Open Journal of Bacteriology
Carrot slice test: A reliable method for evaluating the tumorigenicity of Pseudomonas savastanoi pv. nerii
Attila Fodor, Áron Juhasz, Viktória Vitári and Karacs-Végh Anita*
Cite this as
Fodor A, Juhasz Á, Vitári V, Anita KV (2024) Carrot slice test: A reliable method for evaluating the tumorigenicity of Pseudomonas savastanoi pv. nerii. Open J Bac 8(1): 001-005. DOI: 10.17352/ojb.000026Copyright License
© 2024 Fodor A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Pathogenicity studies on oleander plants take a long time and require significant costs to confirm the identification of isolates and to verify their pathogenicity. In this study, the carrot slice test was used as a rapid method for Pseudomonas savastanoi pv. nerii. The test is suitable for the investigation of tumorigenicity of 25 Pseudomonas savastanoi pv. nerii isolates from different parts of Hungary. On carrot slices, the first characteristic knots were observed 7 days after inoculation and were fully developed 16 days after inoculation. The one-year oleander plants used to confirm the pathogenicity of the bacteria were inoculated with a bacterial suspension to confirm the reliability of the carrot slice test. Characteristic symptoms of knots were observed on all oleander plants 46 days after inoculation. The results showed that the carrot slice method is a straightforward, rapid, and reliable method for testing and confirming tumor formation of Pseudomonas savastanoi pv. nerii without plants.
Introduction
The oleander (Nerium oleander L.) is a popular ornamental plant because of its abundant and long-lasting flowering. Native to the Mediterranean region, it is popular in Central and Western Europe both as a greenhouse plant and as a terrace plant. Oleander knot disease caused by Pseudomonas savastanoi pv. nerii [1,2] is the most common and dangerous disease of oleander (Nerium oleander L.). The typical symptoms are knots or galls on stems, twigs, leaves, and seedcases [2,3] which are incited by phytohormones [4]. The severity of the disease is mainly due to the systematic spread of the pathogen in the plant, which makes prevention and control very difficult [5]. Similar diseases occur on olive (Olea europaea, L) (P. savastanoi pv. savastanoi [1,2,6], on ash (Fraxinus spp., L) (P. savastanoi pv. fraxini [1,2,6], on Spanish broom (Retama sphaerocarpa, L) (P. savastanoi pv. retacarpa [7] and on other different various plants as well [2,7,8]. It is now known that the P. savastanoi pathotypes and Agrobacterium radiobacter [9] differ from most plant pathogen bacterial in that they produce plant hormones to cause knots on susceptible plants [10-12]. The youngest stages tumors caused by A. tumefaciens are very similar to the young knots by P. savastanoi pv. nerii [13]. For tumorigenicity tests of Agrobacterium spp. carrot (Daucus carota subsp. sativus L.) slices were often used [14-22]. What is more, Doksöz and Bozkurt [23] found that the carrot tumorigenicity test of P. savastanoi pv. savastanoi is a sensitive and rapid technique. Therefore, tumorigenicity tests on oleander plants are usually difficult to perform due to the large number of plants and the long time (up to 8 weeks - 10 weeks) needed to evaluate the results [5,24,25], can be replaced by carrot slices. The purpose of this study is to prove that the carrot slice test is a reliable method for evaluating the tumorigenicity of P. savastanoi pv. nerii. To verify this test, the pathogenicity test was also performed on oleander plants.
Material and methods
Bacterial strains
Infected plant samples were collected between 2018 and 2023 from hobby gardeners and public places in Hungary. 25 isolates of P. savastanoi pv. nerii (Table 1.) grown on King’s Medium B (1,5 g K2HPO4; 20 g pepton, 10 g glycerol, 1,5 g MgSO4 X 7 H2O, 15 g agar, 1 l H2O, pH 7.2) for 48 h at 25 ºC were used [35]. In addition, the bacterial culture was stored at -20 ºC and -70 ºC in 20% glycerol preservative liquid for longer storage (3g Beef extract, 5 g pepton, 20 g glycerol, 1 l H2O). The isolates were previously examined by classical methods [25,26]. An Allorhizobium vitis (B.02389) strain [27,28], an Agrobacterium radiobacter (B.01178) strain, an A. rubi (B.01336) strain [29,30], a P. savastanoi (B.01823) strain from National Collection of Agricultural and Industrial Microorganisms (NCAIM, Hungarian University of Agriculture and Life Sciences, Budapest, Hungary), a P. savastanoi pv. retacarpa (CFBP5513) strain and a P. savastanoi pv. fraxini (CFBP5062) strain from the French Collection for Plant-associated Bacteria (CFBP), which is a collection of the International Center for Microbial Resources (CIRM, France) and P. savastanoi pv. savastanoi strain (Table 1) were used as positive controls.
Tumorigenicity test of carrot slices
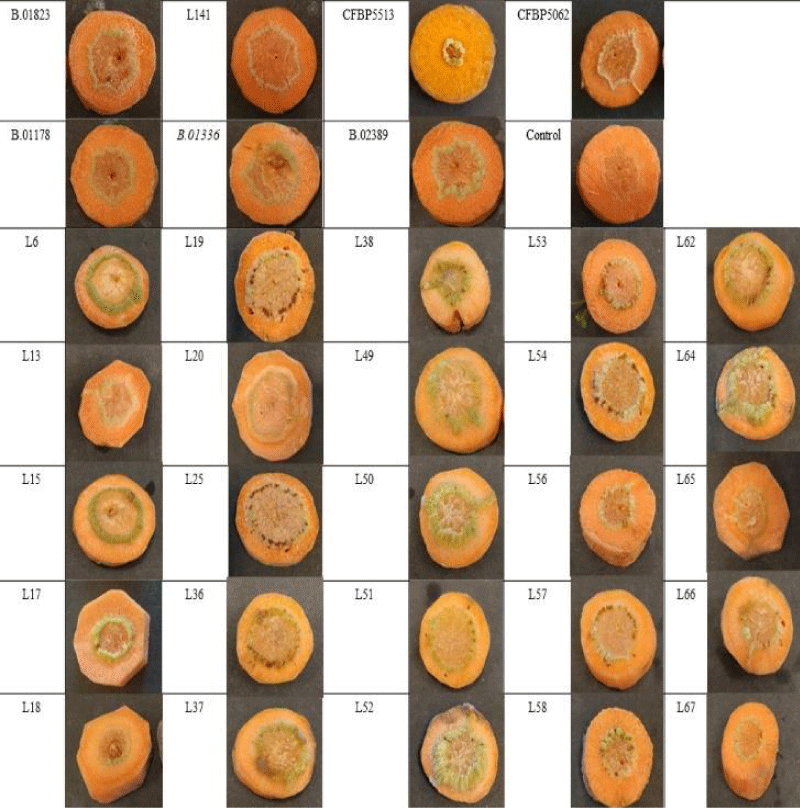
The carrot taproots, which were bought in the supermarket, were washed under running water, next sterilised in 70% ethanol for 5 min, then washed with sterile distilled water, and finally dried on sterile paper. After sterilization, the carrot taproots were peeled, the top and bottom segments were cut off, and were sliced with a sterile scalpel to a thickness of 0.5 cm - 1 cm. Carrot slices were inoculated separately per isolate with pure bacterial cultures using a toothpick and placed in sterile Petri dishes on moistened sterile filter paper. Each carrot slice was punctured once in the middle. For each isolate (Table 1), 3 slices inoculated with bacterial culture and 2 slices inoculated with sterile distilled water were used as negative controls. Petri dishes were placed in a plant growth chamber (PHCBI MLR-352H-PE) at 80% - 85% relative humidity and 25 °C. Knots were observed until 20. days after inoculation and results were visually assessed [16,23,31].
Pathogenicity test on oleander plants
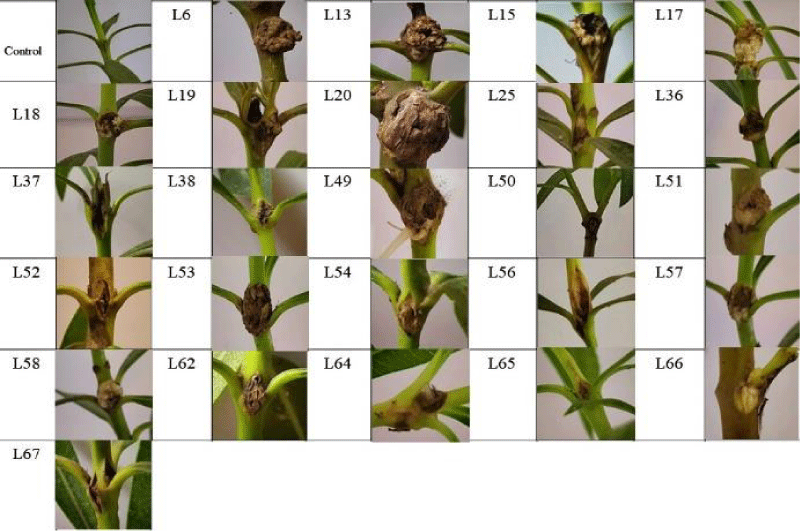
One-year-old home-grown oleander plants were used to verify the safety of the carrot slice assays. Internodes between the first and second, and third and fourth leaves of oleander plants were injected with a sterile syringe needle containing bacterial suspension at a concentration of 5 × 107 cells mL-1, which was determined using a spectrophotometer set at 560 nm. Sterile distilled water was used as a negative control. Inoculated plants were kept in a greenhouse at a relative humidity above 90% for 1 week and observed 60 days after inoculation.
Identification of re-isolated pathogens
The carrot slice and parts of oleander plants were surface-sterilized with 75% ethanol and small pieces of tissues were cut with a sterile scalpel and macerated in Sterile Distilled Water (SDW). A spoonful of the resulting suspension was streaked onto King B agar [35]. The plates were incubated at room temperature (RT) for 48 hours - 72 hours and pure cultures of bacterial isolates were analyzed by PCR (Polymerase chain reaction). The amplification and sequencing of 16S rDNA was achieved using 63F (5’-CAGGCCTAACACATGCAAGTC-3’) and 1389R (5’-ACGGGCGGTGTGTACAAG-3’) universal primer pair [32]. The Polymerase Chain Reaction (PCR) conditions were the following: initial denaturation at 94 °C for 5 min, followed by 35 cycles at 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 90s. The final extension was at 72 °C for 10 min. Amplification was verified on a 1% (w/v) agarose gel in 1 × TBE buffer. The PCR products were cleaned with the High Pure PCR Product Purification Kit (Roche Diagnostics GmbH). The nucleotide sequence of the PCR-amplified DNA fragment was determined and compared with sequences from the National Center for Biotechnology Information (NCBI Genebank) database, using the Basic Local Alignment Search Tool (BLAST) program.
Results and discussion
All tested strains caused small, green tumors on the endodermis of the carrot slides. It was not possible to distinguish our isolates on the basis of typical knot symptoms. The first young knots on carrot slices were observed 7 days after inoculation and the typical knots developed 16 days after inoculation (Figure 1). Positive control strains showed the same symptoms as our isolates. Negative control carrot slices inoculated with sterile distilled water showed no symptoms. The pathogens were re-isolated from the knots and confirmed by PCR. The reaction of the other two pathotypes of P. savastanoi (Ps pv. retacarpa and Ps pv. fraxini) showed a slight delay as the symptoms appeared 9 days after inoculation, but there was no difference in symptoms. In previous studies using carrot slices to test tumorigenicity, the same results were obtained [16,23].
In the pathogenicity test on oleander plants, the first knots were formed on stems 28 days after inoculation. After 46 days the typical knots were observed on all infected plants (Figure 2). Negative control plants inoculated with sterile distilled water showed no symptoms. The pathogens were re-isolated from the knots and PCR confirmed that Pseudomonas savastanoi pv. nerii was responsible for the symptoms.
The tumorigenicity tests on carrot discs and pathogenicity tests on oleander plants resulted in similar tumors on the plant parts. The carrot slice technique is a quite simple and responsible method for testing the tumorigenicity of P. savastanoi pv. nerii without plants. It requires less space and resources, the carrot taproot is easily accessible and the time required for a pathogenicity test can be significantly reduced. This is the first report where carrot slices were used for testing the tumorigenicity of P. savastanoi pv. nerri, P. savastanoi pv. fraxini and P. savastanoi pv. retacarpa.
Conclusion
The results showed that the inoculation of carrot slices technique can be successfully applied to the tumorigenicity test of all Pseudomonas savastanoi pathovar. The tumorigenicity technique with the use of carrot slices may also be applicable to other tumor-forming bacteria.
The research was funded by the “ VP4-10.2.2.-20-Ex situ conservation of genetic resources of rare and endangered plant species and microorganisms”.
- Smith EF. Recent studies on the olive-tubercle organism. Bull Bur Plant Ind US. Dep. Agric. 131: 25- 43.
- Janse JD. Pseudomonas syringae subsp. savastanoi (ex Smith) subsp. nov., nom. rev., the bacterium causing excrescences on Oleaceae and Nerium oleander L. Int. J. Syst. Bacteriol. 1982; 32: 166-169. https://doi.org/10.1099/00207713-32-2-166
- Smith CO. A Bacterial disease of oleander. Bacillus Oleae (Arcang.) Trev. Bot Gaz. 1906; 42: 301-310.
- Surico G, Iacobellis NS, Sisto A. Studies on the role of indole-3-acetic acid and cytokinins in the formation of knots on olive and oleander plants by Pseudomonas syringae pv. savastanoi. Physiolog Plant Pathol. 1985; 26:3; 309-320. https://doi.org/10.1016/0048-4059(85)90006-2.
- Bella P, Catara V, Guarino C, Cirvilleri G. Evaluation of oleander accessions for resistance to Pseudomonas savastanoi pv. nerii. J Plant Pathol. 2006; 88: 273-278.
- Young JM, Saddler GS, Takikawa Y, De Boer SH, Vauterin L, Garden L, Gvozdyak RIN, Stead DE. Names of plant pathogenic bacteria 1864-1995. Review of Plant Pathol. 1996; 75: 721-763.
- De Los Rios JEG. Retama sphaerocarpa (L.) Boiss., a new host of Pseudomonas savastanoi. Phytopathol Mediterr. 1999; 38: 54-60. https://doi.org/10.1111/ppa.12096
- Gardan L, Bollet C, Abu Ghorrah M, Grimont F, Grimont PAD. DNA Relatedness among the pathovar strains of Pseudomonas syringae subsp. savastanoi Janse (1982) and proposal of Pseudomonas savastanoi sp. nov. I Int. J. Syst. Bacteriol. 1992; 42: 606-612. https://doi.org/10.1099/00207713-42-4-606
- Beijerinck MW, Delden VA. Assimilation of free nitrogen by bacteria. Zentbl Bakt Parasitenk Infekt Abt II. 1902; 9: 3-43.
- Surico G, Sparapano L, Lerari, P, Durbin R, Iacobellis N. Cytokinin-like activity in extracts from culture filtrates of Pseudomonas savastanoi. Experientia. 1975; 31: 929-930.
- Comai L, Kosuge T. Involvement of plasmid deoxyribonucleic acid in indoleacetic acid synthesis in Pseudomonas savastanoi. J Bacteriol. 1980 Aug;143(2):950-7. doi: 10.1128/jb.143.2.950-957.1980. PMID: 7204339; PMCID: PMC294399.
- Akiyoshi DE, Regier DA, Gordon MP. Cytokinin production by Agrobacterium and Pseudomonas spp. J Bacteriol. 1987 Sep;169(9):4242-8. doi: 10.1128/jb.169.9.4242-4248.1987. PMID: 3624204; PMCID: PMC213736.
- Smith CO. Oleander bacteriosis in California. Phytopathol. 1928; 18.
- Blakesley D, Chaldecott MA. The role of endogenous auxin in root initiation. Plant Growth Regul. 1993; 13: 77-84. https://doi.org/10.1007/BF00207595
- Aysan Y, Sahin F, Mirik M, Donmez MF, Tekman H. First report of crown gall of apricot (Prunus armeniaca) caused by Agrobacterium tumefaciens in Turkey. J Plant Pathol. 2003; 52: 793-793. https://doi.org/10.1111/j.1365-3059.2003.00902.x
- Abdellatif E, Valentini F, Janse JD, Bouri M, Rhouma A, Chebil S, D’Onghia AM. Occurrence of crown gall of the grapevine in Tunisia and characterization of Tunisian Agrobacterium vitis and A. tumefaciens strains. J Plant Pathol. 2013; 95: 115-126.
- Tolba I, Soliman M. Phenotypic and molecular characterization of tumorigenic Agrobacterium tumefaciens strains isolated from rose plant in Egypt. Middle East Jour. 2014; 3: 1002-1014.
- Basavand E, Khodaygan P, Moradi M, Mousavi SA, Lindström K, Sedaghati N. Characterization of Agrobacterium radiobacter, a new pathogen of pistachio. Australasian Plant Pathol. 2022; 51: 167-174. https://doi.org/10.1007/s13313-021-00831-y
- Khan HM, Manan A, Shah SAH, Raheem MA. A systematic review on isolation, identification, and characterization of Agrobacterium tumefaciens from leguminous plants. MidEast J Appl Sci Tech. 2022; 5:13-17.https://doi.org/10.46431/MEJAST.2022.5403
- Tekiner N, Kotan R. Pathogenicity of different Rhizobium radiobacter (Agrobacterium tumefaciens) Isolates and their diagnosis with classical methods. KSU J. Agric Nat. 2022; 25: 1; 149-157. https://doi.org/10.18016/ksutarimdoga.vi.976158
- Bozkurt İA, Soylu S, Kara M, Doksöz SF, Altan B, Çarpar H. Molecular characterization of stem gall disease caused by Agrobacterium tumefaciens (= Agrobacterium biovar 1) on Citrus trees as a new host, in the Eastern Mediterranean region of Turkey. Journal of Plant Diseases and Protection. 2023; 130: 13-21. https://doi.org/10.1007/s41348-022-00678-5.
- Khaoula H, Hiba Y, Salma B, Abdellatif B, Salma EIEH. Crown gall disease in Moroccan almond trees: tumorigenic bacteria and sustainable management through biological control. Afrimed AJ–Al Awamia. 2023; 140: 01-18. https://doi.org/10.34874/IMIST.PRSM/afrimed-i140.43733
- Doksöz F, Bozkurt İA. A new and simple pathogenicity test using carrot slices for Pseudomonas savastanoi pv. savastanoi, causal disease agent of olive knot. J Plant Pathol. 2020; 102: 1173-1177.
- Mirik M, Aysan Y, Sahin F. Characterization of Pseudomonas savastanoi pv. savastanoi strains isolated from several host plants in Turkey and report of fontanesia as a new host. J Plant Pathol. 2011; 263-270.
- Fodor A, Palkovics L, Végh A. Facts of oleander cancer in Hungary. Plant Protection. 2021; 57: 49-54.
- Fodor A, Palkovics L, Juhász Á, Végh A. Identification and comparison of Hungarian isolates of Pseudomonas savastanoi pv. nerii. Georgikon Agric Multidiscip J. 2022; 26: 219-231.
- Ophel K, Kerr A. Agrobacterium vitis sp. nov. for strains of Agrobacterium biovar 3 from 430 Grapevines. Int J Syst Bacteriol. 1990; 40: 236-241. https://doi.org/10.1099/00207713-40-3-236
- Mousavi SA, Willems A, Nesme X, de Lajudie P, Lindström K. Revised phylogeny of Rhizobiaceae: proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations. Syst Appl Microbiol. 2015 Mar;38(2):84-90. doi: 10.1016/j.syapm.2014.12.003. Epub 2014 Dec 27. PMID: 25595870.
- Hildebrand EM. Cane gall of brambles caused by Phytomonas n. sp. J Agric Res. 1940; 61:685–696.
- Starr MP, Weiss JE. Growth of phytopathogenic bacteria in a synthetic asparagin medium. Phytopathol. 1943; 33: 314–318.
- Babich H, Fox KD. Induction of crown gall on carrot slices. Am Biol Teach. 1998; 60:445–447. https://doi.org/10.2307/4450518
- Osborn AM, Moore ER, Timmis KN. An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ Microbiol.200; 2:39-50. https://doi.org/10.1046/j.1462-2920.2000.00081.x.
- Iacobellis NS, Caponero A, Evidente A. Characterization of Pseudomonas syringae ssp. savastanoi strains isolated from ash. Plant Pathol. 1998; 47:73-83. https://doi.org/10.1046/j.1365-3059.1998.00202.x
- Besenyei E, Hevesi M. Characterization of the Pseudomonas savastanoi pv. forsythiae pv. nov.- A novel pathovar of knot disease bacterium. Plant Protection. 2003; 39:123-128.
- King EO, Wand MK, Raney DE, 1954. Two simple media for the demonstration of pyocanin and fluorescin. J Lab Clin Med 44, 301-307.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
