Open Journal of Bacteriology
Bacteriology of chicken meat samples from Bharatpur, Chitwan, Nepal
Anup Subedee* and Namuna Paude
Cite this as
Subedee A, Paude N (2023) Bacteriology of chicken meat samples from Bharatpur, Chitwan, Nepal. Open J Bac 7(1): 016-024. DOI: 10.17352/ojb.000025Copyright License
© 2023 Subedee A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Many food-borne diseases are associated with the consumption of chicken meat and thus are of public health significance worldwide. A cross-sectional study was done to isolate, identify, and characterize bacteria from chicken meat samples of Bharatpur, Chitwan. A total of 102 samples were randomly collected and processed at the Microbiology laboratory of Birendra Multiple Campus, Chitwan for three months (May-July 2016). One gram of each sample was crushed on 9 ml distilled water in a sterile mortar pestle followed by serial dilution and inoculation of 0.1 ml sample into suitable culture media and incubated at 37 оC for 24 hours. Identification of the isolates was done by microscopic examination and biochemical tests and the antimicrobial susceptibility pattern of the isolates was determined by the Kirby Bauer disc diffusion method. Out of 102 meat samples, the growth positivity rate was 94.0% (n = 96/102) on all of the culture media. 26/48 fresh and 44/54 frozen samples gave positive growth with 36 isolates from fresh and 60 isolates from frozen meat samples, with the occurrence of Staphylococcus aureus 26(27.08%), Pseudomonas spp 6(6.25%), Proteus spp 4(4.16%), Escherichia coli 22(22.91%), Salmonella spp 16(16.66%), Citrobacter spp 8(8.33), Acinetobacter spp 8(8.33%), Streptococcus spp 2(2.08%), Shigella spp 4(4.16%) and Vibrio spp 2(2.08%). Cefalexin (92.85%) was the most effective antibiotic against Gram-positive bacteria followed by Amoxicillin (71.42%) and Methicillin (64.28%). The least effective antibiotic was Ampicillin (50%). Similarly, Gentamicin (76.47%) followed by Nalidixic acid (41.47%) were effective against Gram-negative bacteria, while some showed resistance to all three classes of drugs exhibiting MDR.
Introduction
The consumption of contaminated food is unsafe for health and its consequences have been one of man’s major health problems for a long time. They remain to be a major public health concern globally. Food-borne diseases are responsible for a large occurrence of adult illnesses and deaths; more importantly, as sources of acute diarrheal diseases, they are known to take the lives of many children every day [1]. The problem is severe in developing countries due to difficulties in securing optimal hygienic food handling practices. Evidently, in developing countries, up to an estimated 70% of cases of diarrheal disease are associated with the consumption of contaminated food especially meat and meat products [1]. Transmission of entero-pathogenic bacteria is affected directly or indirectly through objects contaminated with feces. These include food and water indicating the importance of fecal-oral human-to-human transmission [2]. Chicken is a rich source of meat protein and is highly consumed all over the world. However, under a poor hygienic environment, raw chicken meat presents an ideal substrate supporting the growth of pathogenic Escherichia coli and coliform bacteria, indicating the potential presence of other pathogenic bacteria; this may even constitute a major source of food-borne illnesses in humans. Chicken is a nutritious, healthy food that is low in fat and cholesterol compared to other meats but an excellent source of protein. Meat must be of high microbiological quality to ensure that the consumer receives a product that is not spoilt or does not carry food-borne diseases [3]. Special attention in poultry meat production is paid to the fact that live animals are hosts to a large number of different microorganisms residing on their skin, feathers, or in the alimentary tract. During slaughter, most of these microorganisms are eliminated, but subsequent contamination is possible at any stage of the production process, from feather plucking, evisceration, and washing to storage by cooling or freezing [4]. Microorganisms from the environment, equipment, and operators hands can contaminate the meat. The increased prevalence of Salmonella contamination in poultry has gained considerable scientific attention during the last few decades. Poultry is one of the most common reservoirs of Salmonella and contamination of poultry products can occur during the different stages of poultry production. Poultry is a food that has been highly appreciated by man since time immemorial. It is an important, low-cost source of animal protein, rich in nutrients, phosphorus, other minerals, and B-complex vitamins [5]. Food-borne diseases associated with the consumption of poultry meat and its processed products are of public health significance worldwide [6].
Poultry and poultry meat are often found contaminated with potentially pathogenic microorganisms such as Salmonella, Campylobacter, S. aureus, E. coli, and Listeria. Microorganisms introduced from environmental exposure, lack of sanitation in slaughtering premises, equipment, and outfits, and operators’ hands contaminate the meat product [7]. Chicken meat has higher pathogenic and spoilage bacterial counts than most other foods, where meat can be contaminated at several points throughout the processing operation during scalding, de-feathering, and evisceration as well as cross-contamination from other birds and processing equipment. The meat surface does not normally, inherently contain pathogenic organisms but can acquire the organisms from fecal matter or cross-contamination during slaughter. Ensuring a safe food supply has been a continuous challenge following the recognition of more and more pathogenic bacteria. Despite modern innovations in slaughter hygiene and food production techniques, food safety has been at the forefront of public health issues [8]. The organisms tend to remain on the surface or just under it. Meat is an ideal medium for bacterial growth because of its high moisture content, richness in nitrogenous compounds (essential amino acids, proteins), and a good source of minerals, vitamins, and other growth factors. Furthermore, its pH is favorable for the growth of micro-organisms too [9]. The progressive increase in antimicrobial resistance among enteric pathogens in developed and developing countries has become a critical area of concern [10]. Previous studies have shown that food-borne pathogens, such as Escherichia coli and Salmonella, are highly prevalent, and have been isolated in stool samples from humans affected by food-borne illnesses, as well as in the meat and poultry products processed for human consumption [11-14]. Ensuring a safe food supply has been a continuous challenge following the identification of more and more pathogenic bacteria. Despite modern innovations in slaughter hygiene and food production techniques, food safety has been at the forefront of public health issues [8]. The safety of commercially processed poultry products is a major area of concern for producers, consumers, and public health officials worldwide for products excessively contaminated with microorganisms are undesirable from the standpoint of public health, storage quality, and general aesthetics [15]. The contamination of chicken meat with microorganisms during processing, handling, and transportation is undesirable, though inevitable. A higher bacterial load on the carcass could be expected when carcasses are handled unhygienically at the abattoir [16]. Two of the most common etiologic bacterial organisms responsible for causing gastroenteritis, a major public health concern in most regions of Thailand, are Salmonella and E. coli [17,18]
Several studies have reported an outbreak of infections due to consumption of contaminated food and poor hygiene and in most of the cases, data are loosely based on laboratory isolates which do not reflect the actual ratio of food-borne infections. However, a few community-based reports provide evidence of outbreaks caused by Salmonella, Shigella, E. coli, and Listeria spps in different parts of the world [19]. Moreover, antibiotic resistance levels are also elevated among food-borne pathogens such as Salmonella and Shigella [20]. Meat, a good source of animal protein along with sensory attributes, appeals to consumers very easily.
Materials and methods
This was a cross-sectional study carried out at the microbiology laboratory of Birendra Multiple Campus, Bharatpur during a period from May to July 2016, and the samples were collected from different wholesalers and retailer meat shops in Bharatpur, Nepal.
All of the slaughter slabs, and wholesale and retail chicken meat shops in Bharatpur ward no. 7, 8, and 10, Chitwan, Nepal were visited and butchers were interviewed.
Collection of samples
A total of one hundred-two random samples of chicken carcasses were collected from local commercial retail shops and wholesale shops in Bharatpur sub-metropolitan municipality. The collected samples were kept in separate sterile plastic bags and transferred directly to the laboratory in an insulated icebox under complete aseptic conditions without any delay to evaluate their bacteriological quality. Samples with improper labeling and inappropriate collection were also rejected.
Preparation of samples (USDA 2011)
One gram of the examined samples was removed by sterile scissors and forceps after surface sterilization by a hot spatula, transferred to a sterile polyethylene bag, and 9 ml of 0.1 % sterile buffered peptone water was aseptically added to the content of the bag. Each sample was then homogenized by a mortar and pestle to provide a homogenate of 1/10 dilution.
Bacterial culture
The collected samples were immediately processed without storage. Samples homogenized with peptone water were incubated at 37 °C for 5 hours, and then one loopful of the culture was streaked on Mannitol salt agar for Gram-positive bacteria, MacConkey agar for Gram-negative bacteria, Eosin Methylene Blue agar for Coliforms, especially, E. coli and Xylose lysine deoxycholate agar for the identification of Salmonella spp. and incubated at 37 °C for 24 hours. Further, the suspected, isolated colonies were sub-cultured on Nutrient agar and Gram staining for morphological identifications, and different biochemical tests (IMViC, catalase, oxidase, urease, coagulase, etc.) were carried out.
Biochemical characteristic tests
Identification of bacterial isolates was carried out based on their cultural (appearance; pigmentation, consistency, margin, and elevation), morphological (Gram’s staining, size, and shape), and biochemical characteristics (catalase, oxidase, indole, coagulase tests, citrate utilization, Methyl red and Voges- Proskauer tests, Triple sugar iron, SIM tests, etc.) [21-23].
Disc diffusion susceptibility test
Susceptibility tests were performed by the disc diffusion method [24,25]. The turbidity of the inoculums should be adjusted to the equivalent turbidity of 0.5 McFarland standards. An 18 hours culture of test organisms incubated at 37 °C was standardized by diluting to 0.5 McFarland turbidity standard before spreading over the surface of Mueller Hinton agar (MHA) (Titan Biotech Ltd. Bhiwadi-301019, Rajasthan, India.) plates using sterile cotton swab/glass spreader [26] and allowed to dry for 2 to 5 minutes. Using sterile tweezers, antimicrobial discs ampicillin (10 mcg), nalidixic acid (30 mcg), nitrofurantoin (300 mcg), trimethoprim (5 mcg), Gentamycin (10 mcg), methicillin (5 mcg), amoxicillin (30 mcg) and Azithromycin (15 mcg) were placed widely spaced aseptically on the surface of MHA plate. Tweezers were re-flamed after the application of each disc. The plates were then incubated at 37 °C for 24 hours. Following incubation, the Diameter of the Inhibition Zone (DIZ) was measured with a transparent ruler and expressed in millimeters (mm).
Quality control for test
In this study, the quality and accuracy of all tests were maintained by following standard procedures of collection, isolation, and identification. For identification and standardization by the Kirby-Bauer test, a standard culture of E. coli ATCC 25922 was used as a reference strain. For quality control, media, antibiotics, and reagents were prepared, stored, and utilized as recommended by the manufacturing company. Antibiotic discs were stored at refrigerator temperature. For each batch of the test, a positive and negative known culture was used for color reaction, biochemical tests, and antibiotic sensitivity tests.
Statistical analysis
Data entry, management, and analysis were done using SPSS v20. The association between different risk factors and the antibiotic resistivity pattern of isolated bacteria was compared statistically by a Chi-square (χ [2]) test.
Result
The pattern of growth of chicken meat sample
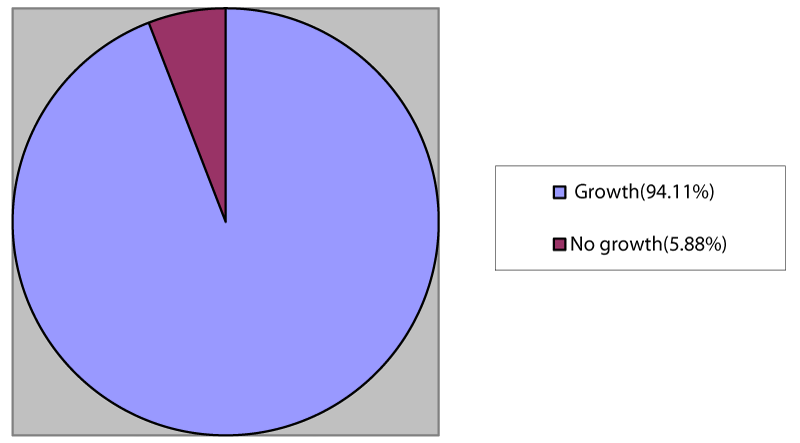
Out of 102 meat samples, growth was observed in 96 (94.11%) samples, and no growth was observed in 6 (5.88%) samples (Figure 1).
Differentiation based on gram’s reaction
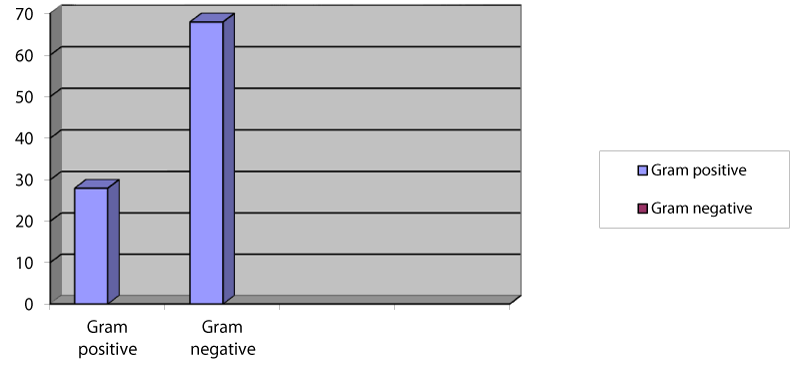
Out of 96 positive cultures, 28 were Gram-positive, while the rest 68 isolates were Gram-negative (Figure 2).
The growth pattern of total isolates
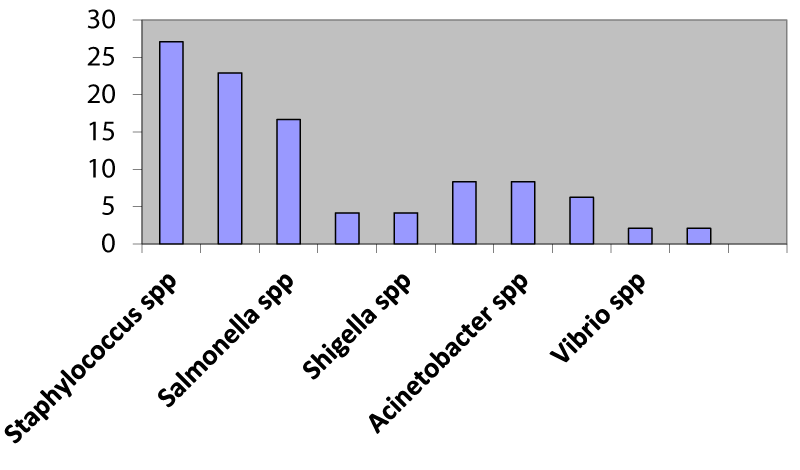
Out of 102 samples, 40 fresh meat (39.21%) and 56 frozen meat (60.78%) samples were taken. Of which 96 bacteria were isolated i.e, 26(27.08%) Staphylococcus aureus spp, 22(22.91%) E. coli spp, 14(14.58%) Salmonella spp, 4(4.16%) Proteus spp, 4(4.16%) Shigella spp, 8(8.33%) Citrobacter spp, 8(8.33%) Acinetobacter spp, 6(6.25%) Pseudomonas spp, 2(2.08%) Vibrio spp, 2(2.08%) Streptococcus spp (Figure 3).
Antimicrobial susceptibility pattern
The antibiotic discs used for Gram-positive isolates were Cefalexin (CN), Amoxycillin (AMX), Methicillin (MET), Azithromycin (AZM), and Ampicillin (AMP) while for Gram-negative isolates, Gentamicin (GEN), Nalidixic acid (NA), Trimethoprim (TR), and Nitrofurantoin (NIT) were used. Cefalexin (92.85%) was the most effective antibiotic against Gram-positive bacteria followed by Amoxicillin (71.42%) and Methicilin (64.28%), while the least effective antibiotic was Ampicillin (50.00%). Similarly, Gentamicin (76.47%) followed by Nalidixic acid (41.71%) were effective against Gram-negative bacteria, while the least effective antibiotic was Trimethoprim (36.76%). All the antibiotics used were broad-spectrum antibiotics (Table 1).
Antimicrobial susceptibility pattern of Staphylococcus aureus
26(27.08%) Staphylococcus aureus was isolated from the meat sample. Of which, Cefalexin (92.30%) followed by Amoxycillin (69.23%) was the most effective drug against the isolated species (Table 2).
Antibiotic susceptibility pattern of Streptococcus spp
2(2.08%) Streptococcus spp were isolated from the meat sample. Of which, Ampicillin (100%) followed by Amoxycillin (100%), Methicillin (100%), Cefalexin (100%), and Azithromycin (100%) was the most effective drug against the isolated species (Table 3).
Antibiotic susceptibility pattern of E. coli
22(22.91%) E. coli were isolated from the meat samples. Of which, Nitrofurantoin (72.72%) was the most effective drug followed by Gentamicin (54.54%), Trimethoprim (36.36%), and Nalidixic acid (36.36%) (Table 4).
Antibiotic susceptibility pattern of Shigella spp
4(4.16%) Shigella spp were isolated from meat samples. Of which, Gentamicin (100%) was the most effective drug (Table 5).
Antibiotic susceptibility pattern of Citrobacter spp
8(8.33%) Citrobacter spp were isolated from meat samples. Of which, Gentamicin (75%) was the most effective drug, followed by Trimethoprim (50%) then Nitrofurantoin (25%), and Nalidixic acid (25%) (Table 6).
Antibiotic susceptibility pattern of Acinetobacter spp
8(8.33%) were isolated from meat samples. Of which, Gentamicin (100%) was the most effective drug, followed by Nitrofurantoin (50%) and Nalidixic acid (25%) (Table 7).
Antibiotic susceptibility pattern of Proteus spp
4(4.16%) of Proteus spp was isolated from the meat samples. Of which, Trimethoprim (100%) was the most effective drug used against Proteus spp followed by Gentamicin (50%) and Nalidixic acid (50%) (Table 8).
Antibiotic susceptibility pattern of Vibrio spp
2(2.08%) of Vibrio spp were isolated from meat samples. Of which Gentamicin (100%) was the most effective drug (Table 9).
Antibiotic susceptibility pattern of Pseudomonas spp
6(6.25%) of Pseudomonas were isolated from the meat samples. Of which Gentamicin (100%) was the most effective drug followed by Nalidixic acid (66.66%) (Table 10).
Antibiotic susceptibility pattern of Salmonella spp
14(14.58%) Salmonella spp were isolated from the meat samples. Of which Gentamicin (85.71%) was the most effective drug, followed by Nalidixic acid (71.42%), and Trimethoprim (50%) (Table 11).
Multi-drug resistant organisms
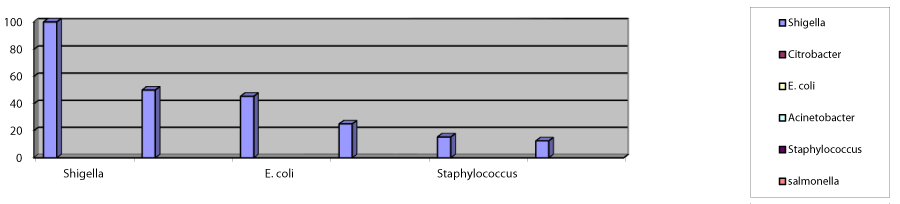
The highest number of MDR isolates was Shigella spp 4(100%) followed by Citrobacter 4(50%), E. coli (45.45%), Acinetobacter 2(25%), Staphylococcus aureus 4(15.38%), and Salmonella 2(12.50%). The prevalence of MDR among total isolates was found to be 27.08% (Figure 4).
Age-wise distribution of the butchers and distribution of bacteria among fresh and frozen meat samples
Among 96 isolates, the highest number of isolates 60(62.5%) was recorded from the age group 16 years - 30 years. The lowest number of isolates were detected from age group 46 - 60 accounting for 4(4.167%). Lastly, 32(33.33%) bacterial isolates were detected from the age group of 31 - 45 summing both fresh and frozen meat samples. The prevalence of bacteria in fresh and frozen meats was not significantly affected by the age of butchers (p > 0.05) (Table 12).
Sex-wise distribution of butchers and distribution of bacteria among fresh and frozen meat samples
Among 96 isolates, 22(22.91%) bacteria were detected from fresh meat handled by male butchers, while 14(14.58%) isolates were detected from fresh meat handled by female butchers. Similarly, 42(43.75%) isolates were detected from frozen meat provided by male meat sellers, while 18(18.75%) bacteria were isolated from frozen meat provided by female meat sellers. From the above data collected, meat collected from male butchers was more highly contaminated than meat sold by female butchers as the number of isolates in the case of a male was quite higher than that of a female. There was no significant association between the sex of the respondents and the quality of the products being sold p > 0.05 (Table 13).
Distribution of bacteria among the type of shops and distribution among fresh and frozen meat samples
Among 96 isolates, 20(20.83%) bacteria were isolated from fresh meat taken from retailer shops, while 48(50%) isolates were detected from frozen meat samples taken from retailer shops. Similarly, 16(16.66%) isolates were detected from fresh meat samples collected from wholesale shops, while 12(12.50%) isolates were detected from frozen meat samples taken from wholesale shops. From the above data collected the number of bacterial isolates was higher from frozen meats which were taken from retail shops. However, there was no significant association between the type of shops and the distribution of bacteria among fresh and frozen meat samples p > 0.05 (Table 14).
Distribution of bacteria among literacy groups and the occurrence of isolates from fresh and frozen meat samples
Among 96 isolates, 20(20.83%) bacteria were isolated from fresh meat provided by literate butchers, while 34(35.41%) bacteria were isolated from frozen meat taken from literate meat sellers. Similarly, 16(16.66%) bacterial isolates were detected from fresh meat provided by illiterate butchers, while 26(27.08%) isolates were identified from frozen meat samples taken from illiterate butchers. The number of bacterial isolates was higher in the case of frozen meat collected from literate meat sellers. There is no significant association between the literacy group and the occurrence of bacteria in fresh and frozen meat samples p > 0.05 (Table 15).
Discussion
Bacterial contamination of chicken meat can be from the chicken itself, workers, tools, and types of equipment as well as hygiene in the slaughterhouse. In the present study performed, the most frequently isolated bacteria were Staphylococcus aureus, 1Escherichia coli, Citrobacter spp, Acinetobacter spp, Shigella spp, and Salmonella spp. respectively. Out of 102 samples, 40 fresh lumps of meat (39.21%) and 56 frozen lumps of meat (60.78%) samples were taken. Of which 96 bacteria were isolated i.e. 26(27.08%) Staphylococcus aureus spp, 22(22.91%) E. coli spp, 14(14.58%) Salmonella spp, 4(4.16%) Proteus spp, 4(4.16%) Shigella spp, 8(8.33%) Citrobacter spp, 8(8.33%) Acinetobacter spp, 6(6.25%) Pseudomonas spp, 2(2.08%) Vibrio spp, 2(2.08%) Streptococcus spp.
In the study conducted in Chitwan, antimicrobial drugs were used for AST. Ampicillin, Amoxycillin, Methicillin, Cefalexin, and Azithromycin were used against Gram-positive isolates while Nitrofurantoin, Gentamicin, Trimethoprim, and Nalidixic acid were used against Gram-negative isolates. Among 28 Gram-positive isolates, 14(50%) were resistant to Ampicillin, 28.68% were resistant to Amoxycillin, 35.71% were resistant to Methicillin, and 7.41% were resistant to Cefalexin and 35.71% were resistant to Azithromycin. Among 68 Gram-negative isolates, 55.88% were resistant to Nitrofurantoin, 17.64% were resistant to Gentamicin, 63.33% were resistant to Trimethoprim and 38.23% were resistant to Nalidixic acid.
Hence, among all the antibiotics used for Gram-positive isolates, most strains of bacteria were resistant to Ampicillin (50%). And in the case of Gram-negative isolates, most bacteria were resistant to Trimethoprim (63.23%).
Among 26 Staphylococcus spp isolated from our study, only 4 of them (15.38%) showed Multi-Drug Resistance (MDR) properties i.e. among 5 antimicrobial drugs used, the isolated spp were resistant to more than 2 classes of drugs. Similarly, out of 22 isolated E. coli 10(45.45%) showed MDR, while 14.28% of Salmonella were multi-drug resistant. Likewise, 100% Shigella spp, 50% Citrobacter, and 25% Acinetobacter were MDR. The highest number of MDR isolates was Shigella spp 4(100%) followed by Citrobacter 4(50%), E. coli (45.45%), Acinetobacter 2(25%), Staphylococcus aureus 4(15.38%), and Salmonella 2(12.50%).
Among 96 isolates, the highest number of isolates 60(62.5%) was recorded from the age group 16-30 years. The lowest number of isolates were detected in the age group 46 - 60 accounting for 4(4.167%). Lastly, 32(33.33%) bacterial isolates were detected from the age group of 31 - 45 summing both fresh and frozen meat samples. The prevalence of bacteria in fresh and frozen meats was not significantly affected by the age of butchers (p > 0.05). Among 96 isolates, 22(22.91%) bacteria were detected from fresh meat handled by male butchers, while 14(14.58%) isolates were detected from fresh meat handled by female butchers. Similarly, 42(43.75%) isolates were detected from frozen meat provided by male meat sellers, while 18(18.75%) bacteria were isolated from frozen meat provided by female meat sellers. From the above data collected, meat collected from male butchers was more highly contaminated than meat sold by female butchers as the number of isolates in the case of the male was quite higher than that of females. There was no significant association between the sex of the respondents and the quality of the products being sold p > 0.05.
Among 96 isolates, 20(20.83%) bacteria were isolated from fresh meat taken from retailer shops, while 48(50%) isolates were detected from frozen meat samples taken from retailer shops. Similarly, 16(16.66%) isolates were detected from fresh meat samples collected from wholesale shops, while 12(12.50%) isolates were detected from frozen meat samples taken from wholesale shops. From the above data collected the number of bacterial isolates was higher from frozen meats which were taken from retail shops. However, there was no significant association between the type of shops and the distribution of bacteria among fresh and frozen meat samples p > 0.05.
Among 96 isolates, 20(20.83%) bacteria were isolated from fresh meat provided by literate butchers, while 34(35.41%) bacteria were isolated from frozen meat taken from literate meat sellers. Similarly, 16(16.66%) bacterial isolates were detected from fresh meat provided by illiterate butchers, while 26(27.08%) isolates were identified from frozen meat samples taken from illiterate butchers. The number of bacterial isolates was higher in the case of frozen meat collected from literate meat sellers. There is no significant association between the literacy group and the occurrence of bacteria in fresh and frozen meat samples p > 0.05.
Meat is the most perishable of all-important foods since it contains sufficient nutrients needed to support the growth of microorganisms. The annual production of chicken meat in Nepal is 16662 metric tons and the annual production of chicken meat in the Chitwan district is 1422 metric tons. So, Chitwan has an 8.5 percent share of chicken meat production annually in Nepal. Chicken meat can be contaminated at several points throughout the processing operations. Moreover, retail cuts could result in greater microbial load owing to a large amount of exposed surface area, more readily available water, nutrients, and greater oxygen penetration which leads to spoilage of meat. Meat-borne zoonotic diseases such as Salmonellosis, Campylobacteriosis, E. coli enteritis, and food poisoning by Clostridium, Staphylococcus, etc. are the major problems encountered by consumers eating contaminated meat. Only little is known about microbial aspects, shelf life, and food safety of commercial retail chicken meat in Chitwan. The poultry slaughtered and dressed under Chitwan conditions carrying high initial contamination would be exhibited to the point the consumers are offered as retail meat. So, retail meat would harbor all the bacteria that are already present in meat as inherent contamination through infection and that are introduced during handling, improper dressing, cleaning, unsanitary conditions, and retailing. To increase meat quality, assurance following microbial load assessment is deemed necessary. Hence, this study was conducted to assess the microbiological situation of fresh chicken meat which can be the reflection of the hygienic condition of meat consumed and the possible hazards to public health. Chicken meat can also act as a reservoir of drug-resistant bacteria. Antimicrobial resistance among E. coli, Salmonella, and other species in chicken meat is of increasing concern due to the potential for the transfer of these resistant pathogens to the human population. Most of these genera are known to be of public health concern and have been associated with cases of gastroenteritis and other food-borne diseases [27]. The sources of these contaminations have been linked to poor hygienic conditions of the handlers, the environment, and cross-processing contaminations [28-30]. Among the 102 meat samples, only two did not produce any microorganisms when incubated at 37 0C. Of the samples, 58 contained Coliform bacteria, 58 contained S. aureus, 56 showed Pseudomonas growth, and 38 of them contained E. coli. Among the samples, 32 out of the 58 samples were S. aureus–positive. The susceptibility results of bacteria isolated from meat samples showed that they are highly resistant to all the antibiotics tested. Gram-negative organisms are more resistant than Gram-positives; this is expected because of the intrinsic nature of the gram-negative cell wall. The gram-negative micro-organisms isolated belong to the Enterobacteriaceae family, this group of organisms is always resistant to various classes of antibiotics [31-53].
Conclusion
A total of 102 chicken meat samples were collected from different retail and wholesale shops, out of which 40 fresh and 56 frozen samples were growth positive while 6 samples were growth negative. The samples were collected from shops with 64 male and 38 female butchers. The results of the present study indicated that the prevalence of common food-borne pathogens in the market samples of chicken meat in Bharatpur, Chitwan was at the higher levels. On antimicrobial susceptibility testing, Ampicillin was the most effective antibiotic against Gram-positive bacteria followed by Amoxicillin, Cefalexin, and Azithromycin. Most of the isolated Staphylococcus were Methicillin-resistant and some exhibited MDR too. Similarly, Gentamicin followed by Trimethoprim and Nalidixic acid were effective against Gram-negative bacteria, while some showed resistance to all three classes of drugs exhibiting MDR. The highest MDR organism isolated was Staphylococcus aureus followed by E. coli and Salmonella spp. There wasn’t a significant association between the type of meat shops and the condition of the meat samples (p > 0.05). Similarly, the association was absent between the age of the meat sellers and the occurrence of pathogens in fresh and frozen meat samples. Hence, we cannot say that the age factor is responsible for causing more contamination of meat products. Likewise, there was no correlation between the sex of butchers and the hygiene of the meat, so we cannot say that male butchers handled meat improperly so the number of isolated bacteria was higher though the collected data suggested that result. The present study provided us with an idea about the occurrence of pathogens in chicken meat in correlation with different risk factors and helped policymakers to build rules and regulations that strengthen the quality of health of people consuming meat and meat products.
- Keener KM, Bashor MP, Curtis PA, Sheldon BW, Kathariou S. Comprehensive Review of Campylobacter and Poultry Processing. Compr Rev Food Sci Food Saf. 2004 Apr;3(2):105-116. doi: 10.1111/j.1541-4337.2004.tb00060.x. PMID: 33430546.
- Sanath Kumar H, Otta SK, Karunasagar I, Karunasagar I. Detection of Shiga-toxigenic Escherichia coli (STEC) in fresh seafood and meat marketed in Mangalore, India by PCR. Lett Appl Microbiol. 2001 Nov;33(5):334-8. doi: 10.1046/j.1472-765x.2001.01007.x. PMID: 11696091.
- Singh S, Yadav AS, Singh SM, Bharti P. Prevalence of Salmonella in chicken eggs collected from poultry farms and marketing channels and their antimicrobial resistance. Food. Research. Intern. 2010; 43: 2027-2030.
- Yang B, Qu D, Zhang X, Shen J, Cui S, Shi Y, Xi M, Sheng M, Zhi S, Meng J. Prevalence and characterization of Salmonella serovars in retail meats of marketplace in Shaanxi, China. Int J Food Microbiol. 2010 Jun 30;141(1-2):63-72. doi: 10.1016/j.ijfoodmicro.2010.04.015. Epub 2010 Apr 29. PMID: 20493570.
- Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010 Jan 15;50(2):241-6. doi: 10.1086/649541. PMID: 20014951; PMCID: PMC2798017.
- Tambekar DH, Hirulkar NB, Waghmare AS. MAR indexing to discriminate the source of fecal contamination in drinking water. Nat Environ Poll Tech. 2005; 4:525–8.
- Montville TJ, Matthews KR. Food microbiology: An introduction 2nd ed. ASM Press, Washington, United States of America. 2008.
- Bauer AW, Kirby WM, Sheris JC, Turck M (1966). Antibiotic susceptibility testing by standardized single disc method. Ame. J. Clin. Pathol. 45:493-496.
- Cheesbrough M. District Laboratory Practices in Tropical Countries. Volume 2 part 2 Cambridge University Press, UK.Crossref. 2006.
- Forbes BA, Sahm DE, Weissfeld AS. Bailey and Scott’s Diagnostic Microbiology. International edition. 12th edn. Mosby, Inc., an affiliate of Elsevier, Inc., USA. 2007.
- Fraizer W, Westhoff D. Food Microbiology. 4th ed. Tata McGraw-Hill Publication Company Ltd, New Delhi, India. 2005.
- FAO. Poultry Meat & Eggs. Investment Centre Division.Vialedelle Terme di Caracalla, 00153 Rome, Italy. 2010.
- Rabie S, Khalifa NO, Radwan MEI, Afify JSA. Epidemiological and Molecular Studies of Salmonella Isolates from Chicken, Chicken Meat and Human in Toukh, Egypt. Global Veterinaria. 2012; 8:128-132.
- Sharaf EM, Sabra SM. Microbiological loads for some types of cooked chicken meat products at Al-Taif Governorate, KSA. World. Appl. Sci. J. 2012; 17:593-597.
- Javadi A, Saeid S. Microbial profile of marketed broiler meat. Middle-East J. Sci. Res. 2011; 9:652-656.
- Ruban SW, Thyageeswaran M, Sharadha R. Isolation and identification of Salmonella spp from retail chicken meat by polymerase chain reaction. Intl. J. Micro. Res. 2010; 1:106-109.
- Rindhe SN, Zanjad PN, Doifode VK, Siddique A, Mendhe MS. Assessment of microbial contamination of chicken products sold in Parbhani city. Vet. World. 2018; 1:208-210.
- Prakash B, Perumal P, Jegadeesh KD, Tamilmani P. Molecular characterization of plasmid mediated esbl resistant Salmonella isolated from poultry environment in Namakkal district (India). Adv. Biotech. 2012; 12:2.
- Ramya P, Madhavarao T, Rao L. Study on the incidence of Salmonella enteritidis in poultry and meat samples by cultural and PCR methods. Vet. World. 2012; 5:541-545.
- Ruban SW, Prabhu NK, Naveen Kumar GS. Prevalence of food borne pathogens in market samples of chicken meat in Bangalore. Int. Food Res. J. 2012; 19:1763-1765.
- Saikia P, Joshi SR. Retail market poultry meats of North-East India – A microbiological survey for pathogenic contaminants. Research. J. Microbiol. 2010; 5:36-43.
- Sengupta R, Das R, Ganguly S, Mukhopadhayay SK. Survey on microbial quality of chicken meat in Kolkata, India. Int. J. Res. in Pure and Appl. Microbiol. 2011; 1:3; 32-33.
- Turtura GC. Enterobacteriaceae and Other Gram Negative Bacteria in Slaughtered Poultry. Microbiology Ailments and Nutrition. 1991; 9(2):139-146.
- Cheesbrough M. Medical Laboratory Manual. Tropical Health Technology, Low priced Edition. Doddington, Cambridgeshire, England. 2003; 20-35.
- Clarence SY, Obinna CN, Shalom NC. Assessment of bacteriological quality of ready to eat food (Meat pie) in Benin City metropolis. Nigeria. Afr. J. Microb. Res. 2009; 3(6): 390-395.
- Dhakal IP, Manandhar P. Isolation of Salmonella in the Pooled Samples of Litter, Food and Water in Chitwan Poultries. In: Proceedings on National Poultry Expo-2005, Chitwan, December 15-19, 2005;43-46.
- Acharya B. Isolation of Salmonella in Poultry meat in Kathmandu, Lalitpur, Bhaktapur, Chitwan district. A B.V.Sc. & A.H. Internship thesis submitted to Tribhuvan University. 2007.
- Tiwari R. Isolation and Identification of Salmonella from Broiler in Chitwan district. Internship thesis submitted to Tribhuvan University. 2002.
- Darshana BD. Thyagarajan R. Richard N. Punniamurthy. Bacterial pathogens in chicken meat. Veterinary and Animal Sciences University, Chennai, India. 2014; 2:3; 1-7: 2348-3148.
- Mead C. Hygiene Problems and Control of Process Contamination. In: Processing of poultry. (Mead G. C., Ed.), Elsevier Science Publishers Ltd. 1989; 183-220.
- White DG, Zhao S, Simjee S, Wagner DD, McDermott PF. Antimicrobial resistance of foodborne pathogens. Microbes Infect. 2002 Apr;4(4):405-12. doi: 10.1016/s1286-4579(02)01554-x. PMID: 11932191.
- Hanson R, Kaneene JB, Padungtod P, Hirokawa K, Zeno C. Prevalence of Salmonella and E. coli, and their resistance to antimicrobial agents, in farming communities in northern Thailand. Southeast Asian J Trop Med Public Health. 2002;33 Suppl 3:120-6. PMID: 12971491.
- APHA. Compendium of Methods for Microbiological Examination of Foods, Washington. 1994.
- Holds G, Pointon A, Lorimer M, Kiermeier A, Raven G, Sumner J. Microbial profiles of carcasses and minced meat from kangaroos processed in South Australia. Int J Food Microbiol. 2008 Mar 31;123(1-2):88-92. doi: 10.1016/j.ijfoodmicro.2007.12.007. Epub 2007 Dec 23. PMID: 18234385.
- Ishizaki N, Kaneko S, Itoh T, Jinbo K, Kataoka J, Kukturbo Y. The detection and sero-typing of Salmonella in commercial meat from 1989-1992 in Tokyo. Ann Rep. Met. Res. Lab. Pub. Health. 1993; 44: 101-104.
- Jerngklinchan J, Koowatananukul C, Daengprom K, Saitanu K. Occurrence of Salmonellae in Raw Broilers and Their Products in Thailand. J Food Prot. 1994 Sep;57(9):808-810. doi: 10.4315/0362-028X-57.9.808. PMID: 31121802.
- Kinsella KJ, Prendergast DM, McCann MS, Blair IS, McDowell DA, Sheridan JJ. The survival of Salmonella enterica serovar Typhimurium DT104 and total viable counts on beef surfaces at different relative humidities and temperatures. J Appl Microbiol. 2009 Jan;106(1):171-80. doi: 10.1111/j.1365-2672.2008.03989.x. Epub 2008 Nov 28. PMID: 19054240.
- Zweifel C, Fischer R, Stephan R. Microbiological contamination of pig and cattle carcasses in different small-scale Swiss abattoirs. Meat Sci. 2008 Mar;78(3):225-31. doi: 10.1016/j.meatsci.2007.06.025. Epub 2007 Jul 14. PMID: 22062274.
- Duffy G, Cloak OM, O’Sullivan MG, Guillet A, Sheridan JJ, Blair IS, McDowell DA. The incidence and antibiotic resistance profiles of Salmonella spp. on Irish retail meat products. Food. Microbiol. 1999; 16:623-631.
- Vázquez-Sánchez D, López-Cabo M, Saá-Ibusquiza P, Rodríguez-Herrera JJ. Incidence and characterization of Staphylococcus aureus in fishery products marketed in Galicia (Northwest Spain). Int J Food Microbiol. 2012 Jul 2;157(2):286-96. doi: 10.1016/j.ijfoodmicro.2012.05.021. Epub 2012 Jun 2. PMID: 22704064.
- EI-Jakee J, Marouf SA, Ata NS, Abdel-Rahman EH, Abd El-Moez SI, Samy AA, Walaa E, El-Sayed WE. Rapid method for detection of Staphylococcus aureus enterotoxins in food. Global Veterinaria. 2013; 3: 335-341.
- Awny NM, Abou Zeid AAM, Abdo MA. Prevalence of toxigenic bacteria in some Egyptian food. Proceeding of Fifth Scientific Environmental Conference. Zagazig Uni. 2010; 107-124.
- Adwan G, Abu-Shanab B, Adwan K. Enterotoxigenic Staphylococcus aureus in raw milk in the North of Palestine. Turkish Journal of Biology. 2005; 29: 229-232.
- Adwan G, Alqarem BR, Adwan KM. Prevalence of food-borne pathogens in meat samples in Palestine. International Food Research Journal. 2015; 22(5):1806-1812.
- Letellier A, Messier S, Paré J, Ménard J, Quessy S. Distribution of Salmonella in swine herds in Québec. Vet Microbiol. 1999 Jul 1;67(4):299-306. doi: 10.1016/s0378-1135(99)00049-8. PMID: 10466505.
- Limawongpranee S, Hayashidani H, Okatani AT, Ono K, Hirota C, Kaneko K, Ogawa M. Prevalence and persistence of Salmonella in broiler chicken flocks. J Vet Med Sci. 1999 Mar;61(3):255-9. doi: 10.1292/jvms.61.255. PMID: 10331198.
- Davies PR, Bovee FG, Funk JA, Morrow WE, Jones FT, Deen J. Isolation of Salmonella serotypes from feces of pigs raised in a multiple-site production system. J Am Vet Med Assoc. 1998 Jun 15;212(12):1925-9. PMID: 9638195.
- Bangtrakulnonth A, Pornreongwong S, Pulsrikarn C, Sawanpanyalert P, Hendriksen RS, Lo Fo Wong DM, Aarestrup FM. Salmonella serovars from humans and other sources in Thailand, 1993-2002. Emerg Infect Dis. 2004 Jan;10(1):131-6. doi: 10.3201/eid1001.02-0781. PMID: 15078609; PMCID: PMC3322742.
- Wilson IG. Salmonella and campylobacter contamination of raw retail chickens from different producers: a six year survey. Epidemiol Infect. 2002 Dec;129(3):635-45. doi: 10.1017/s0950268802007665. PMID: 12558349; PMCID: PMC2869928.
- Vindigni SM, Srijan A, Wongstitwilairoong B, Marcus R, Meek J, Riley PL, Mason C. Prevalence of foodborne microorganisms in retail foods in Thailand. Foodborne Pathog Dis. 2007 Summer;4(2):208-15. doi: 10.1089/fpd.2006.0077. PMID: 17600488.
- Odwar JA, Kikuvi G, Kariuki JN, Kariuki S. A cross-sectional study on the microbiological quality and safety of raw chicken meats sold in Nairobi, Kenya. BMC Res Notes. 2014 Sep 10;7:627. doi: 10.1186/1756-0500-7-627. PMID: 25204564; PMCID: PMC4167279.
- O'Brien TF. Emergence, spread, and environmental effect of antimicrobial resistance: how use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clin Infect Dis. 2002 Jun 1;34 Suppl 3:S78-84. doi: 10.1086/340244. PMID: 11988877.
- Mølbak K. Spread of resistant bacteria and resistance genes from animals to humans--the public health consequences. J Vet Med B Infect Dis Vet Public Health. 2004 Oct-Nov;51(8-9):364-9. doi: 10.1111/j.1439-0450.2004.00788.x. PMID: 15525367.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
