Extraction of silica from natural deposits for the production of silicon in photovoltaic applications
LMESM Laboratory, Physics Department, University of Science and Technology, Mohamed Boudiaf,(USTO-MB), Oran 31000, Algeria
Author and article information
Cite this as
Zeboudj A, Hamzaoui S (2023) Extraction of silica from natural deposits for the production of silicon in photovoltaic applications. Open J Bac. 2023; 7(1): 011-015. Available from: 10.17352/ojb.000024
Copyright License
© 2023 Zeboudj A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The Silica, primarily sourced from silicon dioxide (SiO2) is a common chemical compound abundant in nature, existing in various forms such as quartz, sand, glass, and diverse minerals. It finds extensive use across multiple industries, contributing significantly to glassmaking, ceramics, abrasives, refractory materials, and serving as a crucial component in semiconductor production for electronic chips.
This work provides a comprehensive review of the silica elaboration process aimed at obtaining silicon. The initial stage involves the preparation of raw materials, utilizing Algerian sand and diatomite, which undergo meticulous chemical treatment to eliminate unwanted impurities. This method comprises distinct steps, known as the purification process, wherein the sand or diatomite shell is assessed through dissolution in a caustic alkaline solution. This approach indicates the potential to generate high-quality silica from diatomite using an aqueous chemical process.
This paper explores the prospect of sand or diatomaceous earth as a novel source of premium-grade silica. It discusses the effects of hydrochloric acid as a solvent for purification. This study contrasts treatment methods before and after acid leaching, focusing on samples with larger particle sizes (ranging from 53 to 300 microns), shorter refining durations (1 to 6 hours), and relatively higher temperatures (30 °C to 70 °C). The findings reveal that the highest purity of silica was attained through HCl treatment (25%).
The increased demand for renewable energy sources has spurred extensive research and development in photovoltaic technologies. Silicon, whether in single-crystalline or multi-crystalline wafer form, remains dominant in the photovoltaic industry [1,2] due to its established fabrication processes and desirable electrical properties [3,4].
Silicon (Si) has long been acknowledged as the primary material in photovoltaic devices owing to its exceptional electrical characteristics and widespread availability [5,6].
Silicon primarily derived from silica [7,8], undergoes a purification process. recognizing that the deposits used often contain numerous impurities such as titanium, iron, magnesium, calcium, boron, potassium, and others [9,10]. Consequently, our endeavor involves eliminating these elements to obtain several silicas of varying purities, facilitating diverse applications [11,12].
Silica (SiO2) exists in various forms, either crystalline or amorphous. In nature, quartz represents the most common crystalline form of silica. It is widely present in rocks and belongs to the mineralogical group of silicates. Oxygen and silicon are the most abundant constituents of the Earth’s crust, and quartz makes up approximately 12% of its mass [13,14]. Silica is a refractory material characterized by several aspects [15,16].
- A melting point around 1600 °C
- A density close to 2.2
- Excellent thermal insulation capacity; approximately 1.5 Wm-1 k-1 at 20 °C
- A very low coefficient of thermal expansion around 0.5 x 10-6 from 20 °C to 1000 °C.
Materials and methods
Our aim to acquire highly pure silicon often involves extracting it from silica. that chemically composed of silicon dioxide (SiO2), where one silicon atom is bonded to two oxygen atoms, has been extracted in our case from raw materials like diatomaceous earth and sand. These sources can contain a range of impurities including iron, titanium, calcium, magnesium, copper, potassium, sodium, as well as various inorganic compounds such as carbonates, sulfates, phosphates, and oxides. These impurities can affect the characteristics and quality of silica, especially when intended for applications requiring high purity, like the semiconductor industry or high-end glass manufacturing. To attain highly pure silica, purification methods are used to eliminate or reduce these impurities. This silica, as the compound from which pure silicon is obtained, finds utility in various industrial and technological applications.
- The elaboration of silica:
The purification process encompasses multiple steps aimed at eliminating impurities and isolating silica from other minerals and compounds found in the extracted material.
In our case, we primarily relied on a chemical process involving leaching and chemical reactions to attain a high level of purity. For our experiments, we utilized two types of raw material.
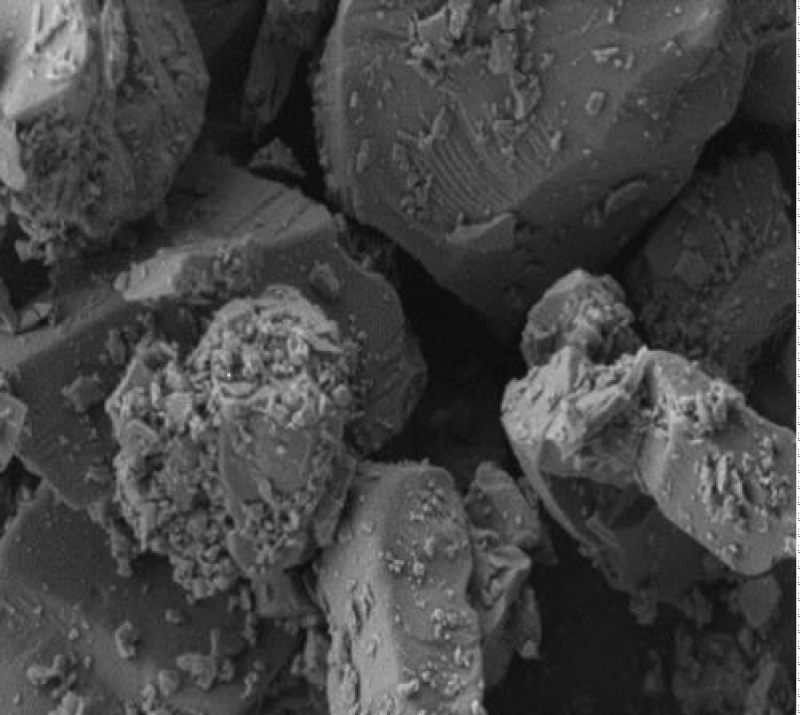
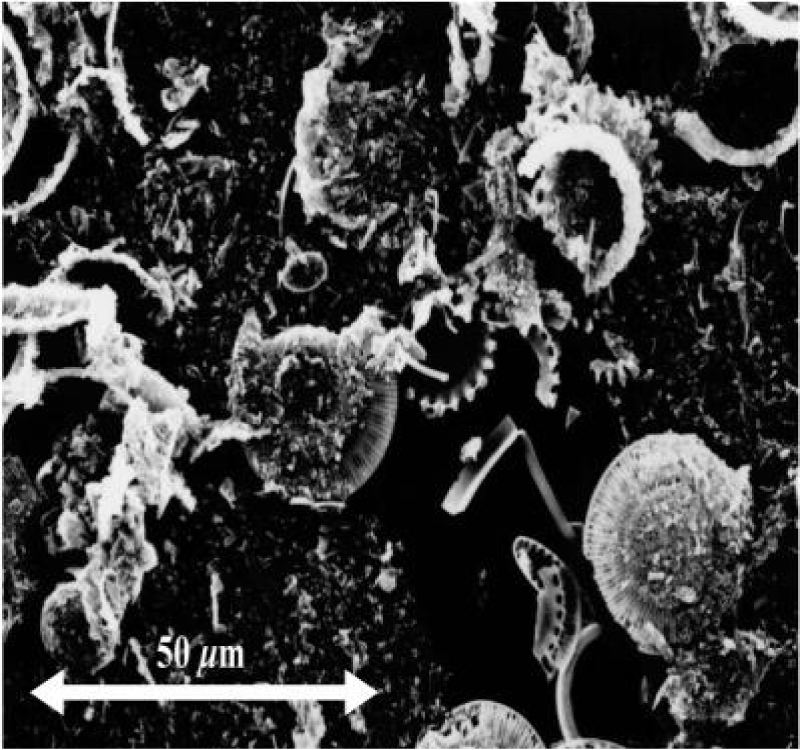
One composed of sand from North algerai which contains 92% silicon dioxide The particle size of this sand ranged between (400 μm and 50 μm) present in Figure 1, and the other is diatomaceous earth diatomaceous earth powder from western Algeria (Figure 2).
Both samples underwent treatment with Hydrochloric acid (HCl). This phase involved the creation of two solutions: the initial solution contained diatomaceous earth powder dissolved in HCl at a conce ntration of 2 mol/L, heated at 100 °C for 1 to 3 hours. The second solution consisted of sand mixed in a 2 mol/L HCl solution at room temperature for 24 hours. Subsequent to this stage, both samples underwent multiple rinses with distilled water to achieve a neutral pH.
The process is described by the following chemical reaction (eq1): [21,22]
SiO2 + 4 HCl → SiCl4 + 2 H2O (1)
The reaction between silicon dioxide and hydrochloric acid results in the formation of silicon tetrachloride (SiCl4) and water (H2O). Specifically, silicon dioxide reacts with the H+ ions from hydrochloric acid to produce hydrated silicon dioxide, which, being unstable, subsequently converts into silicon tetrachloride (SiCl4) and two molecules of water.
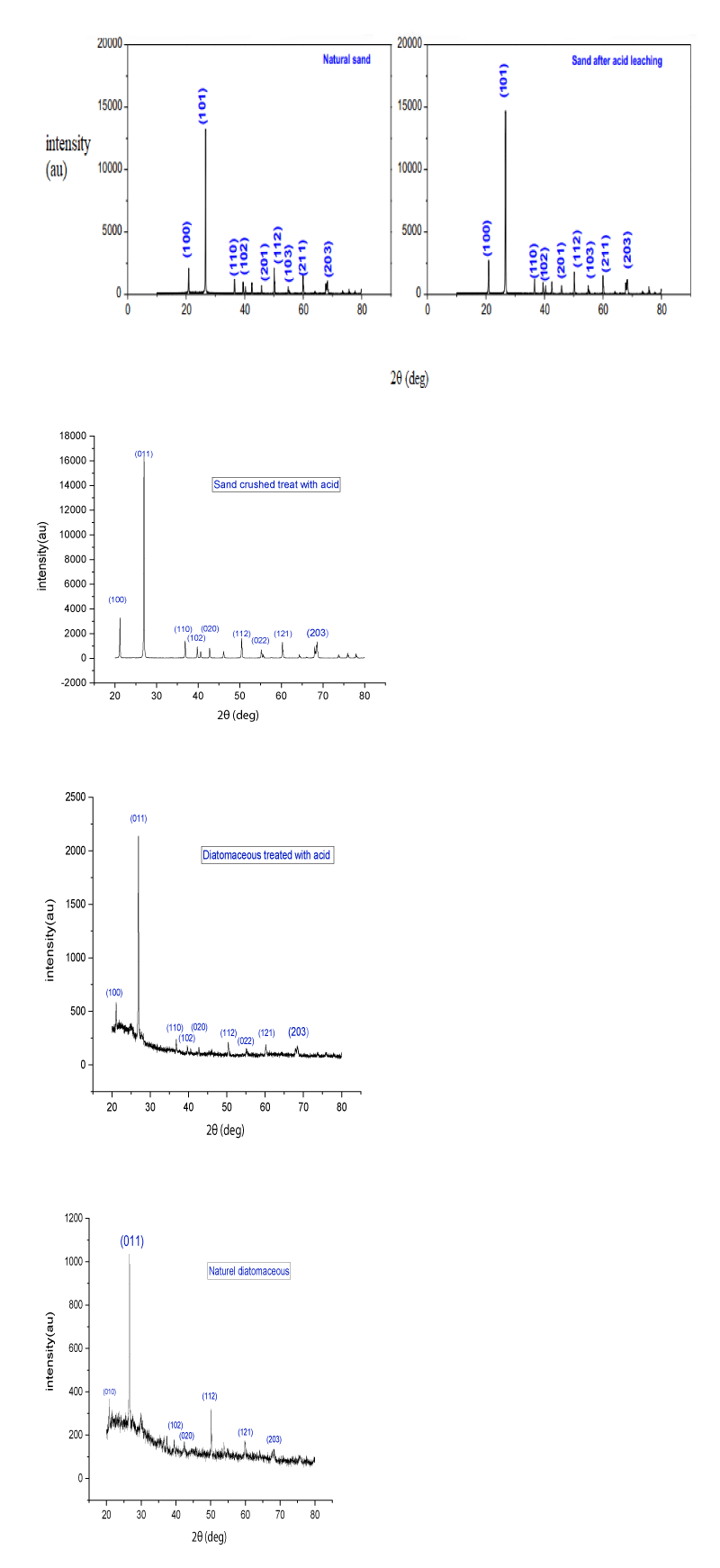
The X-ray Diffraction (XRD) Figure 3 characterization was made using a Shimadzu LabX XRD-6000 diffractometer with CuKα radiation (λ = 1.54059 Å). The diffractograms were recorded in the 2θ range of 20° - 80°, with a step size of 0.02° and 2 deg/min scan speed.
The morphological analysis was investigated by Scanning Electron Microscopy (SEM), using a JSM 6390 apparatus (JEOL Ltd.) Figures 4,5 with an accelerating voltage of 1.8 kV.
Results and discussion
Figure 3 illustrates the outcomes of the X-ray Diffraction pattern (XRD) for the samples. The X-ray analysis results underwent assessment using the X’Pert High Score software with the 2012 database. As per the graphs and database, the initial CaSi2 peaks register at 2θ = 27 and 29 degrees. Additionally, there’s another observed peak close to 2θ = 76 degrees, with the main peak apparent near 2θ = 47.305 degrees.
An additional impurity, composed of iron and silicon, appears in the sample with a chemical formula of Fe0 42Si2.. 67. According to the graph, its primary peaks significantly align at 47, 28, and 56 degrees. Another impurity containing aluminum with the chemical formula Al3.21Si0.47 is also detected in the sample, showing logical alignment at certain points. Moreover, the expansion of the sample peaks occurs due to the overlapping of impurity compounds, resulting in the aggregation of various phase peaks at specific angles, particularly within the 40 to 60-degree range.
A Scanning Electron Microscopy (SEM) picture of a representative The surface morphologies and composition(Table) of sand and diatomaceous sample is shown in Figure 1 and the composition in Table 1.
The XRD peaks for two samples, both before and after undergoing chemical treatment. The peak with the highest intensity was observed at 2θ = 26.68°; notably, its intensity increased following acid treatment (from 1028 to 2150 counts per second) in cas for diatomaceous and (from 11000 to 16000 counts per second) , as did the other peaks. However, the chemical treatment, specifically the acid treatment, did not affect the interplanar distance or the 2θ values (Table 2).
In this research, when comparing the purity levels before and after treatment of sand and diatomaceous earth to earlier studies, it was evident that the purity increased significantly, and impurities were more effectively removed. It was observed that Hydrochloric Acid (HCl) leaching, conducted at a concentration of 25%, selectively eliminated particular impurities. The efficacy of removing impurities from the raw material through chemical processes relies on the reactivity of the silica substrate and the susceptibility of impurities to the acid etching agents.
Conclusion
The current research focuses on the principles and advancements concerning impurity elimination from silica through acid leaching techniques. The overall conclusions and future prospects are summarized as follows:
Impurities found in MG-Si can be categorized into segregation impurities and insoluble impurities. While segregation impurities are easily exposed, insoluble impurities remain partially concealed when the MG-Si bulk is pulverized. Therefore, addressing insoluble impurities is crucial to attain the desired high-purity silicon.
A novel chemical process for refining silica sourced from diatomaceous earth is proposed based on geological and chemical characteristics. This approach involves controlling impurities in amorphous silica through a combination of precipitation separation techniques and pH adjustments. Acid leaching proves effective in reducing the levels of aluminum (Al) and iron (Fe) in amorphous silica. Repetitive chemical processing emerges as a straightforward method for obtaining higher-grade silica.
We aim to optimize the elaboration process by investigating the impact of solution temperature, reaction time, pH, and acid leaching on the final product. The selection of these parameters is driven by the complex chemical reactions involved in the aggregation of silica particles within the solution.
Patents
Author contributions: Contributions Asmaa Zeboudj. wrote the main manuscript text, prepared the figures and performed all experiments. Saad Hamzaoui . oversaw the project and assisted with the writing of the overall manuscript.. Additional Information Competing Interests: Te authors declare no competing interests.
Data availability statement: Data are provided in the figures of the article.
Acknowledgements We would like to thank the LMESM Laboratory, Physics Department, University of Science and Technology Mohamed Boudiaf in “Instrumentation aux limites”.
- Jiang T, Xua X, George Z. Chen. Silicon is prepared by electroreduction in molten salts as new energy materials. Journal of Energy Chemistry. 2019.
- Tahir R, Wahab AW, Nafie NL, Raya I. Synthesis of mesoporous silica SBA-15 through surfactant set-up and hydrothermal process. Rasayan J Chem. 2019.
- Gotze J, Mockel R. Quartz: Deposits, Mineralogy and Analytics. Springer Geology. 2012. DOI: 10.1007/978-3-642-22161-3_2 Springer-Verlag Berlin p 29-51.
- Lai H, Huang L, Lu C, Luo X. Leaching behavior of impurities in Ca alloyed metallurgical grade silicon. Hydrometallurgy. 2015; 156:173-181.
- Ali M, Ul Haq E, Abdul Karim MR, Ahmed S, Ibrahim A, Ahmad W, Baig WM. Effect of leaching with 5-6 N H2SO4 on thermal kinetics of rice husk during pure silica recovery. J Adv Res. 2016 Jan;7(1):47-51. doi: 10.1016/j.jare.2015.01.007. Epub 2015 Jan 28. PMID: 26843969; PMCID: PMC4703416.
- Tabatabaei S, Shukohfar A, Aghababazadeh R, Mirhabibi A. Experimental study of the synthesis and characterization of silica nanoparticles via the sol-gel method. Journal of Physics. 2006.
- Munasir, Sulton A, Triwikantoro, Zainuri M, Darminto. Synthesis of silica nanopowder produced from Indonesian natural sand via alkali fusion route. AIP Conference Proceedings. 2013.
- Yang X, Yasuda K, Nohira T, Hagirawa R, Homma T. The role of granule size on the kinetics of electrochemical reduction of SiO2 granules in molten CaCl2. Metall Mater Trans B. 2015. 47: 788-797.
- Sdiri A, Higashi T, Bouaziz S, Benzina M. Synthesis and characterization of silica gel from siliceous sands of southern Tunisia. Arab J Chem. 2014; 7:486–493.
- Sharma A, Leib-Day AR, Thakur MM, Penumadu D. Effect of Particle Morphology on Stiffness, Strength and Volumetric Behavior of Rounded and Angular Natural Sand. Materials (Basel). 2021 Jun 2;14(11):3023. doi: 10.3390/ma14113023. PMID: 34199526; PMCID: PMC8199643.
- Sdiri A, Higashi T, Bouaziz S, Benzina M. Synthesis and characterization of silica gel from siliceous sands of southern Tunisia. Arab J Chem. 2014; 7:486–493.
- Slatni I, Elberrichi FZ, Duplay J, Fardjaoui NEH, Guendouzi A, Guendouzi O, Gasmi B, Akbal F, Rekkab I. Mesoporous silica synthesized from natural local kaolin as an effective adsorbent for removing of Acid Red 337 and its application in the treatment of real industrial textile effluent. Environ Sci Pollut Res Int. 2020 Nov;27(31):38422-38433. doi: 10.1007/s11356-020-08615-5. Epub 2020 May 8. PMID: 32385816.
- Altuhafi FN, Coop MR, Georgiannou VN. Effect of Particle Shape on the Mechanical Behavior of Natural Sands. Journal of Geotechnical and Geoenvironmental Engineering. 2016; 142(12).
- Muñoz-Ibáñez A, Delgado-Martín J, Grande-García E. Acoustic emission processes occurring during high-pressure sand compaction. First published in Journal Name. 2018, September 20. DOI: 10.1111/1365-2478.12691
- Ni H, Lu S, Chen C. Modeling and simulation of silicon epitaxial growth in Siemens CVD reactor. Journal of Crystal Growth. October 15, 2014; 404:89-99.
- Zhong W, Yue F, Ciancio A. Fractal Behavior of Particle Size Distribution in the Rare Earth Tailings Crushing Process under High Stress Condition. Appl. Sci. 2018; 8(7):1058.
- Carvalho Y, Almeida JMAR, Romano PN, Farrance K, Demma Carà P, Pereira N Jr, Lopez-Sanchez JA, Sousa-Aguiar EF. Nanosilicalites as Support for β-Glucosidases Covalent Immobilization. Appl Biochem Biotechnol. 2017 Aug;182(4):1619-1629. doi: 10.1007/s12010-017-2422-7. Epub 2017 Feb 2. PMID: 28155169.
- Boualem A, Leontie L, Gomez Lopera SA, Hamzaoui S. Synthesis and Characterization of Mesoporous Silica from Algerian River Sand for Solar Grade Silicon: Effect of Alkaline Concentration on the Porosity and Purity of Silica Powder. Silicon. 2021.
- Securing a sustainable raw materials supply basis for Europe. ETP SMR Policy Document for the EIP on Raw Materials, Brussels. November; 2011; 8
- Evaluation of High Purity Resources (I), Laboratory Analyses. Brochure, Dorfner Anzaplan. P.5.
- Gotze J, Mockel R. Quartz: Deposits, Mineralogy and Analytics. Springer Geology. Springer-Verlag Berlin. 2012; 29-51. DOI: 10.1007/978-3-642-22161-3_2
- Tobergte DR, Curtis S. Rate processes of extractive metallurgy. Miner Eng. 2013; 53(9):1–286
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
