Open Journal of Bacteriology
Detection rate of pulmonary tuberculosis by direct and concentrated microscopy techniques with Lowenstein-Jensen Culture as standard: South-South Nigeria Scenario
Ekom Ndifreke Edem1*, Anthony Nathaniel Umo2, Olajide Joseph Akinjogunla3, Adel AE Elahmar4 and David Arome5
2Department of Medical Microbiology and Parasitology, College of Health Sciences, University of Uyo, Uyo, Nigeria
3Department of Microbiology, Faculty of Science, University of Uyo, Nigeria
4Hamad Medical Corporation, Qatar
5Health News.ng, Nigeria
Cite this as
Edem EN, Umo AN, Akinjogunla OJ, Elahmar AA, Arome D (2022) Detection rate of pulmonary tuberculosis by direct and concentrated microscopy techniques with Lowenstein-Jensen Culture as standard: South-South Nigeria Scenario. Open J Bac 6(1): 001-005. DOI: 10.17352/ojb.000021Copyright License
© 2022 Edem EN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Objective: This study seeks to identify a more sensitive smear microscopy method for the detection of Mycobacterium tuberculosis in low-resource centers.
Materials and methods: One hundred and forty sputum specimens were collected and direct smears were prepared as the specimen was submitted. The specimens were afterward digested/decontaminated using the modified Petroff’s method. Both smear methods were stained by the Ziehl-Neelsen technique and examined. All specimens (direct and concentrated) were cultured on a Lowenstein-Jensen medium and results were considered the gold standard to calculate sensitivity.
Results: Out of 140 specimens, 27 (19%) and 34 (24%) were positive by direct and concentrated methods respectively. The number of culture positives by the direct and concentrated method was 26 (19%) and 31(22%) respectively. The sensitivity of direct and concentrated methods was 92.3% and 100% respectively. The negative culture and contamination rate was minimum with the concentrated method. The diagnostic accuracy of direct and concentrated methods was 88.6% and 97.1%.
Conclusion: In summary, results showed concentrated techniques were able to detect more true positive Mycobacterium tuberculosis than the direct smear method. Therefore, the study region with AFB smear microscopy can utilize the concentrated smear method for AFB smear microscopy to improve the case-finding strategy.
Introduction
Globally, Tuberculosis (TB) continues to be one of the most fatal infections despite a well-planned therapeutic regimen recommended by the World Health Organization (WHO) and if left untreated, each person with active TB will infect an average of 10 and 15 people every year. This outlines the importance of correct and accurate TB diagnosis to prevent the further spreading of infection and help control the prevalence and incidence of TB infections [1]. A study in Uyo recorded a 3.1% case of multi-drug tuberculosis (MDR-TB) [2] and this was possible through accurate active case findings. This resonates with the need for a more accurate TB screening for all TB centers or resource constraints TB centers [3]. Delayed and inaccurate diagnosis is associated with worse clinical outcomes and increased transmission [4], especially in communities with resource constraints. Diagnosis of TB is hampered due to the presence of different bacteria and fungi in a patient’s sputum as contaminants [5] and this results in the delayed time of TB diagnosis which in turn interferes with treatment initiation. The contaminated sputum specimens slow down the process of M. tuberculosis detection indicating a pressing need for establishing an inexpensive method for accurate identification [5]. Smear microscopy plays an important role in the initial diagnosis of tuberculosis and is still the most used among all methods employed worldwide in the diagnosis of TB due to its low cost and minimal requirement of equipment and technical skills. Though it lacks sensitivity since a load of about 5,000 to 10,000 bacilli/ml of the specimen is required to give a positive result after Ziehl –Neelsen staining [6]. However, it has been shown that concentration/decontamination of sputum improves the sensitivity of AFB smears [7], especially for centers where microscopy is their only diagnostic option. Since sputum microscopy is the cornerstone of TB diagnosis in some resource-constraint facilities, a more sensitive smear microscopy method would be useful in these facilities to achieve increased, accurate, and rapid TB diagnosis [8]. In the present study, smear sensitivities for MTB detection were obtained. In this study, the Mycobacterium tuberculosis positivity rates from direct (prepared directly from respiratory specimens) and concentrated/decontaminated (prepared from the concentrate of the sputum specimens) Acid Fast Bacilli (AFB) smear microscopies were compared in South-South Nigeria.
Materials and methods
Area of study
The study was conducted at the TB laboratory, St, Luke’s Hospital, Uyo, and TB laboratory, University of Portharcourt Teaching Hospital, Port Harcourt, Rivers State from May 2019 to August 2019 after getting approval from the Akwa Ibom State Ethical Committee.
Research design, sample size and laboratory analysis
In this comparative study, 140 sputum samples of suspected pulmonary tuberculosis cases were selected for the study. Early morning samples of sputum were collected in sterile wide-mouth containers in the laboratory [9]. Only sputum samples with a volume of 5 ml or more were accepted [10] and were macroscopically labeled as saliva, mucoid, mucopurulent, and purulent. Each sample was divided into two halves for direct and concentrated/decontaminated procedures. Smears were immediately prepared from the samples for direct analysis, stained with Ziehl-Neelsen (Z-N) method, and graded according to IUATLD/WHO guidelines (2000: 2008) as AFB negative, scanty (1 to 9 AFB/100 fields), 1+ (10 to 99/100 fields), 2+ (1 to 10 AFB/field), or 3+ (more than 10 AFB/field). The samples (direct and concentrated/decontaminated) were stored in a refrigerator (2°C - 4°C) and transported for culture at the TB laboratory, University of Portharcourt Teaching Hospital, Port Harcourt, Rivers State. At the TB laboratory, University of Portharcourt Teaching Hospital, samples for direct analysis were cultured without decontamination on Lowenstein Jensen (LJ) medium at 37°C for eight weeks while samples for concentration/decontamination analysis passed through decontamination (Modified Petroff’s method) process in a biosafety cabinet. Smears were prepared after concentration/decontamination, stained with Ziehl-Neelsen (Z-N) method, and graded according to IUATLD/WHO guidelines [11,12]. The concentrated/decontaminated samples were also inoculated directly on Lowenstein Jensen (LJ) medium at 37 °C for eight weeks.
Procedure for decontamination: The sputum samples for concentration/decontamination analysis were processed for culture using the Modified Petroff’s method, where 3-5 ml of the sputum sample was homogenized for 15 min in a shaker using an equal volume of 4% NaOH. After centrifugation at 3,000 rpm for 15 min, the deposit was neutralized with 20 ml of sterile distilled water. The samples were again centrifuged. After centrifugation, the supernatant was decanted under a Biosafety Cabinet (BSC) into a container with tuberculocidal disinfectant [13]. From the sediment, 0.1 ml was used to make serial dillusions before inoculation on LJ media.
Inclusion criteria
(a) All pulmonary non-sputum samples, (b) Both inpatients and outpatients, (c) All age groups of patients.
Exclusion criteria
Those with lower volumes (< 5 ml) of sputum
Statistical analysis
Recorded data were analyzed using the statistical package version 22 (SPSS). Qualitative data were expressed as frequency and percentage. The Chi-square (X2) test was used to compare proportions between two qualitative parameters and to also investigate the association between selected background characteristics. p value p < 0.05 was considered significant.
Evaluation of diagnostic performance:
- Sensitivity = (true positive)/ [(true positive) + (false negative)].
- Specificity = (true negative) / [(true negative) + (false positive)].
- PPV (Positive Predictive value) = (true positive) / [(true positive) + (false positive)].
- NPV (Negative Predictive value) = (true negative)/ [(true negative) + (false negative)]
- Diagnostic Accuracy = [(true positive) + (true negative)] / Total Participantsa
Ethics statement
Ethical approval for this study was obtained from the Akwa Ibom State Ministry of Health, Akwa Ibom State, Nigeria with Ref: MH/PRS/99/Vol.V/631. Participants were required to submit informed consent.
Results
Out of 140 specimens, 27 (19%) were found to be AFB positive and 113 (81%) were found AFB negative by direct AFB smear. 34 (24%) were found to be AFB positive and 106 (76%) were found AFB negative by concentrated AFB smear. Specimens cultured directly had 25 (18%) culture positives, 104 (74.3%) culture negatives, 3 (2%) nontuberculous Mycobacteria (NTM) and 8 (5.7%) contaminants while specimens concentrated before culture had 31 (22%) culture positive, 105 (75%) culture negative, 3 (2%) nontuberculous Mycobacteria (NTM) and 1 (1%) contaminants (Table 1).
The specimen microscopy showed mucoid samples to have a substantially higher proportion of MTB positives by both direct and concentrated methods, 20 (81.2%) and 29 (92.6%) respectively, followed by salivary with 4 (12.9%) by the direct method. Mucopurulent had the least with 2 each by both direct and concentrated methods (Table 2).
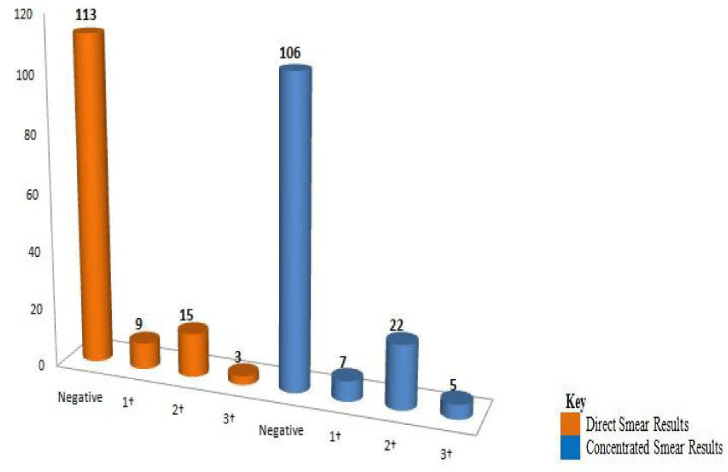
Among 27 positive specimens on direct microscopy, 9 (33.3%), 15 (55.6%) and 3 (11.1%) were found to be 1+, 2+ and 3+ respectively. Of the 34 positive specimens on concentrated microscopy, 7 (20.6%), 22 (64.7%), and 5 (14.7%) were found to be 1+, 2+ and 3+ respectively (Figure 1).
Of the 140 specimens, 27 (19%) specimens were positive both on direct and concentrated methods, and 7 (5%) samples were positive on the concentrated method which was negative on direct smear. More than 70% of the samples were found to be negative on both methods. The direct smear method had 88% sensitivity, 95.3% specificity, 84.5% Positive Predictive Value (PPV), and 97.1% Negative Predictive Value (NPV) when compared with direct culture results. However, when the concentrated smear method was compared with concentrated culture results, the sensitivity, specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) were 100%, 97.2%, 91.2% and 100% respectively (Table 3).
Discussion
There has been evidence that the concentrated method can be superior to the direct method, however, it is not being performed in low-income countries because of the feasibility of centrifugation in settings with irregular power supply; limited human and financial resources; inadequate training capacity; potential biohazard posed by lack of proper biosafety arrangements [14]. Nonetheless, it is important to note that sputum decontamination methods are used to kill oral bacteria present in the specimen [15] thereby yielding positive results for Mycobacteria. The present study has shown that the concentrated smear technique is better than the older direct smear technique as the direct smear was less sensitive, though in a close range than the concentrated smear in detecting culture-positive specimens (88 and 100%, respectively). A Study by Uddin, et al. [14] which had 70.5% and 82.9% sensitivity for direct and concentrated smear methods respectively was in agreement with our study. A similar study conducted by Peterson, et al. [16] in two different laboratory settings (a tertiary-care laboratory and several local outpatients clinics) found that in a tertiary-care hospital, the direct smear was significantly less sensitive than the concentrated smear (28% and 51%, respectively) and in local outpatients clinics, the direct smear was still less sensitive than that made from the concentrated specimen (82 vs. 93%, respectively).
The samples were cultured and 25 (18%) samples were positive for MTB by direct technique. Seven samples were found to be negative for MTB by the direct smear technique, but positive by the concentrated smear technique. However, these 7 samples constituted 3 samples found to be harboring nontuberculous Mycobacteria (NTM). The probable reason for this might be that smear microscopy only indicates the presence of Acid-Fast Bacilli (AFB) which could be Mycobacteria sp and or Nocardia sp [17], thereby yielding false-positive results, compared to a culture that distinguishes only living Mycobacteria sp [18]. A study at UCIMC by Peterson, et al. [16], agrees with our study as 10% was positive by the direct smear technique, whereas 23% was positive by the concentrated technique when cultured.
It is observed in this study that the appearance of a specimen cannot be used as a criterion to discard sputum samples as it could yield AFB [19-22]. In this study, salivary samples for both direct and concentrated techniques yield AFB positive. Our study agrees with a study by Yoon, et al. [23] with a salivary sample positivity rate of 17.6% (19 of 108). In addition, the number of bacilli in smear-positive smears can be underestimated in macroscopic poor-quality specimens [24]. From our study, it can be observed in Table 2 that a purulent sample seen to be 1+ using the direct technique was 3+ using the concentrated technique which reaffirms the low detection rate by the direct technique.
In light of our study findings, it can be seen, with respect to the relative sensitivity, that the concentrated technique is more efficient in detecting AFB than the direct technique.
Conclusion
In conclusion, since some facilities lack the necessary resources for Xpert MTB/RIF and Xpert Ultra as initial tests, and a single sputum-positive case missed by direct smear microscopy can infect 10-15 persons/year, this study was necessary for the study region. This study showed that smears prepared from concentrated specimens (concentrated AFB smears) had higher sensitivity to smears prepared directly from respiratory specimens (direct AFB smears). We strongly recommend the concentrated smear method to be used for AFB smear microscopy in low-resource centers as it helps identify a higher number of true positive cases. However, it requires more processing time than the direct smear method, and the resources needed to practice the concentrated smear technique remain a problem in many laboratories.
- Kakoma LN, Mukesi M, Moyo SR. Effectiveness of GeneXpert Technology in the Diagnosis of Smear-Negative Pulmonary Mycobacterium tuberculosis in HIV Positive Patients in Namibia. Open J Med Micro. 2016; 6:133-141.
- Edem EN, Umoh A, Olaniyan UOO, Anikpe JN. Genexpert MTB/RIF Diagnostic Yield of Mycobacterium tuberculosis and Rifampicin Resistance in Uyo, Nigeria. Clin Med. 2021; 3(2): 1034.
- Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, Deng S, Chen M. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect. 2012 Jun;64(6):580-8. doi: 10.1016/j.jinf.2012.02.012. Epub 2012 Feb 27. PMID: 22381459.
- Falzon D, Jaramillo E, Schünemann HJ, Arentz M, Bauer M, Bayona J, Blanc L, Caminero JA, Daley CL, Duncombe C, Fitzpatrick C, Gebhard A, Getahun H, Henkens M, Holtz TH, Keravec J, Keshavjee S, Khan AJ, Kulier R, Leimane V, Lienhardt C, Lu C, Mariandyshev A, Migliori GB, Mirzayev F, Mitnick CD, Nunn P, Nwagboniwe G, Oxlade O, Palmero D, Pavlinac P, Quelapio MI, Raviglione MC, Rich ML, Royce S, Rüsch-Gerdes S, Salakaia A, Sarin R, Sculier D, Varaine F, Vitoria M, Walson JL, Wares F, Weyer K, White RA, Zignol M. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011 Sep;38(3):516-28. doi: 10.1183/09031936.00073611. Epub 2011 Aug 4. PMID: 21828024.
- Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006 Oct;6(10):664-74. doi: 10.1016/S1473-3099(06)70602-8. PMID: 17008175.
- Rasool G, Khan AM, Mohy-Ud-Din R, Riaz M. Detection of Mycobacterium tuberculosis in AFB smear-negative sputum specimens through MTB culture and GeneXpert® MTB/RIF assay. Int J Immunopathol Pharmacol. 2019 Jan-Dec;33:2058738419827174. doi: 10.1177/2058738419827174. PMID: 30791749; PMCID: PMC6360468.
- Miörner H, Ganlöv G, Yohannes Z, Adane Y. Improved sensitivity of direct microscopy for acid-fast bacilli: sedimentation as an alternative to centrifugation for concentration of tubercle bacilli. J Clin Microbiol. 1996 Dec;34(12):3206-7. doi: 10.1128/jcm.34.12.3206-3207.1996. PMID: 8940473; PMCID: PMC229484.
- Tuberculosis Division International Union Against Tuberculosis and Lung Disease. Tuberculosis bacteriology--priorities and indications in high prevalence countries: position of the technical staff of the Tuberculosis Division of the International Union Against Tuberculosis and Lung Disease. Int J Tuberc Lung Dis. 2005 Apr;9(4):355-61. PMID: 15830740.
- Murphy ME, Phillips PPJ, Mendel CM, Bongard E, Bateson ALC, Hunt R, Murthy S, Singh KP, Brown M, Crook AM, Nunn AJ, Meredith SK, Lipman M, McHugh TD, Gillespie SH; REMoxTB Consortium. Spot sputum samples are at least as good as early morning samples for identifying Mycobacterium tuberculosis. BMC Med. 2017 Oct 27;15(1):192. doi: 10.1186/s12916-017-0947-9. PMID: 29073910; PMCID: PMC5658986.
- Warren JR, Bhattacharya M, De Almeida KN, Trakas K, Peterson LR. A minimum 5.0 ml of sputum improves the sensitivity of acid-fast smear for Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2000 May;161(5):1559-62. doi: 10.1164/ajrccm.161.5.9908063. PMID: 10806154.
- International Union against Tuberculosis and Lung Disease (IUATLD). Technical guide: sputum examination for tuberculosis by direct microscopy in low-income countries, 5th ed. International Union against Tuberculosis and Lung Disease, Paris, France. 2000
- WHO. Anti-tuberculosis drug resistance in the world. The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. http://www.who.int/tb/publications/2008/drs_report4_26feb08.pdf. World Health Organization, Geneva, Switzerland. 2008.
- Buijtels PC, Petit PL. Comparison of NaOH-N-acetyl cysteine and sulfuric acid decontamination methods for recovery of Mycobacteria from clinical specimens. J Microbiol Methods. 2005 Jul;62(1):83-8. doi: 10.1016/j.mimet.2005.01.010. PMID: 15823396.
- Uddin MK, Chowdhury MR, Ahmed S, Rahman MT, Khatun R, van Leth F, Banu S. Comparison of direct versus concentrated smear microscopy in detection of pulmonary tuberculosis. BMC Res Notes. 2013 Jul 25;6:291. doi: 10.1186/1756-0500-6-291. PMID: 23885922; PMCID: PMC3733684.
- Peres RL, Maciel EL, Morais CG, Ribeiro FC, Vinhas SA, Pinheiro C, Dietze R, Johnson JL, Eisenach K, Palaci M. Comparison of two concentrations of NALC-NaOH for decontamination of sputum for Mycobacterial culture. Int J Tuberc Lung Dis. 2009 Dec;13(12):1572-5. PMID: 19919781.
- Peterson EM, Nakasone A, Platon-DeLeon JM, Jang Y, de La Maza LM, Desmond E. Comparison of direct and concentrated acid-fast smears to identify specimens culture positive for Mycobacterium spp. J Clin Microbiol. 1999 Nov;37(11):3564-8. doi: 10.1128/JCM.37.11.3564-3568.1999. PMID: 10523552; PMCID: PMC85691.
- Bayot ML, Mirza TM, Sharma S. Acid Fast Bacteria. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021: 30725806.
- Tripathi K, Tripathi PC, Nema S, Shrivastava AK, Dwiwedi K, Dhanvijay AK. Modified Petroff’s Method: an Excellent Simplified Decontamination Technique in Comparison with Petroff’s Method. International Journal of Recent Trends in Science and Technology. 2014:10 (3): 461-464.
- Alisjahbana B, van Crevel R, Danusantoso H, Gartinah T, Soemantri ES, Nelwan RH, van der Meer JW. Better patient instruction for sputum sampling can improve microscopic tuberculosis diagnosis. Int J Tuberc Lung Dis. 2005 Jul;9(7):814-7. PMID: 16013780.
- Khan MS, Dar O, Sismanidis C, Shah K, Godfrey-Faussett P. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet. 2007 Jun 9;369(9577):1955-60. doi: 10.1016/S0140-6736(07)60916-7. PMID: 17560448.
- Murray P, Baron EJ, Jorgenson JH, et al. Manual of Clinical Microbiology. 9th edition. Washington, DC: ASM Press; 2007:1–319.
- Edem EN, Umo AN, Akinjogunla OJ, Akereuke UE. Pus cell as an indicator for Mycobacterium tuberculosis diagnostic yield by GeneXpert MTB/RIF in South-South Nigeria: A prospective study. J Clin Sci. 2022; 19:62-6.
- Yoon SH, Lee NK, Yim JJ. Impact of sputum gross appearance and volume on smear positivity of pulmonary tuberculosis: a prospective cohort study. BMC Infect Dis. 2012 Aug 1;12:172. doi: 10.1186/1471-2334-12-172. PMID: 22853561; PMCID: PMC3449203.
- Ramsay A, Bonnet M, Gagnidze L, Githui W, Varaine F, Guérin PJ. Sputum, sex and scanty smears: new case definition may reduce sex disparities in smear-positive tuberculosis. Int J Tuberc Lung Dis. 2009 May;13(5):613-9. PMID: 19383195.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
