Journal of Clinical Microbiology and Biochemical Technology
Immobilization and Biochemical Properties of Purified Xylanase from Bacillus amyloliquefaciens SK-3 and Its Application in Kraft Pulp Biobleaching
Sharad Kumar1,2, Izharul Haq1, Ashutosh Yadav3, Jyoti Prakash2 and Abhay Raj1*
2Amity Institute of Biotechnology, Amity University, Lucknow Campus, Uttar Pradesh, India
3Department of Environmental Microbiology, Babasaheb Bhimrao Ambedkar University (A Central University), Vidya Vihar, Uttar Pradesh, India
Cite this as
Kumar S, Haq I, Yadav A, Prakash J, Raj A (2016) Immobilization and Biochemical Properties of Purified Xylanase from Bacillus amyloliquefaciens SK-3 and Its Application in Kraft Pulp Biobleaching. J Clin Microbiol Biochem Technol 2(1): 026-034. DOI: 10.17352/jcmbt.000012In the present study, we studied the production and immobilization xylanase from Bacillus amyloliquefaciens strain SK-3. Isolate produced highest xylanase activity (56.2±1.6 IU/mol) at pH=8 and 40°C after 48 h incubation in presence of 1% wheat bran. Immobilization studies of purified xylanase showed that 3.0% sodium alginate and 0.2 M calcium chloride was found to be optimum. Characterization of immobilized beads by SEM and FTIR showed significant changes on the surface morphology and structure. The immobilization increases the time reaction for xylan degradation from 15-30 min and pH activity 8.0 to 9.0 whereas temperature 60-70°C with reference to free enzyme. After immobilization, thermostability of enzyme increased and retained more than 70% of its original activity after 5 h at 50°C as compared with free enzyme which showed only 20% of residual activity. Also, immobilized enzyme showed better storage stability and reusability. Overall performance of immobilized enzyme has attractive biochemical properties that make it a potential promising candidate for application in the kraft pulp pretreatment.
Abbreviations
SEM: Scanning Electron Microscopy; FTIR: Fourier Transform Infrared
Introduction
Xylanase are widely used in industrial applications due to their ease production, substrate specificity and degradation of xylan in plant cell wall. Xylan is the second most polysaccharides after cellulose in nature consisting β-1-4-linked D-xylose backbone and branches of arabinose, glucuronic acid, mannose, or acetyl residues [1,2]. In recent year the increase in the global demand of xylanase production for various industrial processes such as in animal feed, food processing, textiles, pharmacy, and pulp and paper industry [3]. Xylanases required high temperature and pH for application in pulp and paper industry for bleaching application, where they reduce toxic wastes and make environmentally friendly [4,5]. Xylanases are produced by different microorganisms including bacteria, fungi, and actinomycetes [6-8].
Application of xylanase avoids the use of chemicals that are expansive and causes pollution [3]. However, with several benefits, the use of enzyme with various processes is often limited by losing enzyme activity because of insufficient stability at various temperatures, unsatisfactory operational and storage stability, and high sensitivity to the environmental conditions [9,10].
As per industrial requirement the free enzyme may, however, be limited by its high cost and low stability. Immobilization technique can be used for the improvement of enzyme thermostability and recycling capability at elevated temperature and can be reduced the high cost of enzyme by reusing it through its immobilization on to an insoluble support [11-13]. The immobilization of enzymes on various supports is considered to be impressive and economical strategy to improve the thermostability and reuse of the enzyme and reduction of auto-digestion [14]. The enzyme stability can be improved by immobilizing them within various synthetic and non-synthetic matrices [9,15]. Sodium alginate is a natural polysaccharide composed of 1, 4 linked β-d mannuronic and α-l-guluronic acid residues, and in presence of calcium ions, alginate produces insoluble gel-like structure which is capable of tolerating high temperature and is biocompatible with most of the enzymes [16]. The use of calcium alginate beads for various industrial applications for the entrapment of enzyme [17]. So, immobilized xylanase is likely to have significant advantage over its soluble counterpart for applications such as kraft pulp bleaching.
In the present study xylanase produced by B. amyloliquefaciens strain SK-3 was immobilized within calcium alginate matrix using entrapment technique. The biochemical properties of immobilized enzymes were studied including optimum pH and temperature, thermostability, storage stability and reusability in terms of recycling efficiency.
Materials and Methods
Chemicals
Beechwood xylan, 3, 5-dinitrosalicylic acid (DNSA), Congo red, and D-xylose were purchased from Sigma (St. Louis, MO, USA). DEAE-Cellulose and sodium alginate were from Merck Bioscience. All other chemicals and solvents used in this work were of analytical grade and obtained from S. D. Fine Chem. Ltd., Mumbai, India. Microbiological culture media and media ingredient were obtained from HiMedia (Mumbai, India). The wheat bran purchased from local market in Lucknow U.P. India.
Microorganism and culture conditions
Bacillus amyloliquefaciens strain SK-3 [Gene Bank Accession: KU877335] used in the current study was previously isolated in our lab from soil for xylanase production. The xylanase production studied was performed at different culture conditions in basal medium in batch experiment in 250 mL Erlenmeyer flasks. The ingredient of basal medium (g/L) was: NaNO3 3.0, K2HPO4 0.5, MgSO4.7H2O 0.2, MnSO4.H2O 0.02, FeSO4.H2O 0.02, and CaCl2.2H2O 0.02, agar powder 15.0 and yeast extract 5.0 [18], adjusted to pH=7.2 using 2.0% Na2CO3, and 1.0% wheat bran (w/v) as a source of carbon. Production studies were performed at 37°C and 120 rpm in incubator shaker (Innova-4230, New Brunswick, USA).
Optimization of culture conditions on xylanase production
Effect of various culture conditions on xylanase production such as inoculum size, carbon and nitrogen source (organic and inorganic compounds), pH and temperature were studied. The effect of inoculum size on xylanase production was studied with different amounts (0.5, 1.0%, 2.5% and 5.0% v/v) of 24 h old pre-culture. Effect of various carbon sources (beechwood xylan, wheat bran, rice bran, oat bran @ 1.0%, w/v) on xylanase production was studied at pH=7.2.0, 37°C and 120 rpm. Effect of both organic and inorganic nitrogen sources (ammonium sulphate, sodium nitrate, urea, peptone, tryptone or beef extract @ 0.5%, w/v) on xylanase production was studied in presence of best carbon source. Effect of initial pH of medium on xylanase production was studied at pH ranged from pH 4.0-10.0. Xylanase production was also investigated at various temperatures (35, 40 and 45°C). The effect of best substrate for xylanase production (wheat bran) was also studied at various concentrations (0.1, 0.25, 0.50, 0.75, 1.0, 2.0 and 2.5%). After, optimization of culture conditions, enzyme production was performed at optimized conditions in 1-lt Erlenmeyer flasks containing 500 mL medium. Time course bacterial growth and enzyme activity was measured. Bacterial growth was estimated by measuring optical density (OD) of the culture broth at 600 nm.
Xylanase assay and protein estimation
Xylanase activity was assayed using lab established protocol [19]. Briefly, 0.25 mL of crude enzyme was mixed with 1.0 mL of 1.0% (w/v) beechwood xylan prepared in 100mM phosphate buffer pH 7.0 and the mixture was incubated at 50°C. After 15 min, 1mL DNS reagent was added and boiled at 100°C for 10 min in water bath [20]. Xylanase activity of immobilized enzyme was measured same method with slight modification. Immobilized beads (0.5g) were mixed with 1.0 ml of substrate and incubated for 15 min at 50 °C. From this tube, 1.0 ml reaction mixture was taken in another tube containing DNSA solution and rest of the protocol was followed exactly the same as mentioned for free enzyme. The color developed after boiling of the solution was measured at 540nm (UV/visible 2300 spectrophotometer, Techcomp, Korea) against reagent blank, and xylanase activity was calculated using standard curve prepared from D-xylose (Sigma). One unit (IU) of xylanase activity was defined as the amount of enzyme required to produce 1µM of xylose per min under the assay conditions. Total soluble protein was measured according to Lowry’s method [21]. Protein concentration was determined using bovine serum albumin (BSA) as a standard. The protein content of the crude and immobilized enzyme was measured by monitoring the optical density at 280 nm.
Enzyme immobilization studies
Xylanase was purified to five folds through ion exchange chromatography using DEAE cellulose (data not shown) and used for immobilization studies. The immobilization of purified xylanase was performed by mixing equal volume (1:1 ratio) of sodium alginate solution (3.0%) with purified enzyme. This mixture was added drop-wise in optimized CaCl2 solution (0.2 mM) at 4°C which leads to the formation of calcium alginate beads. The Ca-alginate beads were subjected for hardening by storing overnight in the same solution at 4°C. Finally, the calcium alginate beads were washed with deionized water to remove untrapped enzyme and stored in buffer (50 mM phosphate buffer, pH 8.0) for further process. Effect of different concentrations of sodium alginate and calcium chloride on immobilization was investigated by varying both to obtain stable calcium alginate beads (sodium alginate from 1.0 to 5.0% and calcium chloride 0.05 to 0.5 M).
SEM and FTIR analysis
The surface morphology of calcium alginates beads with and without enzyme was investigated by scanning electron microscopy (SEM). Samples (dried beads) were placed on a conducting carbon tape over aluminium stubs and coated with platinum in a sputter coater (Model SC 7620, Quorum Technology Ltd, UK) and micrograph were taken using scan electron microscope (SEM, QUANTA 450 FEG, FEI, Netherland). SEM images of calcium alginate beads were taken at 50X magnifications at an accelerating voltage of 10 KV. The FTIR spectra of calcium alginates beads with and without enzyme were taken with NicoletTM 6700 (Thermo Scientific, USA) spectrophotometer. For this method the samples were crushed with potassium bromide (KBr) to form a very fine powder. This powder is then compressed into thin pellet for analysis. Spectral scanning was done in the range of 500-4000 cm-1.
Characterization of free and immobilized xylanase beads
The optimum pH and temperature of free and immobilized enzyme was determined at different pH (pH=4.0-11.0) and temperatures (30-90°C). pH activity was assayed after incubation of enzyme with 1.0% (w/v) beechwood xylan prepared in 100mM of citrate buffer (pH 4.0-6.0), phosphate buffer (pH 6.0-8.0), tris-HCl buffer (pH 8.0-9.0), or glycine-NaOH buffer (pH 9.0-11.0) at 50°C for 15 min. The pH stability was determined by pre-incubating the free and immobilized xylanase in above mention buffer of pH 4.0-11.0 at 50°C without substrate, and the remaining activity was measured in phosphate buffers (100 mM, pH 8.0) at 50°C.The effect of temperature stability of free and immobilized xylanase was performed by incubating the free and immobilized enzyme in phosphate buffers (100 mM, pH 8.0) for 30 min pre-incubation h at different temperatures ranging from 30 to 80°C. Reaction time progress was investigated by incubating substrate and enzymes for different time intervals (5-90 min) at their optimal reaction conditions. To determine the storage stability of the enzyme preparations was stored at 4°C. The samples were incubated over a 30 days period. The aliquots were withdrawn at different time interval and residual activity was measured. Thermo stability of free and immobilized enzyme was investigated by pre-incubating of all three forms of enzymes at various temperatures (50, 60 and 70°C) at pH=9.0 for 5 h. Percent residual activity were measured under standard assay conditions. Several successive hydrolysis operating cycle were performed by beechwood xylan reaction with immobilized xylan. Immobilized beads (1.0 g) were incubated with beechwood xylan substrate (1%) at 40°C for 15 min. At the end of each reaction, the immobilized beads were recovered and washed with three times by phosphate buffer (100mM and pH=8.0). The residual activity of xylanase was calculated by taking the enzyme activity of the first cycle as 100% and the resultant reducing sugar was estimated under standard assay conditions. The recycling process was repeated several times and calculated as: residual activity (%) = enzyme activity in nth cycle X 100/enzyme activity in 1st cycle.
Pre-treatment of kraft pulp
Pulp pre-treatment of studies with free and immobilized enzyme was performed on hardwood unbleached Kraft pulp collected from Star Paper Mill, Saharanpur (Uttar Pradesh, India). Pulp samples were washed extensively to remove the alkali. Pre-treatment studies were carried out at pH 9.0, 60°C and 120 rpm. During this study, enzyme dose, incubation temperature and reaction time were studied in a water bath under shaking at 100 rpm. Reducing sugars released from pulp with and without enzyme treatment were measured [20]. Kappa number, (lignin content in pulp), was estimated by reaction of pulp with acidified potassium permanganate [22].
Results and Discussion
Xylanase production by B. amyloliquefaciens
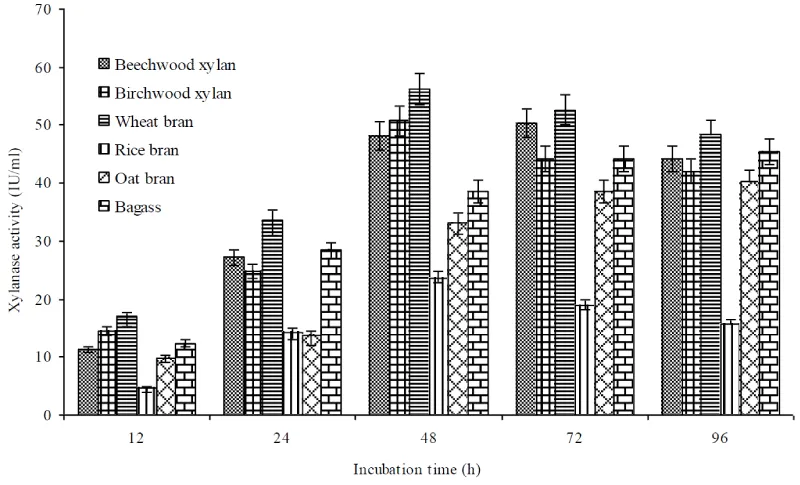
Among various agroindustrial tested, highest xylanase production was observed in presence of wheat bran (56.2±1.6 IU/mL) followed by birchwood xylan (50.8± 0.9 IU/mL) after 48 h (Figure 1). Low levels of xylanase activity were recorded when isolate was cultivated in presence of rice bran, oat bran and bagasse. The higher levels of xylanolytic activity in wheat bran medium could be due to improved bacterial growth and presence of 45% hemicellulose, which may fulfill the role of inducer which is essential for protein synthesis [23]. High production of xylanase in wheat bran medium has also been reported earlier by Bacillus pumilus strains [24-26]. Further, xylanase production studies on wheat bran in presence of various organic and inorganic nitrogen sources suggested that yeast extract was the best organic nitrogen source for xylanase production by isolate (Table 1). A combination of yeast extract + NaNO3 was supportive for higher xylanase production (68.2IU/mL) compared to single nitrogen source. Earlier studies also reported enhanced xylanase production in presence of wheat bran + yeast extract by B. mojavensis, S. maltiphilia and B. subtilis [6,26,27].
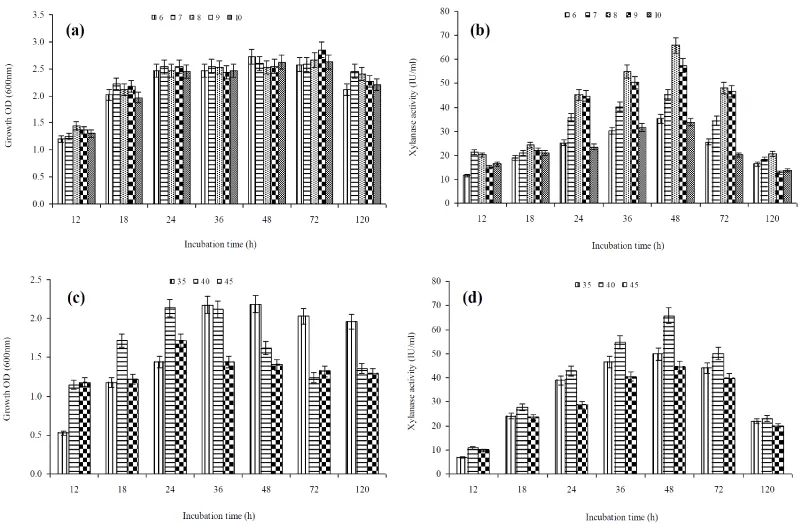
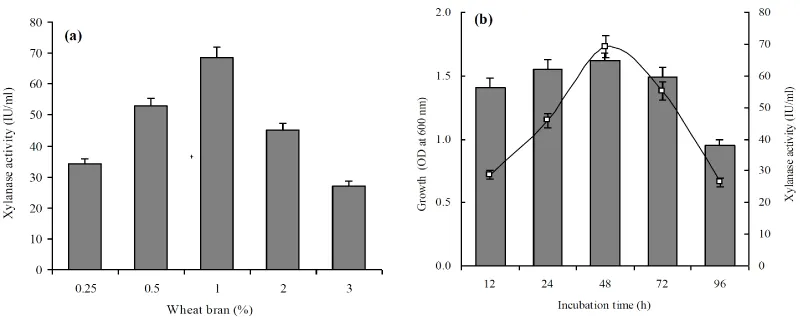
Furthermore, xylanase production was observed in all pH tested but bacterial growth (A620=2.62) and enzyme production (65.8IU/ml) was highest at pH=8.0 (Figure 2a,b) indicating growth associated xylanase production. Similar to this study, pH=8.0 was optimum for growth and xylanase production by S. maltophilia and B.mojavensis AG 137 [26,27]. Further, B. amyloliquefaciens SK-3 showed highest growth at 35°C and enzyme production at 40°C (Figure 2c,d), which is agreement with previous study of Sepahy et al. [27]. Also, xylanase production at differed wheat bran concentrations (0.25-3.0% w/v) suggested maximum xylanase production (68.6IU/ml) at 1% wheat bran and thereafter gradually declined (Figure 3a). This may possibly be due to increased viscosity of medium leading to problems in aeration and nutrient distribution [26]. Enzyme production studies at optimized culture conditions (40°C, pH=8.0, 1% wheat bran and 0.5% yeast extract) showed growth-associated xylanase production (Figure 3b). Bacterial growth and xylanase production was started simultaneously and reached 69.2 IU/ml maximum on 48 h and thereafter started decline. It has been reported that time required for maximum xylanase production is dependent upon the type of the organism, culture/environmental conditions and genetic makeup of the organism [28]. Other studies reported maximum xylanase production for B. licheniformis and Bacillus sp. after 72 h on wheat bran [28,29].
Effect of sodium alginate and CaCl2 concentration on xylanase immobilization
Enzyme entrapment in beads depends on the concentration of sodium alginate and calcium chloride ions. In this study, 3% of sodium alginate concentration was suitable for enzyme immobilization (Table 2). This may be due to the formation of strong cross linked gel and entrapment of enzyme [30,31]. Studies of different concentration (0.05-0.5 M) of CaCl2 showed that 0.2 M retained maximum immobilization yield (Table 2). However further increase the concentration of CaCl2 decreased the immobilization yield. Previously studies also reported that the enzyme activity of entrapped pectinase was decreased with the increased of CaCl2 concentration [30,31].
Enzyme characterization
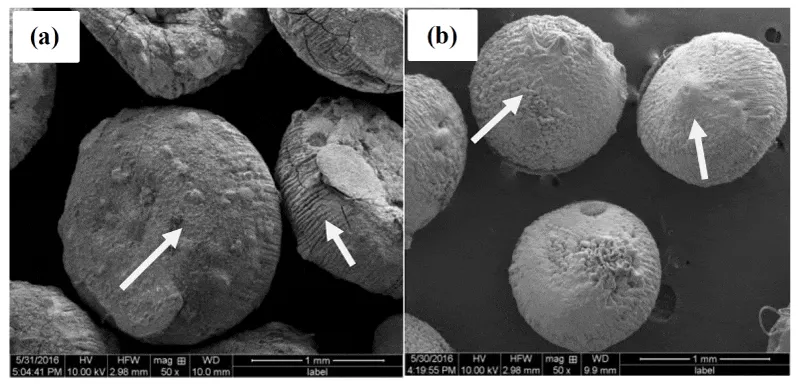
SEM was used to determine the surface morphologies of calcium alginate beads immobilized with and without enzyme. SEM images of both beads suggested that beads immobilized with and without xylanase were different from each other in shape and size (Figure 4). The control beads (without enzyme) shape were irregular with cracks on the surface (Figure 4a), whereas enzyme immobilized beads shape were regular and crackles with rough surface (Figure 4b). Further, the control beads were larger than the enzyme immobilized. The rough surface of immobilized beads image was observed was due to the action of xylanase entrapped with Ca-alginate beads. The smaller beads with xylanase immobilization possibly may be due to the addition xylanase enzyme. Similar observations were also reported on immobilization of xylanase on Ca-alginate beads [32,33].
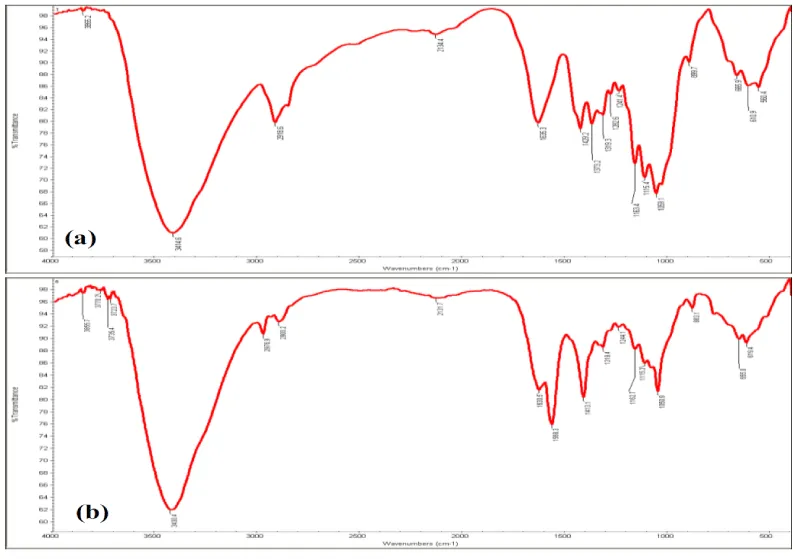
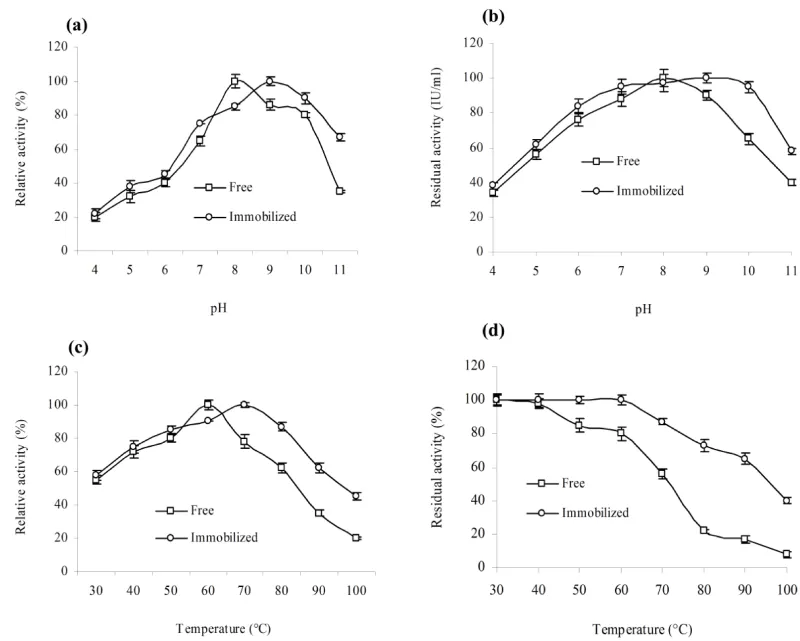
The FTIR spectroscopy of free and immobilized beads showed the structural and functional group changes (Figure 5). The increasing the banding and stretching pattern was observed in immobilized beads at different absorbance as compare to control beads (Figure 5a,b). A peak of amines N–H stretch is established around 3430 cm-1 in free and immobilized beads. An extra peak was observed in near 1600-1500 cm-1 range with aromatic C=C bending in immobilized beads (Figure 5b). The similar pattern was also reported by Bagewadi et al. 2016, [34].The effect of pH on activity and stability of free and immobilized xylanase suggested that the immobilized xylanase had higher activity as compare to free form. Optimum pH activity for free enzyme and immobilized enzyme were pH=8 and pH=9, respectively (Figure 6a). Improved pH optima of immobilized enzyme may be due to the surface and residual charge interaction [31,35]. The stability assayed of free and immobilized enzyme in pH range of 4.0-11.0 for 30 min preincubation showed that immobilized enzyme retained more than 90% activity at pH=10.0 (Figure 6b), However, free enzyme found at pH=9. Increased pH optima of xylanase, peroxidase and amylase immobilized in calcium alginate beads and immobilized enzymes have also been reported [1, 31]. The effect of temperature on free and immobilized enzyme at various temperatures (30-100°C) suggested improved activity of immobilized enzyme at higher temperatures (Figure 6c). The gradual decline in relative activity was observed in both cases at higher temperature. The temperature stability values for free and immobilized beads were 60°C and 70°C respectively (Figure 6d). The increase in temperature stability of immobilized enzyme may be the result of improvement in enzyme rigidity upon immobilization by covalent binding [1,31,36]. Earlier reports also observed the extant of improvement varied from matrix to matrix and with the kind of interaction between enzyme and matrix [1,31,37].
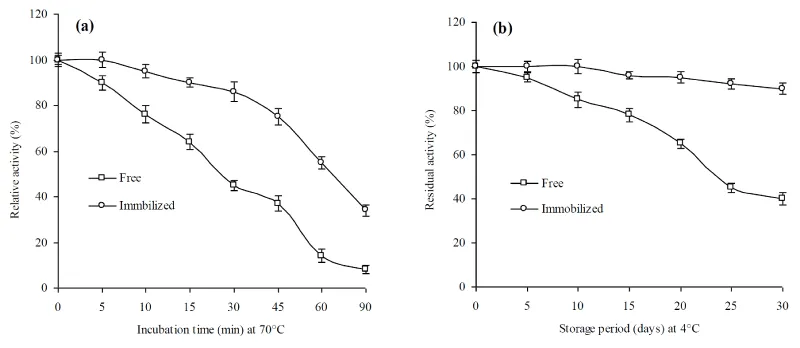
The reaction progress of free enzyme for maximum activity was increased 10 to 30 min after immobilization (Figure 7a). Increased reaction time may be due to the diffusion of substrate molecules into calcium alginate beads which require greater time to reach the substrate binding site of immobilized enzyme [30,31]. Time reaction was increased due to the resistance to diffusion faced by high molecular weight substrate. The storage stability of free and immobilized enzyme showed that immobilized enzyme retained 90% activity as compared to 40% of free enzyme after 28 d storage at 4°C (Figure 7b). The enhancement of the storage stability could be due to the physical contacts of charged residues by the interaction involving the enzyme and matrix [31,38]. The results are agreement with previous reports [38-40].
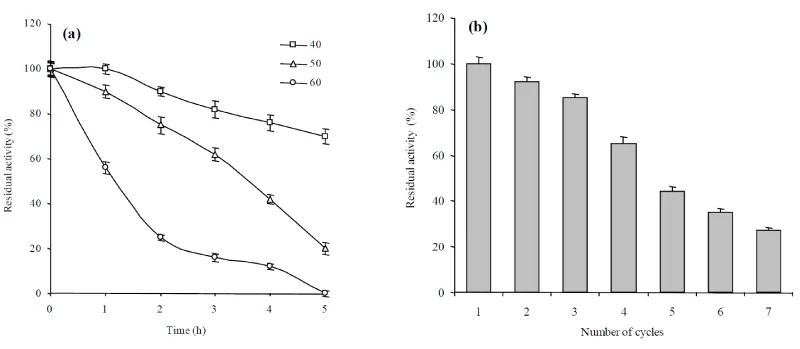
Thermostability studies of free and immobilized enzyme showed that the immobilized enzyme retained >70% its original activity at 40°C after 5 h incubation (Figure 8a). Whereas, free enzyme lost its activity completely at 60°C after 5 h of pre-incubation. It was noted that immobilized xylanase is capable to stand a wide range of temperatures for relatively longer time period in comparison with free enzyme. These results were also agreement with those earlier reports [31,32]. It is possible that elevated temperature may denature the catalytic function of an enzyme in the micro environment of the matrix used. Enhanced the thermal stability can be attributed to the nature and type of the matrix which could prevent the conformational changes and in return stabilize the enzyme activity against different temperature levels [40].
Reusability of immobilized enzyme was important parameter for industrial applications. The operational stability of immobilized enzyme in alginate beads was assessed by reusing the immobilized enzyme for seven cycles (Figure 8b). Up to five cycles, the activity retention by immobilized beads was 50% and afterwards the activity retention decreased. After seven cycles, 30% of the initial activity was retained by immobilized enzyme. The loss of activity of entrapped enzyme is may be due to leakage of enzyme from the calcium alginate beads as results of washing of beads at the end of each cycle or conformational changes by repeated uses [30,32]. This is higher value of the operational stability as compared to that reported previously by using same support for xylanase, pectinase immobilization under same [30,31,40].
Pre-treatment of kraft pulp using free and immobilized enzymes
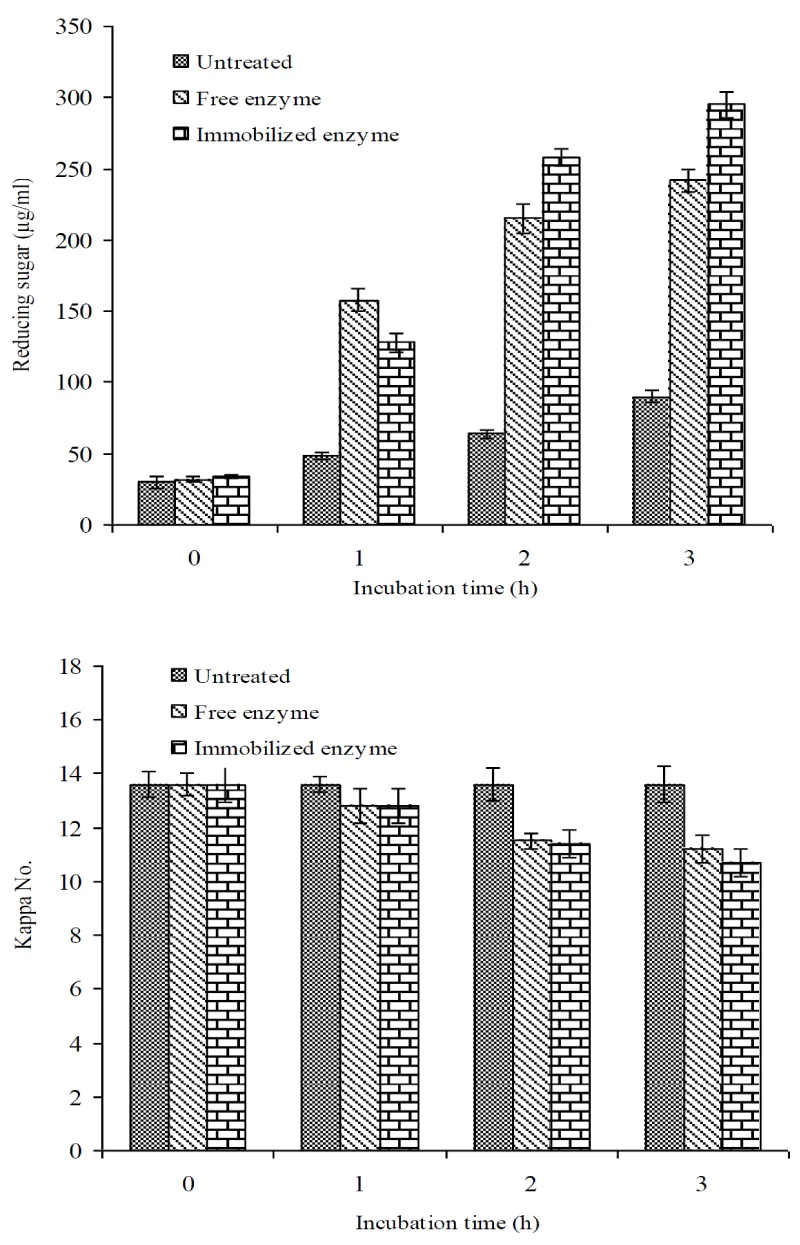
Pre-treatment of kraft pulp with free and immobilized enzyme was studied using 20IU/g (oven dried pulp) at 60°C and pH=9.0 for 3 h and results are given in Figure 9. The amount of reducing sugar present in filtrate of untreated, free and immobilized xylanase pulp was 90, 242 and 295µg/mL respectively after 3h treatments (Figure 9a). The Kappa number (lignin content in pulp) in untreated pulp, free and immobilized treated pulp after 3h treatment was reduced by 2.4 and 2.9 points compared to untreated pulp (Figure 9b). This reduction in kappa number was correlated with xylanase-mediated bleaching of pulp. The direct bleaching effect of xylanase on kraft pulp was reported in previous studies [19,41]. The other applications of immobilized enzyme in juice yield and clarity has been reported earlier after enzymatic treatment of fruit pulp with xylanase alone [1,28]. In total, the above results clearly indicated that the immobilized enzyme showed better results in applications purpose as compare to free form.
Conclusions
Xylanase from B. amyloliquefaciens strain SK-3 was immobilized with calcium alginate matrix and characterized. The immobilized enzyme exhibited improved stability and recycling capability toward pH, temperature, storage stability and reusability. The enzymes which have attractive characteristics such as the maximum reusable activity at high temperature and pH have vast potential for kraft pulp bleaching. Based on the observations, it could be suggested that xylanase from B. amyloliquefaciens SK-3 can be exploited at industrial scale for the production of xylanase. Taken together the overall performance of immobilized enzyme, the xylanase from B. amyloliquefaciens SK-3 may be a potential promising candidate for biotechnological applications.
- Pal A, Khanum F (2011) Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: Characterization of immobilized enzyme. Process Biochem 46: 1315-1322. Link: https://goo.gl/wrrXrt
- Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6: 219-228. Link: https://goo.gl/8n0nBq
- Dhiman SS, Sharma J, Battan B (2008) Industrial applications and future prospects of microbial xylanases: a review. Bio Resources 3: 1377-1402. Link: https://goo.gl/m9wBRZ
- Srinivasan MC, Rele MV (1999) Microbial xylanases for paper industry. Current Science 77: 137-142. Link: https://goo.gl/wLG92O
- Kulkarni N, Rao N (1996) Application of xylanases from alkalophilic thermophilic Bacillus sp. NCIM 59 in biobleaching of bagasse pulp. J Biotechnol 51: 167-173. Link: https://goo.gl/TE58xh
- Sanghi A, Garg N, Sharma J, Kuhar K, Kuhad RC, Gupta VK (2008) Optimization of xylanase production using inexpensive agro-residues by alkalophilic Bacillus subtilis ASH in solid-state fermentation. World J Microbiol Biotechnol 24:633-640. Link: https://goo.gl/SaI1pM
- Bajaj BK, Singh NP (2010) Production of xylanase from an alkalitolerant Streptomyces sp. 7b under solid-state fermentation, its purification, and characterization. Appl Biochem Biotechnol 162: 1804-1818. Link: https://goo.gl/sJxOoo
- Kumar KS, Manimaran A, Permaul K, and Singh S (2009) Production of 𝛽-xylanase by a Thermomyces lanuginosus MC 134 mutant on corn cobs and its application in biobleaching of bagasse pulp. J Biosci Bioeng 107: 494-498. Link: https://goo.gl/PtVzlP
- Cowan DA and Fernandez-Lafuente R (2011) Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb Technol 49:326-346. Link: https://goo.gl/rOJV3K
- Quiroga E, Illanes CO, Ochoa NA, Barberis S (2011) Performance improvement of araujiain, a cystein phytoprotease, by immobilization within calcium alginate beads. Process Biochem 46:1029-1034. Link: https://goo.gl/JRTvBN
- Kumar L, Nagar S, Mittal A, Garg N, Gupta VK (2014) Immobilization of xylanase purified from Bacillus pumilus VLK-1 and its application in enrichment of orange and grape juices. Food Sci Technol 51:1737-1749. Link: https://goo.gl/CBfiF6
- Elleuche S, Schröder C, Sahm K, Antranikian G (2014) Extremozymes- biocatalysts with unique properties from extremophilic microorganisms. Curr Opin Biotechnol 29: 116-123. Link: https://goo.gl/efLwJG
- Fernandez-Lafuente R (2009) Stabilization of multimeric enzymes: strategies to prevent subunit dissociation. Enzyme Microb Technol 45:405-418. Link: https://goo.gl/uudMJ1
- Bautista FM, Bravo MC, Campelo JM, Garcia A, Luna D, et al. (1998) Covalent immobilization of porcine pancreatic lipase on amorphous AlPO4 and other inorganic supports. J Chem Technol Biotechnol 72: 249-254. Link: https://goo.gl/fxN4gV
- Qader SAU, Aman A, Syed N, Bano S, Azhar A (2007) Characterization of dextran sucrase immobilized on calcium alginate beads from Leuconostoc mesenteroides PCSIR-4. Ital J Biochem 56:158-162. Link: https://goo.gl/ggQ4rF
- Blandino A, Macías M, Cantero D (1999) Formation of calcium alginate gel capsules: influence of sodium alginate and CaCl2 concentration on gelation kinetics. J Biosci Bioeng 88: 686-689. Link: https://goo.gl/azY839
- Kar S, Mandal A, Mohapatra PKD, Samanta S, Pati BR, et al. (2008) Production of xylanase by immobilized Trichoderm reesei SAF3 in Ca-alginate beads. J Ind Microbiol Biotechnol 35:245-249. Link: https://goo.gl/XpzMYn
- Berg B, Hofsten BV, Pettersson G (1972) Growth and cellulase formation by Cellvibrio fulvus. J Appl Bacteriol 35: 201-214. Link: https://goo.gl/vh3VHb
- Raj A, Kumar S, Singh SK, Kumar M (2013a) Characterization of a new Providencia sp. strain X1 producing multiple xylanases on wheat bran. ScientificWorldJournal 2013: 386769. Link: https://goo.gl/rhNvOu
- Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31: 426-428. Link: https://goo.gl/E7PbfN
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin-phenol reagent. J Biol Chem 193: 265-275. https://goo.gl/qmmAzi
- Tappi method T 236 cm-8 (1985) Kappa Number of Pulp, Tappi Press, Atlanta, Ga, USA. Link: https://goo.gl/pW3SB6
- Babu KR, Satyanarayana T (1996) Production of bacterial enzymes by solid state fermentation. J Scientific Industrial Res 55: 464-467. Link: https://goo.gl/ZlGDTO
- Sharma A, Adhikari S, Satyanarayana T (2007) Alkali-thermostable and cellulase-free xylanase production by an extreme thermophile Geobacillus thermoleovorans. World J Microbiol Biotechnol 23: 483-490. Link: https://goo.gl/lSxXkj
- Kapoor M, Nair LM, Kuhad RC (2008) Cost-effective xylanase production from free and immobilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochem Engineg J 38: 88-97. Link: https://goo.gl/T7A39n
- Raj A, Kumar S, Singh SK (2013b) A highly thermostable xylanase from Stenotrophomonas maltophilia: purification and partial characterization, Enzyme Res 429305. Link: https://goo.gl/9Xr26Y
- Sepahy AA, Ghazi S, Sepahy MA (2011) Cost-Effective production and optimization of alkaline xylanase by indigenous Bacillus mojavensis AG137 fermented on agricultural waste. Enzyme Res 593624. Link: https://goo.gl/6wVHV3
- Bajaj BK, Manhas K (2012) Production and characterization of xylanase from Bacillus licheniformis P11 (C) with potential for fruit juice and bakery industry. Biocat Agri Biotechnol 4: 330-337. Link: https://goo.gl/O5Z8G
- Gupta U, Kar R (2009) Xylanase production by a thermo-tolerant Bacillus sp. under solid-state and submerged fermentation. Braz Arc Biol Technol 52: 1363-1371. Link: https://goo.gl/K4Z8Eb
- Rehman HU, Aman A, Silipo A, Qader SAU, Molinaro A, et al. (2013) Degradation of complex carbohydrate: immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Food Chem 139:1081-1086. Link: https://goo.gl/LlnmMh
- Bhushan B, Pal A, Jain V (2015) Improved enzyme catalytic characteristics upon glutaraldehyde cross-linking of alginate entrapped xylanase isolated from Aspergillus flavus MTCC 9390. Enzyme Res 210784. Link: https://goo.gl/svS0lJ
- Bibi Z, Qader SAU, Aman A (2015) Calcium alginate matrix increases the stability and recycling capability of immobilized endo‑β‑1, 4‑xylanase from Geobacillus stearothermophilus KIBGE‑IB29. Extremophiles 19: 819-827. Link: https://goo.gl/Rnmy2a
- Wang Q, Peng L, Li G, Zhang P, Li D, et al. (2013) Activity of laccase immobilized on TiO2-montmorillonite complexes. Int J Mol Sci 14: 2520-12532. Link: https://goo.gl/6gD3pS
- Bagewadi ZK, Mulla SI, Shouche Y, Ninnekar HZ (2016) Xylanase production from Penicillium citrinum isolate HZN13 using response surface methodology and characterization of immobilized xylanase on glutaraldehyde-activated calcium-alginate beads. 3 Biotech 6: 164. Link: https://goo.gl/i9fAc2
- Sardara M, Roy I, Gupta MN (2000) Simultaneous purification and immobilization of Aspergillus niger xylanase on the reversibly soluble polymer EudragitTM L-100. Enzyme Microb Technol 27: 672-679. Link: https://goo.gl/Ye3gHY
- Ortega N, Perez-Mateos M, Pilar MC, Busto MD (2009) Neutrase immobilization on alginate-glutaraldehyde beads by covalent attachment. J Agric Food Chem 57: 109-115. Link: https://goo.gl/1GkW7T
- Gawande PV, Kamat MY (1998) Preparation, characterization and application of Aspergillus sp. xylanase immobilized on Eudragit S-100. J Biotechnol 66: 165-175. Link: https://goo.gl/LUTRfy
- Landarani-Isfahani A, Taheri-Kafrani A, Amini M, Mirkhani V, Moghadam M, et al. (2015) Xylanase immobilized on novel multifunctional hyperbranched polyglycerol-grafted magnetic nanoparticles: An Efficient and Robust Biocatalyst. Langmuir 31: 9219-9227. Link: https://goo.gl/8Hg10c
- Nwagu TN, Aoyagi H, Okolo BN, Yoshida S, (2012) Immobilization of a saccharifying raw starch hydrolyzing enzyme on functionalized and non-functionalized sepa beads. J Mol Cat B: Enz 78: 1-8. Link: https://goo.gl/UXyQ1t
- Shah P, Sridevi N, Prabhune A, Ramaswamy V (2008) Structural features of penicillin acylase adsorption on APTES fuctionalized SBA-15. Micropor Mesopor Mat 116: 157-165. Link: https://goo.gl/JrqUGx
- Battan B, Sharma J, Dhiman SS, Kuhad RC (2007) Enhanced production of cellulase-free thermostable xylanase by Bacillus pumilus ASH and its potential application in paper industry. Enzyme MicrobTechnol 41: 733-739. Link: https://goo.gl/Ohdeh5

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
