Open Journal of Bacteriology
The Antibiotic Resistance Patterns of Klebsiella pneumoniae Clinic Isolates: A Comprehensive Meta-Analysis
Elmas Pınar Kahraman1* and İhsan Hakkı Çiftci1,2
2Ministry of Health Sakarya University Training and Research Hospital, Department of Medical Microbiology Laboratory, Sakarya, Turkey
Cite this as
Kahraman EP, Çiftcia IH (2017) The Antibiotic Resistance Patterns of Klebsiella pneumoniae Clinic Isolates: A Comprehensive Meta-Analysis. Open J Bac 1(1): 021-026. DOI: 10.17352/ojb.000005Background/Purpose: Recently, Klebsiella pneumoniae has become a health care concern due to its production of extended-spectrum beta-lactamase (ESBL) and its resistance to carbapenem. In Turkey, systematic meta-analyses investigating antibiotic resistance in K. pneumoniae are lacking.
Methods: Consequently, we performed a systematic review of the literature followed by a meta-analysis to investigate antibiotic resistance in K. pneumoniae. This study was designed and conducted in accordance with the PRISMA guidelines. We identified observational studies published from 2000 to 2015 using the various search engines. In total, 2,225 articles were published during this study period, but we only included 25 in our meta-analysis because of eligibility criteria.
Results: We observed a significant increase in antibiotic resistance (>40%) to the following antibiotics: cefazolin, amoxicillin-clavulanic acid, cefuroxime, cefepime, ceftriaxone, and ceftazidime. Unfortunately, the majority of these antibiotics were commonly prescribed for the treatment of K. pneumoniae infections. The rate of bacterial ESBL production has been steadily increasing and in this study was calculated at 39.66% ± 12.46%. In this study, we observed >30% resistance to ciprofloxacin. Furthermore, the rates of resistance to imipenem and meropenem were 5.1% and 3.4%, respectively.
Conclusion: The data obtained from this study may be beneficial for prescribing appropriate antibiotics and preventing their unnecessary use. The frequent checks of resistance rates with new detailed report may suggest to the development of National Antibiotic Stewardship.
Introduction
After the extended-spectrum beta-lactamase (ESBL) enzymes were shown, the number of infections caused by ESBL-producing organisms in hospitalized patients increased remarkably [1]. In the following process, the phrase multi-drug resistance (MDR) started to be used commonly and MDR gram-negative bacilli (MDR-GNB) caused by nosocomial outbreaks were reported [2]. The most common view held about MDR is resistance to at least three antibiotics among cephalosporin (ceftazidime or cefepime only), aminoglycoside, fluoroquinolone, carbapenem and piperacillin groups [3]. It is stated in the literature that one of the biggest factors of outbreaks caused by MDR-GNB is Klebsiella pneumoniae. In K. pneumoniae, MDR develops due to modification in the outer membrane permeability, activation of efflux system and AmpC beta-lactamase, ESBL and carbapenemase enzymes [4].
It is stated in the studies that resistance to antibiotics especially the ones used in nosocomial outbreaks caused by K. pneumoniae has increased gradually and that antibiotics that are effective on isolates in different regions have become limited [5]. Moreover, it is emphasized that patients treated in intensive care units are at risk in terms of MDR K. pneumoniae infections and this situation is associated with the frequent use of carbapenems in intensive care units [6,7]. For, imipenem (IPM) and meropenem (MEM) are the antibiotics that are most commonly used for patients infected with K. pneumoniae in intensive care units [8].
Another problem that needs to be focused on is the colonization of MDR K. pneumoniae isolates. For, it is emphasized that the colonization time of resistant isolates may be longer than a year and it is stated that resistance problem can be experienced more intensely in the near future [9]. More importantly, there are no data regarding Turkey in the study of K. pneumoniae antibiotic resistance covering a number of countries conducted in 2015 by the World Health Organization [10].
Acting upon the idea that a significant amount of information can be collected by bringing together systematically similarly themed works made by independent researchers between 2000 and 2015, we planned our study, which is the first one in which notifications of antibiotic resistance to K. pneumoniae in Turkey were brought together and analyzed through meta-analysis.
Materials and Methods
This systematic meta-analysis was formed in accordance with the instructions in PRISMA Statement [11]. Our study consists of the phases of data sources and keywords, qualitative analysis of the study, determination of choice and rejection criteria and data analysis.
Data sources and keywords
The studies to be used for meta-analysis were determined by searching the databases of Google Scholar, PubMed, Sciencedirect and Turk Medline. While searching databases, the following keywords were used: “Klebsiella pneumoniae”, “Klebsiella pneumoniae antibiotic resistance” and “Klebisella pneumoniae antibiotic resistance Turkey”.
Qualitative analysis of the studies
Our meta-analysis study was prepared based on quinolone resistance within the framework of PRISMA statement guidelines [12]. The methodological evaluation of research was done with the criteria are shown in Table 1. However, the quality of the studies was not regarded as an exclusion criterion.
Qualitative examination of the studies included in the meta-analysis was subjected to scoring with a checklist designed with a critical evaluation by two independent reviewers. They were assessed in terms of the age groups of the patients, scope of the studies, identification methods of strains, methods for determination of antibiotic activity, number of strains, the time period of conduction of the study and healthcare provider steps are shown in [Table 1].
Eligibility criteria
The eligibility criteria for the meta-analysis were as follows: it had to be a scientific study in Turkish or English; it had to examine the state of resistance/susceptibility at least 30 K. pneumoniae isolates based on NCCLS CLSI or EUCAST criteria between 2000 and 2015 in Turkey and the data had to be consistent. On the other hand, the multicenter studies that were made before 2000, whose full texts could not be accessed, that provided data for Klebsiella spp., that provided data for that Enterobacteriaceae family without making distinction between species, that assessed fewer than 30 isolates, whose ESBL results were not reported and that did not have detailed descriptions were excluded.
Data analysis
The data were divided into three groups as 2000-2004, 2005-2009 and 2010-2015; they were examined by two independent investigators; disputes between writers were decided by unanimous votes for eligibility criteria. The names of the first researchers of the articles, their regions, the places where the samples were taken from, the total number of isolates, the methods used in the study, the properties of the patient populations, especially antibiotic resistance data were collected; and tables were formed. In the tables, the states of antibiotic resistance were shown in numbers; thus, all of the studies were enabled to be assessed by a common unit. The resistance data reported in the articles were converted to digital resistance data by ratio-proportion method. The antibiotic resistance or susceptibility data of K. pneumoniae isolates were analyzed by taking CLSI M100-S25 and M100-S-20-U into consideration. Besides, notifications related to resistance were grouped based on National Antibiotic Stewardship (NAS) [13].
Statistical analysis
In the meta-analysis, “forest plot” analysis was performed by using the program Comprehensive Meta-Analysis (CMA) (Biostat, USA). The study was assessed through Cochran Q test to find out whether studies showed similar effects or not. The homogeneity of the data was examined by the value I2. The value I2≥50 was accepted as limit value for homogeneity. The changes of K. pneumoniae isolates in the state of antibiotic resistance over the years were assessed statistically by using the program SPSS 20.0 version (SPSS, Inc., USA) through One Way ANOVA method. In the calculations, the value of p≤0.05 was expressed as the difference at significant level statistically.
Results
During the literature review conducted based on specified criteria, a total of 2225 articles between the years 2000 and 2015 that could potentially be used related to our topic were determined. 25 scientific studies having the eligibility criteria were included in the meta-analysis. In the qualitative examinations, studies were graded between 8 and 15. The qualitative average score of the studies was calculated as 11.24 ±1.98 are shown in Table 2.
In the calculations made with the ESBL data presented in the study, the rate was determined as 39.66% ±12.46. In the “forest plot” analysis made in 95% confidence intervals (CI) with CMA, the ESBL rate that can be encountered at any time based on the fixed effects model [14], was found to be 37.9% (95% CI, 37.9 to 40.8%) and the ESBL rate that can be encountered at any time based on the random effects model was found to be 39.8% (95% CI, 32.8 to 47.2%) (Q = 579.8, P <0.0001, I2=95.5) are shown in Table 2.
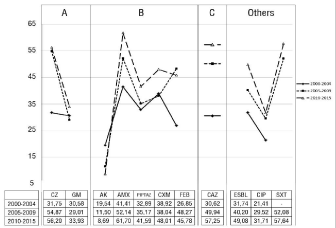
In the assessment made within the framework of NAS, all isolates were reported to be resistant to ampicillin (AM), which is among group A antibiotics to be tested primarily for K. pneumoniae. The average resistance rates to other antibiotics in this group were found to be 58.79%±22.47 and 27.93%±17.68 for cefazolin (CZ) and gentamicin (GM) respectively.
The average rates of resistance for amikacin (AK), amoxicillin-clavulanate (AMX), piperacillin-tazobactam (PIPTAZ), cefuroxime (CXM), cefepime (FEP), cefoxitin (FOX) and ceftriaxone (CRO), which are in Group B, were calculated as 7.59%±14.25, 44.97%± 29.43, 34.39%±4.90, 37.79%±3.44, 39.61%±9.93, 12.83%±9.26 and 48.75%±13.81 respectively. Calculation was not made for ampicillin-sulbactam (SAM) because sufficient data were not presented in the study for it. It was observed that a rate of 49,59%±13,07 was reported for ceftazidime (CAZ), which is in group C. No notification was made for the antibiotics in group U in any study.
Resistance rates for trimethoprim-sulfamethoxazole (SXT), ciprofloxacin (CIP), imipenem (IPM) and meropenem (MEM), which are not included in NAS groups, were calculated as 55,29%±14,23, 30,18%±14,79, 1,59%±2,66 and 1,31%±3,86 respectively.
In the “forest plot” analysis made in 95% CI with CMA for IPM resistance, the rate that can be encountered at any time based on the fixed effects model was found as 5.1% (95% CI; 3,9-6,7%) and the rate that can be encountered at any time based on the random effects model was found as 1.8% (95% CI, 0,9-3,7%) (Q =103,7, P < 0.0001, I2=79,8). In the “forest plot” analysis made in 95% CI with CMA for MEM resistance, the rate that can be encountered at any time based on the random effects model was found as 1.5% (95% CI, 0.6-3.4%) (Q=32.3, P <0.0001, I2=69.5).
In the analysis made based on years, an increase in the rates of resistance to all antibiotics except AK was determined. However, despite a significant reduction in the rate of resistance to AK, no statistically significant difference was found (p=0.083). CZ and CAZ showed the highest resistance rate increase in the period between 2000-2004 and 2010-2015. This was not found to be statistically significant (p=0.094). Change in resistance over the years could not be calculated for FOX and CRO since there were not sufficient data about them. Changes in the resistance rates based on years are shown in Figure 1.
It was determined that ESBL rates increased and that ESBL positivity reached the rate of 49.08% ± 12.93 in 2010-2015 period. This difference observed between ESBL rates over the years was not found to be statistically significant (p = 0.346). It was determined that the CIP resistance passed the 30% band and showed a resistance increase of 10% between 2000-2004 and 2010-2015 periods. SXT could not be assessed due to lack of sufficient data between 2000 and 2004. However, the SXT level of resistance was observed to be 57.64%±9.89 during 2010-2015 period.
No statistically significant difference was found among regions in terms of resistance rates developing against antibiotics assessed within the NAS framework in the analyses made for regions.
Discussion
The most important component of the studies intended to prevent the spread of antibiotic resistance is to enable more institutions and people to have access to the data obtained through evidence-based practices of medicine. It is not enough to report only the antibiotic resistance/susceptibility in studies because to make notifications about all of the antibiotics that are dealt with while reporting might not always lead to accurate results. That is why, it is necessary to be selective about the notifications within the framework of preventive practices of the development of resistance and to apply the NAS policy [13]. The most important objective of this practice is to test for the most appropriate antimicrobials, to report the most suitable options for the treatment within the framework of test results in a limited way and to prevent the development of resistance as much as possible. In our study, which was planned as a meta-analysis, different results on antibiotic resistance of K. pneumoniae found by independent researchers who did not know each other were assessed systematically in order to form data based on evidence. Most of the work done in the databases was excluded because there was no certain standardization when the resistance reported. Otherwise, studies outside of our criteria would increase the standard deviation of the results and the results would not be meaningful.
ESBL-producing bacteria show resistance to beta-lactam antibiotics along with antibiotics of different groups and hold a significant place among MDR microorganisms [39-41]. A significant increase in ESBL producing organisms has been reported recently in many countries [42]. In multicenter studies, the rate of ESBL was found to be 48.7% in K. pneumoniae isolates [43]. In our meta-analysis, the rate of ESBL determined in the last 5 year period is 49.08%; as determined as factors in the analysis the rate of ESBL that can be encountered at any time determined as a factor in K. pneumoniae isolates was found to be 37.9% based on the fixed effects model in our “forest plot” analysis. Under these circumstances, we think it is necessary to add the notification of ESBL positivity to NAS statements so that the success of antibiotic treatment based on the personal experience of clinicians will increase, the ESBL rate will be brought under control and awareness will be raised.
As a result of the assessment based on NAS framework, a marked resistance to CZ among group A antibiotics, to AMX, CXM, FEP and CRO among group B antibiotics, and to CAZ among group C antibiotics was observed in the following three periods: 2000-2004, 2005-2009 and 2010-2015. This was associated with the frequent use of these antibiotics in the treatment of K.pneumoniae infections in general and the increase in ESBL activity. The fact that the rate of resistance of beta-lactam antibiotics in all three groups was about 40% indicates that it is necessary to make new guiding studies for combined medication therapy that can be used to treat K. pneumoniae infections.
According to Prescribing Information System (PIS) data, 34.94% of the prescriptions written by family physicians, who are in the first step, contained antibiotics [44]. “Quinolones are indicated for the treatment of milder infections including upper respiratory tract infections.” Acting upon the quote above, it can be said that CIP treatment preferred contrarily to the principle of evidence-based medicine awaits solution as one of the most important problems in the spread of the resistance. We believe that the most important cause of the 10% increase in resistance to CIP, which is shown by our study and which is not included among NAS groups, developed by K. pneumoniae isolates over the years is the frequent and uncontrolled use of this antibiotic.
Carbapenems, which have not been included in NAS groups yet, are the first preferred antibiotics for the treatment of serious infections caused by ESBL-producing bacteria. In vitro studies show that ESBL-positive bacteria are quite sensitive to carbapenems [45]. However, there is an increase in the rate of resistance due to the increased or irresponsible use of carbapenems. Especially the increase in carbapenem resistance in parallel with the rate of ESBL between 2010 and 2015 is quite remarkable. In our study, the IPM and MEM resistance rates that can be encountered at any time were found to be 5.1% and 3.4% respectively. However, there are not enough MEM data for the period between 2005 and 2009.
When the regional distribution of the 25 scientific studies evaluated in meta-analysis is examined, it is seen that the most notifications came from the Western Anatolia region, followed by the Central Anatolia region, and the fewest notifications came from the Eastern Anatolia region. Although there are differences among regions, these differences are statistically insignificant; therefore, they can contribute to the holistic examination of the issue of resistance in K. pneumoniae clinical isolates and to its solution.
There are no data related to Turkey in terms of carbapenem resistance in the study called The State of the World’s Antibiotics. According to the classification of the study The State of the World’s Antibiotics, the carbapenem resistance rate in Turkey is between 2.1% and 5.9% in the light of the data we obtained. However, no resistance notification for K. pneumoniae clinical isolates was made after 2013; therefore, it should be kept in mind that this rate might have increased.
Conclusion
Consequently, these antibiotics, which are used in order to treat K. pneumoniae experimentally, should be revised and the clinical in use of antibiotics whose therapeutic indications are known should be restricted. In particular, restrictions on the use of carbapenem and/or antibiotic use policies should be developed. More notifications should be done about the resistance state of K. pneumoniae clinical isolates; NAS to should be taken into consideration while making notifications in order to support evidence-based medical practices. Additionally, each country should establish a NAS Networks for states and territories and private and pediatric sectors.
- Kfoury JNS, Araj GF (2003) Recent development in β-lactamases and extended spektrum β lactamases. BMJ 327: 1209-1213. Link: https://goo.gl/DlacxC
- Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, et al. (2003) Antibiotic resistance among gram-negative bacilli in US intensive care units. JAMA 289: 885-888. Link: https://goo.gl/g6NhjA
- Falagas ME, Bliziotis IA, Kasiakou SK, Samonis G, Athanassopoulou P, et al. (2005) Outcome of infections due to pandrug-resistant (PDR) gram-negative bacteria. BMC Infect Dis 5: 24. Link: https://goo.gl/W5Iaqs
- Kallen AJ, Hidron AI, Patel J, Srinivasan A (2010) Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006-2008. Infect Control Hosp Epidemiol 31: 528-531. Link: https://goo.gl/XnvXbV
- Gulay Z (2014) Molecular epidemiology of carbapenemases in Enterobacteriaceae. ANKEM 28: 73-76. Link: https://goo.gl/sxMJGk
- Kuvat N, Pehlivanoglu F, Bayrak F, Aksoy N, Gungor N, et al. (2013) Yetiskin yogun bakim unitesinde invaziv arac iliskili hastane infeksiyonlarının bir yillik sonuclari. ANKEM 27: 18. Link:
- Zorakolu P, Hascelik G, Unal S (2006) Antimicrobial susceptibility pattern of nosocomial gram negative pathogens: Results from MYSTIC study in Hacettepe University Adult Hospital (2000-2004). Mikrobiol Bul 40: 147-154. Link: https://goo.gl/EEjEYI
- Ozsut H (1998) Yogun bakim unitesinde infeksiyon sorunu: Direncli bakteriler ve antibiyotik kullanimi. Hastane İnfek Derg 2: 5-14. Link: https://goo.gl/5WQhYQ
- Apisarnthanarak A, Bailey TC, Fraser VJ (2008) Duration of stool colonization in patients infected with extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin Infect Dis 46: 1322-1323. Link: https://goo.gl/76oy94
- Gelbrand H, Miller-Petrie M, Pant S, Gandra S, Levinson J, et al. (2015) The state of the world's antibiotics 2015. Wound Healing Southern Afr 8: 30-34. Link: https://goo.gl/cBYflz
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151: 264-269. Link: https://goo.gl/a6uN21
- Kanık EA, Tasdelen B, Erdogan S (2011) Randomization in clinical trials. Marmara Medical Journal 24: 149-155. Link: https://goo.gl/D7Pka7
- TC Saglik Bakanligi Ulusal Mikrobiyoloji Standartları (UMS) (2014) Antibiyogram yorumlama kriterleri ve kisitli bildirim kurallari, AMD-TB-03, Surum: 1.0.
- Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G (2015) Ciprofloxacin resistance in community and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis 15: 545.Link: https://goo.gl/AfHdwZ
- Gonullu N, Aktas Z, Salcioglu M, Bal C, Ang O (2001) Comparative in vitro activities of five quinolone antibiotics, including gemifloxacin, against clinical isolates. Clin Microbiol Infect 7: 499-503. Link: https://goo.gl/A29abO
- Yavuzdemir S, Aysev AD, Guriz H (2001) Resistance of hospital originated, ESBL (+) 50 Klebsiella pneumoniae strains to some antibiotics and the importance of the distance between discs in determining ESBL. Flora 6: 196-200.
- Özkan C, Oldacay M, Erdem G (2002) Frequency of extended spectrum beta-lactamases in Escherichia coli and Klebsiella pneumoniae strains isolated from nosocomial infections. ANKEM 16: 65-68.
- Tolun V, Kucukbasmaci O, Torumkuney AD, Catal Ç, Ang KM, et al. (2004) Relationship between ciprofloxacin resistance and extended-spectrum β-lactamase production in Escherichia coli and Klebsiella pneumoniae strains. Clin Microbiol Infect 10: 72-75. Link: https://goo.gl/P1ysA0
- Aydemir H, Yalcı A, Piskin N, Gurbuz Y, Turkyilmaz R (2006) The ratio of extended spectrum b-lactamase production and antibiotic resistance in Escherichia coli and Klebsiella pneumoniae. Klimik Journal 19: 63-68.
- Okaygun E, Kipritci Ö, Akman A, Aydın D (2006) Toplum ve hastane kaynakli üriner sistem enfeksiyonlarinda etken mikroorganizmalar ve antibiyotik direnci. ANKEM 20: 13.
- Pullukcu H, Tasbakan MI, Aydemir S (2006) The bacteria isolated from urine cultures and their in-vitro antibiotic susceptibility. ANKEM 20: 26-30.
- Cetin ES, Kaya S, Pakbas İ, Demirci M (2007) Microorganisms isolated from patients in intensive care units and their antibiotic susceptibilities. İnönü Universitesi Tip Fakultesi Dergisi 14: 69-73. Link: https://goo.gl/yzQQpT
- Guducuoglu H, Baykal S, İzci H, Berktas M (2007) Antimicrobial resistance of Escherichia coli and Klebsiella pneumoniae strains that produce extended spectrum beta-lactamase. ANKEM 21: 155-160.
- Muhtaseb MA, Kaygusuz A (2008) The frequency of extended-spectrum beta-lactamases (ESBL) in escherichia coli and Klebsiella pneumoniae strains isolated from blood cultures. ANKEM 22:175-182.
- Gur D, Gulay Z, Arikan Akan O, Aktaş Z, Kayacan CB, et al. (2008) Resistance to newer beta-lactams and related ESBL types in gram-negative nosocomial isolates in Turkish hospitals: results of the multicentre hitit study. Mikrobiol Bul 42: 537-544. Link: https://goo.gl/vc6nO4
- Isik F, Arslan U, Tuncer İ (2008) Extended-spectrum beta-lactamase production in Klebsiella pneumoniae strains isolated from blood cultures and their antibiotic susceptibilities. Mikrobiol Bul 42: 131-136.
- Taşbakan MI, Pullukçu H, Sipahi OR, Aydemir S, Arda B, et al. (2008) Extended-spectrum beta-lactamase production and antimicrobial resistance patterns of Klebsiella pneumoniae strains isolated from nosocomial bacteremic patients: evaluation of the results of 2001-2005 period. Mikrobiol Bul 42: 1-7. Link: https://goo.gl/IhGU64
- Tunccan OG, Keten DT, Dizbay M, Hizel K (2008) Antimicrobial susceptibility of ertapenem comparing with other antibiotics against nosocomial Escherichia coli and Klebsiella strains. ANKEM 22: 188-192.
- Koksal F, Sirekbasan S, Ak K, Kucukbasmacı O, Samasti M (2009) Prevalence and antimicrobial resistance patterns of extended-spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae strains isolated from blood cultures. Turk Mikrobiyol Cem Derg 39: 31-35. Link: https://goo.gl/Quaqbj
- Yılmaz N, Agus N, Kose S, Yurtsever SG, Oner O (2009) Antimicrobial susceptibility of extended spectrum beta-lactamase producing Escherichia coli and Klebsiella pneumoniae isolates to ertapenem and other antimicrobials. Turk Mikrobiyol Cem Derg 39: 80-84.
- Nazik H, Ongen B, Sarikaya A, Kuvat N, Ilktac M (2011) CTX-M type beta-lactamase frequency and antibiotic co-resistance in extended spectrum beta-lactamase producing Klebsiella pneumoniae strains. Turkiye Klinikleri J Med Sci 31: 300-306. Link: https://goo.gl/5Xg43i
- Uyanik MH, Hanci H, Yazgi H, Karamese M (2010) ESBL production and susceptibility to various antibiotics including ertapenem in Escherichia coli and Klebsiella pneumoniae strains isolated from blood cultures. ANKEM 24: 86-91. Link: https://goo.gl/6t4kXu
- Agca H (2011) Extended spectrum beta lactamase production and antibiotic susceptibilities of Escherichia coli and Klebsiella pneumoniae strains. DEU Medical Journal 25: 169-173.
- Ardic N, Karakas A (2012) Antimicrobial resistance status of e. coli and K. pneumoniae strains isolated from inpatients: five-year data. Turk J Phys Med Rehab 58: 189-193. Link: https://goo.gl/YzV8Eg
- Dal T, Tekin A, Tekin R, Deveci O, Can S, et al. (2012) The distribution according to the species of Gram-negative bacteria isolated from hospitalized patients's urine specimens and their antimicrobial susceptibility. Turkish Bulletin of Hygiene and Experimental Biology 69: 193-200. Link: https://goo.gl/tLxNhE
- Parlak M, Cikman A, Bektas A, Berktas M (2012) Extended spectrum beta-lactamase production and antibiotic resistance in Escherichia coli and Klebsiella pneumoniae strains: five-year follow up results. Sakaryamj 2: 11-15. Link: https://goo.gl/owg4Lo
- Simsek M, Caglar K, Sultan N (2012) Klinik örneklerden izole edilen Klebsiella pneumoniae suslarinda karbapenemaz, İBL ve GSBL yapiminin incelenmesi. 1. Antibiyotik Farkındalık Sempozyumu, November 2012, Kırıkkale. Kırıkkale University Journal of Advances in Science 1: 37-43.
- Terzi HA, Karakece E, Ciftci İH (2013) The evaluation of antibiotic susceptibilities of Klebsiella spp. isolates. Journal of the Acibadem University of Health Sciences 4: 68-71.
- Güler Ö, Aktas O, Uslu H (2008) Investigation of presence of beta-lactamases and susceptibilities to various antibiotic groups of bacteria isolated from clinical samples. ANKEM 22: 72-80.
- Bradford PA (2001) Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14: 933–951. Link: https://goo.gl/KAB0Dh
- Vahaboglu H, Oztürk R, Aygün G, Coşkunkan F, Yaman A, et al. (1997) Widespread detection of PER 1-type extended-spectrum beta-lactamases among nosocomial Acinetobacter and Pseudomonas aeruginosa isolates in Turkey: a nationwide multicenter study. Antimicrob Agents Chemother 41: 2265-2269. Link: https://goo.gl/ebvbz2
- Bush K (2008) Extended-spectrum beta-lactamases in North America, 1987-2006. Clin Microbiol Infect 14: 134-143. Link: https://goo.gl/eO1B6q
- Korten V, Ulusoy S, Zarakolu P, Mete B, Turkish MYSTIC Study Group (2007) Antibiotic resistance surveillance over a 4-year period (2000–2003) in Turkey: results of the MYSTIC program. Diagn Microbiol Infect Dis 59: 453-457. Link: https://goo.gl/1KP65K
- Aksoy M (2014) Verilerle antibiyotik kullanımı. Akılcı İlaç Kullanimi ve Farkindalik Sempozyumu, 19 November 2014, İstanbul. Symposium Handbook: 11. Link: https://goo.gl/yg8xCo
- Mody RM, Erwin DP, Summers AM, Carrero HA, Selby EB, et al. (2007) Ertapenem susceptibility of extended spectrum beta-lactamase-producing organisms. Ann Clin Microbiol Antimicrob 6: 1-6. Link: https://goo.gl/qveq0e

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
