Apr 26. PMID: 33900535; PMCID: PMC8159842.
- Tamang A, Swarnkar M, Kumar P, Kumar D, Pandey SS, Hallan V. Endomicrobiome of in vitro and natural plants deciphering the endophytes-associated secondary metabolite biosynthesis in Picrorhiza kurrooa, a Himalayan medicinal herb. Microbiol Spectr. 2023 Dec 12;11(6):e0227923. doi: 10.1128/spectrum.02279-23. Epub 2023 Oct 9. PMID: 37811959; PMCID: PMC10715050.
- Walia V, Chaudhary SK, Kumar Sethiya N. Therapeutic potential of mangiferin in the treatment of various neuropsychiatric and neurodegenerative disorders. Neurochem Int. 2021 Feb;143:104939. doi: 10.1016/j.neuint.2020.104939. Epub 2020 Dec 17. PMID: 33346032.
- Pokhriyal A, Prakash S, Patni B. Comparative Study on the Biochemical Profile and Antioxidant Activity of Picrorhiza kurrooa Rolye ex Benth. Obtained from Uttarakhand. Evid Based Complement Alternat Med. 2023 Dec 7;2023:8792414. doi: 10.1155/2023/8792414. PMID: 38099237; PMCID: PMC10721352.
- Sharma T, Sharma U, Kumar S. Iridoid glycosides from Picrorhiza genus endemic to the Himalayan region: phytochemistry, biosynthesis, pharmacological potential and biotechnological intercessions to boost production. Crit Rev Biotechnol. 2022 Oct 2:1-16. doi: 10.1080/07388551.2022.2117681. Epub ahead of print. PMID: 36184806.
- Kant K, Walia M, Agnihotri VK, Pathania V, Singh B. Evaluation of Antioxidant Activity of Picrorhiza kurroa (Leaves) Extracts. Indian J Pharm Sci. 2013 May;75(3):324-9. doi: 10.4103/0250-474X.117438. PMID: 24082348; PMCID: PMC3783750.
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999 Jan;12(1):147-79. doi: 10.1128/CMR.12.1.147. Erratum in: Clin Microbiol Rev 2001 Jan;14(1):227. PMID: 9880479; PMCID: PMC88911.
- Weber WARaDJ. Disinfection, Sterilization, and Control of Hospital Waste. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 2014. doi: 10.1016/B978-1-4557-4801-3.00301-5. PubMed Central PMCID: PMC70996623309.e4.
- Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 2004 Nov;37(5):790-6, 798-802. doi: 10.2144/04375RV01. PMID: 15560135.
- Gorman SP, Scott EM, Russell AD. Antimicrobial activity, uses and mechanism of action of glutaraldehyde. J Appl Bacteriol. 1980 Apr;48(2):161-90. doi: 10.1111/j.1365-2672.1980.tb01217.x. PMID: 6780502.
- Smith DR, Wang RS. Glutaraldehyde exposure and its occupational impact in the health care environment. Environ Health Prev Med. 2006 Jan;11(1):3-10. doi: 10.1007/BF02898201. PMID: 21432369; PMCID: PMC2723614.
- Leung HW. Aerobic and anaerobic metabolism of glutaraldehyde in a river water-sediment system. Arch Environ Contam Toxicol. 2001 Oct;41(3):267-73. doi: 10.1007/s002440010248. PMID: 11503062.
- Jordan SL, Russo MR, Blessing RL, Theis AB. Inactivation of glutaraldehyde by reaction with sodium bisulfite. J Toxicol Environ Health. 1996 Feb 23;47(3):299-309. doi: 10.1080/009841096161807. PMID: 8604152.
- Cao-Ngoc P, Leclercq L, Rossi JC, Hertzog J, Tixier AS, Chemat F, Nasreddine R, Al Hamoui Dit Banni G, Nehmé R, Schmitt-Kopplin P, Cottet H. Water-Based Extraction of Bioactive Principles from Blackcurrant Leaves and Chrysanthellum americanum: A Comparative Study. Foods. 2020 Oct 16;9(10):1478. doi: 10.3390/foods9101478. PMID: 33081198; PMCID: PMC7602794.
- AlZain S. Effect of 0.5% glutaraldehyde disinfection on surface wettability of elastomeric impression materials. Saudi Dent J. 2019 Jan;31(1):122-128. doi: 10.1016/j.sdentj.2018.10.002. Epub 2018 Oct 15. PMID: 30723366; PMCID: PMC6349997.
- Kowalska-Krochmal B, Dudek-Wicher R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens. 2021 Feb 4;10(2):165. doi: 10.3390/pathogens10020165. PMID: 33557078; PMCID: PMC7913839.
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001 Jul;48 Suppl 1:5-16. doi: 10.1093/jac/48.suppl_1.5. Erratum in: J Antimicrob Chemother 2002 Jun;49(6):1049. PMID: 11420333.
- Chitarra LG, Langerak CJ, Bergervoet JH, van den Bulk RW. Detection of the plant pathogenic bacterium Xanthomonas campestris pv. Campestris in seed extracts of Brassica sp. Applying fluorescent antibodies and flow cytometry. Cytometry. 2002 Feb 1;47(2):118-26. doi: 10.1002/cyto.10058. PMID: 11813202.
- Jayashree BS, Alam A, Nayak Y, Kumar DV. Synthesis of 3-methylflavones and their antioxidant and antibacterial activities. Med Chem Res. 2012;21:1991-6.
- Yewale S, Farash Z, Kolhe S, Sakkan S, Bhope S, Ambekar P, Padmanabhan S. Benefits of Soleris® over the Conventional Method for Enumeration of Microbial Load in Salacia Herbal Extract. Pol J Microbiol. 2020 Dec;69(4):453-462. doi: 10.33073/pjm-2020-048. Epub 2020 Dec 27. PMID: 33574873; PMCID: PMC7812356.
- Khan I, Rahman H, Abd El-Salam NM, Tawab A, Hussain A, Khan TA, Khan UA, Qasim M, Adnan M, Azizullah A, Murad W, Jalal A, Muhammad N, Ullah R. Punica granatum peel extracts: HPLC fractionation and LC MS analysis to quest compounds having activity against multidrug resistant bacteria. BMC Complement Altern Med. 2017 May 3;17(1):247. doi: 10.1186/s12906-017-1766-4. PMID: 28468660; PMCID: PMC5415797.
- Patil JS, Suresh S, Sureshbabu AR, Rajesh MS. Development and validation of liquid chromatography-mass spectrometry method for the estimation of rifampicin in plasma. Indian J Pharm Sci. 2011 Sep;73(5):558-63. doi: 10.4103/0250-474X.99014. PMID: 22923869; PMCID: PMC3425068.
- Ooh KF, Ong HC, Wong FC, Sit NW, Chai TT. High performance liquid chromatography profiling of health-promoting phytochemicals and evaluation of antioxidant, anti-lipoxygenase, iron chelating and anti-glucosidase activities of wetland macrophytes. Pharmacogn Mag. 2014 Aug;10(Suppl 3):S443-55. doi: 10.4103/0973-1296.139767. PMID: 25298659; PMCID: PMC4189257.
- Maurya A, Kumar S, Singh BK, Chaudhari AK, Dwivedy AK, Prakash B, Dubey NK. Mechanistic investigations on antifungal and antiaflatoxigenic activities of chemically characterised Carum carvi L. essential oil against fungal infestation and aflatoxin contamination of herbal raw materials. Nat Prod Res. 2022 Sep;36(17):4569-4574. doi: 10.1080/14786419.2021.1994566. Epub 2021 Oct 21. PMID: 34672233.
- Amir M, Zafar A, Ahmad R, Ahmad W, Sarafroz M, Khalid M, Ghoneim MM, Alshehri S, Wahab S, Ahmad S, Mujeeb M. Quality Control Standardization, Contaminant Detection and In Vitro Antioxidant Activity of Prunus domestica Linn. Fruit. Plants (Basel). 2022 Mar 6;11(5):706. doi: 10.3390/plants11050706. PMID: 35270176; PMCID: PMC8912893.
- Garg V, Dhar VJ, Sharma A, Dutt R. Facts about standardization of herbal medicine: a review. Zhong Xi Yi Jie He Xue Bao. 2012 Oct;10(10):1077-83. doi: 10.3736/jcim20121002. PMID: 23073189.
- Ghisleni DD, Braga Mde S, Kikuchi IS, Braşoveanu M, Nemţanu MR, Dua K, Pinto Tde J. The Microbial Quality Aspects and Decontamination Approaches for the Herbal Medicinal Plants and Products: An in-Depth Review. Curr Pharm Des. 2016;22(27):4264-87. doi: 10.2174/1381612822666160623070829. PMID: 27339428.
- Govindaraghavan S, Sucher NJ. Quality assessment of medicinal herbs and their extracts: Criteria and prerequisites for consistent safety and efficacy of herbal medicines. Epilepsy Behav. 2015 Nov;52(Pt B):363-71. doi: 10.1016/j.yebeh.2015.03.004. Epub 2015 Apr 18. PMID: 25899015.
- Trampuz A, Piper KE, Steckelberg JM, Patel R. Effect of gamma irradiation on viability and DNA of Staphylococcus epidermidis and Escherichia coli. J Med Microbiol. 2006 Sep;55(Pt 9):1271-1275. doi: 10.1099/jmm.0.46488-0. PMID: 16914659.
- Simões LC, Lemos M, Araújo P, Pereira AM, Simões M. The effects of glutaraldehyde on the control of single and dual biofilms of Bacillus cereus and Pseudomonas fluorescens. Biofouling. 2011 Mar;27(3):337-46. doi: 10.1080/08927014.2011.575935. PMID: 21512918.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
