Open Journal of Bacteriology
Diagnostic accuracy of dipsticks test among clinically suspected urinary tract infected patients at National Hospital, Tanzania
Leonard Katunzi1, George Msema Bwire1*, James Kalabashanga2, Lilian Nkinda3, Akili Mawazo4 and Kennedy Daniel Mwambete1
2Department of Microbiology and Immunology, Muhimbili National Hospital, Central Pathology Laboratory, P.O. Box 6500, Dar es Salaam, Tanzania
3Department of Microbiology and Immunology, School of Medicine, Muhimbili University of Health and Allied Sciences, P.O. Box 6500, Dar es Salaam, Tanzania
4Department of Microbiology and Immunology, Institute of Health and Allied Sciences, Ministry of Health, Community Development, Gender, Elderly and Children, P.O. Box 65001, Dar es Salaam, Tanzania
Cite this as
Katunzi L, Bwire GM, Kalabashanga J, Nkinda L, Mawazo A, et al. (2019) Diagnostic accuracy of dipsticks test among clinically suspected urinary tract infected patients at National Hospital, Tanzania. Open J Bac 3(1): 003-007. DOI: 10.17352/ojb.000009Background: Dipstick test is a rapid test for diagnosis of urinary track infections (UTIs). Rapid test should be accurate so that does not give a lot of false results that may subject patients to wrong treatments especially when used alone. Therefore, this study was set to determine the diagnostic performance of dipsticks tests by comparing with a standard urine culture
Methods: Microbiological investigations of urine samples suspected of UTIs were performed at Central Pathology laboratory, Muhimbili National Hospital, Dar es Salaam. Dipsticks tests (leucocyte esterase and nitrite) were immediately performed from the submitted urine samples. Both positive and negative samples by dipsticks were subjected to quantitative urine culture using cysteine lactose electrolyte deficient agar (CLED). Diagnostic performance such as sensitivity, specificity and predictive values were determined by cross tabulation using GraphPad prisms whereas the distributions of the culture-isolated bacteria were expressed in frequency and percentage. Type 1 error for significance was 0.05.
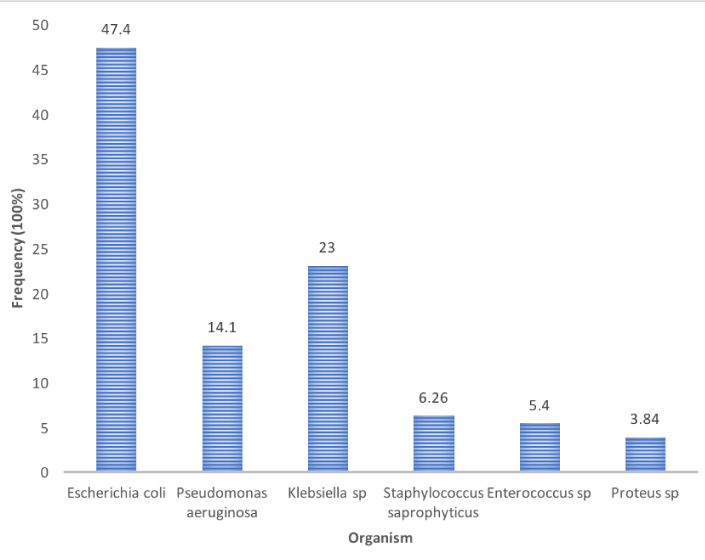
Results: Overall 300 urine samples were simultaneously tested for dipsticks and urine culture. Of 300 urine samples, 218 (73%) tested dipsticks positive while 134 (45%) had significant culture growth. Of 134 culture positive samples, Escherichia coli were mostly isolated by 47.4% while Proteus species were less isolated by 3.8%. Staphylococcus saprophyticus were the most isolated gram-positive bacteria by 6.3%. The sensitivity, specificity, positive and negative predictive values of dipsticks tests were 56%[95%CI (confidence interval); 49%-62%)], 85%(95%CI; 76%-91%), 91%(95%CI; 85%-95%) and 42%(95%CI; 35% - 50%) respectively. The diagnostic accuracy of dipsticks test was 1 (p = 0.1213).
Conclusion: The study found a moderate accuracy of dipsticks tests. Almost half of the patients diagnosed with UTIs by dipsticks tests were not found infected by urine cultures. The study recommends that, in resource available settings, urine culture should be done to confirm the dipsticks tests results.
Abbreviations
Cfu/ml: Colon Forming Units per Milliliter; CLED: Cysteine Lactose Electrolyte Deficient; LE: Leucocyte Esterase; MNH: Muhimbili National Hospital; MUHAS: Muhimbili University of Health and Allied Sciences; NIT: Urine Nitrite; NPV: Negative Predictive Value; PPV: Positive Predictive Value; UTIs: Urinary Tract Infections; CI: Confidence Interval
Introduction
Urinary tract infection, which affects the kidney, bladder or urethra, is one of the most common bacterial infections of public concern [1]. They are common in community and the second cause of bacteremia in hospitalized patients [2]. Children especially female and pregnant women contribute to about 80% of all urinary tract infections UTIs [3]. Early detection and treatment of UTIs is recommended and may help to reduce the incidences and its associated complications [4]. On other hand, inaccurate diagnosis of UTIs exposes patient to a wrong and unnecessary treatment [3,5]. There are several tests available for diagnosis of UTIs such as gram stain, dipstick, automated assay and urine culture remains [2]. Of all the tests urine culture remains to be the standard method for diagnosis of UTIs especially in low and middle-income countries [5].
However, urine culture is an expensive and time-consuming procedure, which needs an equipped microbiology laboratory, high skilled/experienced personnel and may take up to 72 hours to establish the diagnosis [3]. In resource-limited settings the most practical and convenient method for diagnosis of UTIs is urine dipstick test, this method allows early detection of UTIs, inexpensive; require less time and expertise making it reliable and rapid diagnostic test in high-risk patients [3,4].
In the past two decades, studies have examined the accuracy of dipsticks tests as a rapid test for detection of bacteria in urine but none found an adequate explanation in terms of their accuracy [6-11]. Moreover, poor accuracy of any test subject patients to unnecessary treatment hence provoking antibiotic resistance [9,11,12]. In addition to that, several meta-analysis have reported major heterogeneity of diagnostic accuracy of dipsticks across studies hence stimulates a continuing debate on the use of dipsticks tests as a sole test in diagnosis of UTIs [12,13].
Therefore, this study was conducted to determine the diagnostic performance of dipsticks tests from the clinically UTIs suspected patients.
Materials and Methods
Study design and area: This was a cross-sectional study conducted between January and May 2018 at Muhimbili National Hospital (MNH) in Dar es salaam, Tanzania (MNH) is a National Referral Hospital and University Teaching Hospital with 1,500-bed facility, attending 1,000 to 1,200 outpatients per day, admitting 1,000 to 1,200 inpatients per week.
Study population: Both in and out patients of all age groups with suspected UTIs receiving medical/health care services at MNH and submitted urine sample for laboratory analysis following clinicians request [14].
Sample size and sampling technique: A total of 300 samples were randomly selected from patient’s urine sample suspected to have UTIs.
Laboratory investigations
Urine sample collection
Mid-stream urine samples in a provided sterile, wide mouthed bottle were collected from patients suspected to have UTIs.
Gram stain and culture
Patients suspected to have UTIs were instructed by the attending clinician to collect mid-stream urine samples in a provided sterile, wide mouthed bottle. Immediately after receiving a sample, direct gram stain films were performed to examine the presence of microorganisms, epithelial cells, neutrophils and macrophages for guiding culture procedures [15-20].
Dipstick test
Leukocyte esterase (LE) and tested for LE and nitrite test (NIT) were immediately (within 10min) performed by using dipsticks (COMBI-11SL, UK) strip calibrated to deliver 0.02μl of urine. Nitrite was considered positive if there was a change in color of dipstick from colorless towards pink within 60 seconds. Leukocyte esterase was considered as positive if there was a change in color from off-white towards purple within 2 minutes. Dipstick tests were regarded positive when both nitrate and leukocyte esterase tested positive.
Urine culture
Both negative and positive dipstick tests were subjected to urine culture. Urine culture was performed as previously described [21]. Briefly, using sterile 10μl wire loops, urine samples were inoculated on cysteine lactose electrolyte deficient agar (CLED agar) (BDTM CLED Agar, Germany) by streaking method followed by aerobic overnight incubation at 370C.
Growth counts and identification
Culture growths of 105 colony forming unit/ml (Cfu/ml) were considered significant bacteriuria while less than 104 Cfu/ml were considered non-significant, those with growth between 104 - 105 Cfu/ml and those contaminated plates counted 105 Cfu/ml their culture were repeated [21]. All organisms isolated were identified based on colonial morphology, Gram staining and relevant biochemical tests [22].
Quality control
The American Type Culture Collection (ATCC) standard bacteria corresponding to each clinical isolate were used as control microorganisms [22].
Data management and analysis
Urine samples tested positive in dipstick (both LE and nitrate) and showed a significant growth in urine culture were termed as true positive (TP), urine samples tested negative in dipstick (both LE and nitrate) and showed no significant growth in urine culture were termed as true negative (TN), urine samples tested positive in dipstick but showed no significant growth in urine culture were termed as false positive (FP), those which tested negative in dipstick but showed a significant growth in urine culture were termed as false negative (FN). Using cross tabulation sensitivity, specificity, predictive value and likelihood ratios were determined using Prism 7 software (GraphPad Software, USA). The diagnostic accuracy (DA) was explored from receiver-operating characteristics (ROC curve) and the area under the curve was assumed to be DA. Likelihood ratios for positive and negative test result (LR+, LR-) were considered good when LR+ >10, and LR- <0.1, for a test with poor diagnostic accuracy, Youden’s index was equal to 0, and in a perfect test Youden’s index was equal 1 [23]. DA value of 1 indicated perfect test while the p value of less than 0.05 was considered statistically significant.
Ethical consideration
The ethical clearance to conduct this study was obtained from Muhimbili University of Health and Allied Sciences, Research and Publications Senate subcommittee. Additionally, permission to access MNH, Central Pathology lab was requested from MNH, Research and Publications committee. For confidentiality codes were used both during data collection and analysis.
Results
Distribution of bacteria identified from significant urine cultures
Overall 300 urine samples analyzed only 134 (45%) showed significant bacteria growth. Bacteria, which showed significant growth, were purely isolated and identified prior to antibiotic susceptibility testing (Figure 1).
Diagnostic performance of dipsticks test
Of 300-tested urine samples 218 (73%) were reported to have UTIs (diseased) by dipsticks tests but only 56% (122) [95%CI (confidence interval);(49%-62%)] were truly diseased while 82 of samples reported to be non-diseased by dipsticks tests but only 85% (82) (95%CI; 76%-96%) were truly non-diseased. The probability that urine samples-tested dipsticks positives were 91% (95%; 85%-95%) whereas for negative were 42% (95%CI; 35%-50%). The dipsticks tests recorded bad LR+ and LR- of 4 and 0.4 respectively. Additionally, DA was found to be 1 (p = 0.1213). The diagnostic odds ratio (DOR), which measured the odds of positive in patients with disease to those without a disease, was found to be 7.4. This indicated that Dipsticks tests are good when used as a screening test (Table 1).
Discussion
Tanzania as of developing countries lack facility that support urine culture for diagnosis of UTIs, most of dispensaries, health centers and some districts hospitals rely on rapid dipsticks tests results for establishing diagnosis of UTIs [15]. The use of dipsticks tests has been reported to have shortcoming such as poor accuracy but the test has some advantages especially in resources limited settings as it takes short time, less expensive when compared to urine culture and may also help in earlier initiation of treatment especially for the people who are at high risk such as children and pregnancy women [7-12].
We report the findings from the study conducted at MNH (tertiary hospital) between January and June 2018 from the urine samples suspected to have UTIs. In this study, urine culture and dipstick results were compared where urine culture was considered as a gold standard [3,5]. Results from this study showed that urine dipstick test might be considered for rapid test to diagnose UTIs but not as a definitive test.
The current study found the sensitivity of dipstick tests against culture to be 56% while specificity was 85%; PPV and NPV were 91% and 42% respectively. These findings are in line with low accuracy of dipsticks reported from a study conducted on 2013 at MNH on 382 febrile children below five years who were admitted in the general pediatric. Sensitivity and specificity of leukocyte esterase was 76.6% and 85.9% respectively, and nitrite was 68.8% and 92.4% respectively. Positive predictive value and negative predicting value of leukocyte esterase was 52.1% and 94.8% respectively and nitrite was 64.7% and 93.6% [19].
Also, study conducted in Duzce University Turkey, were 250 morning urine specimens tested by using dipstick and microscopic and cultured. 35% of patient had positive urine cultures with 105 colonies/mL or greater. Sensitivity and specificity of dipstick were 80% and 60% respectively. Positive predictive value (PPV) and Negative predictive value (NPV) for dipstick were 52% and 84% respectively [16].
A study done at Bugando Medical Centre, conducted among 370 febrile children. The prevalence of positive urine culture was 39.7% with sensitivity of leukocyte esterase and nitrate was 8.8% and 21.7% with specificity of 99.1% and 97% respectively [3].
The above studies with either combined or separated LE and NIT as the parameters of dipsticks tests have shown heterogeneity in accuracy hence exclude dipsticks tests in ruling out UTIs [3,16,17].
Nevertheless, in resource-limited setup, where the facility of culture is not available, urine dipstick can be used to rule out urinary tract infection in order to avoid unnecessary use of antimicrobials, support from this finding found that only 73% of all UTIs suspected patients were found positive when dipstick was used, so in place of dipstick about 27% suspected to have UTIs could have been saved from irrational use of antimicrobials.
On the other hand, we found that, bacteria showed significant growth, Gram-negative bacteria where Escherichia coli were mostly isolated. Gram-positive bacteria isolated and identified were Staphylococcus saprophyticus and Enterococcus sp. These findings are close to study conducted at MNH in pregnancy women between January 2007 and December 2009 which found E. coli a leading causative [24]. This study has therefore found Gram-negative bacteria to be more responsible for UTIs, similar findings have been reported in rural Tanzania [25] as well as elsewhere [25-28].
Conclusion
The study found a moderate accuracy of dipsticks tests. Almost half of the patients diagnosed to have UTIs by dipsticks tests were not found infected by urine cultures. In resource available settings, urine culture should be done to confirm the dipsticks tests results.
We are grateful to MNH for granting permission to conduct this study and thank all Clinicians and Laboratory personnel at MNH for their different forms of support during the phase of data collection.
- Taneja N, Singh M, Sivapriya S, Sharma M, Chatterjee SS, et al. (2010) Validity of quantitative unspun urine microscopy, dipstick test leucocyte esterase and nitrite tests in rapidly diagnosing urinary tract infections. J Assoc Physicians India 58: 485-487. Link: https://bit.ly/2XmQzkP
- Sundvall PD, Gunnarsson RK (2009) Evaluation of dipstick analysis among elderly residents to detect bacteriuria: a cross-sectional study in 32 nursing homes. BMC Geriatr 9: 32. Link: https://bit.ly/2YAbzBj
- Masinde A, Gumodoka B, Kilonzo A, Mshana S (2009) Prevalence of urinary tract infection among pregnant women at Bugando Medical Centre, Mwanza, Tanzania. Tanzan J Health Res 11: 154-159. Link: https://bit.ly/30cXzxN
- Jido TA (2014) Urinary tract infections in pregnancy: evaluation of diagnostic framework. Saudi J Kidney Dis Transpl 25: 85-90. Link: https://bit.ly/2YAbCgt
- Demilie T, Beyene G, Melaku S, Tsegaye W (2014) Diagnostic accuracy of rapid urine dipstick test to predict urinary tract infection among pregnant women in Felege Hiwot Referral Hospital, Bahir Dar, North West Ethiopia. BMC Res Notes 7: 481. Link: https://bit.ly/2KRBiSs
- Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbines JC, et al. (1989) Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 73: 576-582. Link: https://bit.ly/2KPL4o1
- Wolfhagen MJHM, Hoepelman IM, Verhoef J (1990) Urineweginfectie bij ouderen, wat is de betekenis? [Urinary tract infection in elderly people: what does it mean?]. Ned Tijdschr Geneeskd 134: 470-472.
- Cochat P, Dubourg L, Koch Nogueira P, Peretti N, Vial M (1998) French: Urine analysis by dipstick. Arch Pédiatr 5: 65-70.
- Lohr JA (1991) Use of routine urinalysis in making a presumptive diagnosis of urinary tract infection in children. Pediatr Infect Dis J 10: 646-650. Link: https://bit.ly/2xmQQVr
- (1990) Screening for asymptomatic bacteriuria, hematuria and proteinuria. The U.S. Preventive Services Task Force. Am Fam Physician 42: 389-395. Link: https://bit.ly/2xrwv1j
- Fihn SD (1992) Lower urinary tract infection in women. Curr Opinion Obstet Gyneco 4: 571-578. Link: https://bit.ly/2xqcCaS
- Pelgrom J, de Maeseneer J, Huisarts Nu (1995) De dipstickmethode: vaarwel urinesediment? Academic Bibliography 24: 8-11. Link: https://bit.ly/305MsGu
- Gorelick MH, Shaw KN (1999) Screening tests for urinary tract infection in children: A meta-analysis. Pediatrics 104: e54. Link: https://bit.ly/2NrO6AQ
- (2017) National Treatment Guidline and List of Essential Medicine, Tanzania.
- Holly AW, Peter BB (2005) Dispensary level pilot implementation of rapid diagnostic tests: an evaluation of RDT acceptance and usage by providers and patients – Tanzania, 2005. Malaria Journal 7: 239. Link: https://bit.ly/2NozL8h
- Yildirim M, Şahin İ, Küçükbayrak A, Öksüz Ş, Acar S, et al. (2008) The Validity of the Rapidly Diagnostic Tests for Early Detection of Urinary Tract Infection. Düzce Tıp Fakültesi Derg 3: 39-42. Link: https://bit.ly/2NtjYoP
- Glissmeyer EW, Korgenski EK, Wilkes J, Schunk JE, Sheng X, et al. (2014) Dipstick screening for urinary tract infection in febrile infants. Pediatrics 133: e1121-1127. Link: https://bit.ly/2XibOPE
- Najeeb S, Munir T, Rehman S, Amira Hafiz MG (2015) Comparison of Urine Dipstick Test with Conventional Urine Culture in Diagnosis of Urinary Tract Infection. J Coll Physicians Surg Pak 25: 108-110. Link: https://bit.ly/2Xn8Cr6
- Fredrick F, Francis JM, Fataki M, Maselle SY (2013) Aetiology Antimicrobial susceptibility and predictors of urinary tract infection among febrile under-fives at Muhimbili National Hospital, Dar es Salaam-Tanzania. African Journal of Microbiology Research 7: 1029-1034. Link: https://bit.ly/2KPa6DG
- Mwambete KD, Stephen WS (2015) Antimicrobial Resistance Profiles of Bacteria Isolated from Chicken Droppings in Dar es Salaam. Innovare Academic Sciences 7: 7–10. Link: https://bit.ly/322yuqT
- Koneman EW, Illevt SD, Janda WM, Schreckenberger RC, Winn WC (1997) Part II; Guidelines for collection, transport, processing, analysis, and reporting of cultures from specific specimen sources. In: Koneman EW, Alien SD, Janda WM, Schreckenberger RC, Winn W, editors. Color Atlasand Textbook of Diagnostic Microbiology. Philadelphia: Lippincott. Introduction to microbiology 121-170.
- Collee JG, Miles RS, Watt B (1996) Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marimion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. Worldcat 1: 131-115. Link: https://bit.ly/2KU9kWa
- Šimundić AM (2009) Measures of Diagnostic Accuracy: Basic DefinitiŠimundić. EJIFCC 19: 203–211. Link: https://bit.ly/2WQEpzp
- Moyo SJ, Said A, Mabula K, Maselle SY (2010) Bacterial isolates and drug susceptibility patterns of urinary tract infection among pregnant women at Muhimbili National Hospital in Tanzania. Tanzan J Health Res 12: 236-240. Link: https://bit.ly/2xpFa4d
- Blomberg B, Jureen R, Manji KP, Tamim BS, Mwakagile DSM, et al. (2005) High rate of fatal cases of pediatric septicemia caused by Gram-negative bacteria with extended-spectrum beta-lactamases in Dar es Salaam, Tanzania. J Clin Microbiol 2: 745–749. Link: https://bit.ly/2NnFZ8u
- Delzell JE, Lefevre ML (2000) Urinary tract infections during pregnancy. Am Fam Physician 61: 713-721. Link: https://bit.ly/2XPMEfX
- Gebre S (1998) Asymptomatic bacteriuria in pregnancy: epidemiological, clinical and microbiological approach. Ethiop Med J 36: 185-192. Link: https://bit.ly/323jetP
- Nicolle LE (2001) Epidemiology of urinary tract infection. Infections in Medicine 18: 153-162.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
