Open Journal of Proteomics and Genomics
Mechanistic and Therapeutic Advances in Colon Cancer: A Systematic Review
Xuena Li1,2, Ying Han2, Aihua Zhang2, Jianhua Miao1, Hui Sun2, Guangli Yan2, Fangfang Wu1,2 and Xijun Wang1,2*
2Sino-America Chinmedomics Technology Collaboration Center, National TCM Key Laboratory of Serum Pharmacochemistry, Chinmedomics Research Center of State Administration of TCM, Laboratory of Metabolomics, Department of Pharmaceutical Analysis, Heilongjiang University of Chinese Medicine, Heping Road 24, Harbin, China
Cite this as
Li X, Han Y, Zhang A, Miao J, Sun H, et al. (2019) Mechanistic and Therapeutic Advances in Colon Cancer: A Systematic Review. Open J Proteom Genom 4(1): 001-012. DOI: 10.17352/ojpg.000008Colon cancer is a common gastrointestinal malignancy in daily life and is the third most common cancer. So far, the global incidence of colon cancer has risen every year. Colon cancer mortality accounts for half of the incidence, colon cancer mortality ranks fifth in malignant tumor mortality, which seriously threatens human survival and health. This article refers to the latest domestic and foreign related articles related to colon cancer. It summarizes common and emerging screening methods for colon cancer which can make early detection and early treatment of the disease for reducing the deterioration and metastasis of colon cancer. It discusses the diagnostic markers of colon cancer found out in recent years and its mechanism of action, to improve the level, accuracy and scientificity of diagnosis. This article points out that the metastasis of colon cancer is the main cause of death in patients with colon cancer and to discuss the mechanism of its metastasis. It also summarizes the therapy thereof and advantages and disadvantages of colon cancer from different aspects such as intestinal flora, targeted therapy, traditional Chinese medicine and western medicine. Finally, the article summaries some methods that can effectively prevent colon cancer, reduce the occurrence of colon cancer from the source and improve people’s quality of life.
Introduction
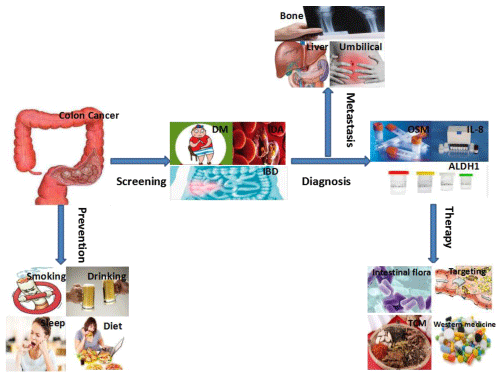
Colon cancer is one of the most common gastrointestinal malignancy in the world, with high incidence rate and death rate [1,2], and the incidence of it is increasing year by year [3]. The region with the highest incidence is Europe and the United States [4]. Recently, it has been reported that the incidence and mortality of colon cancer in China are 28.08/100,000 and 13.41/100,000 respectively, and the incidence of colon cancer accounts for the third place in the incidence of malignant tumors [5], and the mortality rate is about half of the morbidity. According to statistics, one third of the deaths from colon cancer are mostly people over 80 years old [6,7]. The early clinical symptoms of colon cancer often manifest as abdominal pain, diarrhea, adenomatous polyps, blood in the stool, anemia, thrombosis, spleen cyst, intestinal obstruction, etc., and intestinal obstruction is a complication of advanced cancer, which seriously affect people’s life and health [8-11]. The procedure of screening, diagnosis, metastasis, therapy and prevention of colon cancer is shown in figure 1. Screening for colon cancer refers to the examination of related symptoms and sites by colonoscopy, occult blood test in feces, and computed tomography (CTC) [12-14]. The diagnosis of colon cancer is through continuous research and found that more diagnostic markers such as serum oncostatin M (OSM), interleukin-8 (IL-8), aldehyde dehydrogenase 1 (ALDH1), etc., accurately determine the patient’s the situation and determine whether cancer is metastatic [15,16]. Metastasis of colon cancer to vital organs such as the liver and lungs is a major cause of death in colon cancer patients [17,18]. The treatment of colon cancer can be studied from the intestinal flora, targeted therapy, traditional Chinese medicine, western medicine and other aspects [19,20].
Screening of colon cancer
The development of colon cancer is generally from the normal colonic mucosa to the staging of the adenoma. With the deepening of the research, this staged progression can be screened and treated early. The classic screening method is divided into non-invasive and invasive. Including occult blood test in feces, fecal immunochemical test, flexible sigmoidoscopy and colonoscopy, early detection of early treatment can reduce the mortality of patients due to the deterioration of the disease [21,22]. CTC is a non-invasive and easy-to-use medical imaging technique for identifying colorectal polyps and colon cancer masses. It has become a routine diagnosis and treatment method in Western countries. I hope that CTC can be widely used for colon cancer screening in the future [23]. Colon cancer patients often have gastrointestinal bleeding, which can lead to iron deficiency anemia (IDA). Teng et al. had explored that IDA is closely related to colon cancer in adult male and postmenopausal women, and early colonoscopy can be diagnosed early and treatment to improve survival rate [24]. Intestinal flora imbalance is associated with the development of many gastrointestinal diseases, such as inflammatory bowel disease (IBD), however patients with IBD, including Crohn’s disease (CD) and ulcerative colitis (UC), the risk of colon cancer is high and should be screened in time [25,26]. In addition to screening for patients with colon cancer-related symptoms, there are some special populations with high rates of cancer and should be examined accordingly. Bloom’s syndrome (BS) is an example. BS is due to genetic diseases caused by mutations in the BLM gene, patients with BS have a higher risk of developing various malignancies, and therefore early screening for colon cancer should also be performed in patients with BS [27]. Diabetes mellitus (DM) affects the structure and function of the colon and increases the risk of colon cancer. Therefore, DM patients should also be properly screened [28]. Heo et al. had found that supportive liver abscess (PLA) is associated with colon cancer. Colonoscopy of cryptogenic PLA may detect hidden colon tumors, which can be transmitted to the liver through the tumor, so screening for PLA patients should also be performed [29]. In addition to conventional screening methods, as technology advances, some new screening methods have been discovered, such as mean platelet volume (MPV), and Li et al. have known that patients with colon cancer have a higher level of MPV, the level of MPV after surgery is reduced, so perhaps MPV can be used as a new diagnostic screening tool for colon cancer [30].
Diagnosis
Prospective radial array echogenic endoscopy can be used to diagnose colon cancer, but it has been concluded that this method is not suitable for locally advanced colon cancer, nor is it suitable for assessing peripheral lesions of colon cancer, and its accuracy also needs to be expanded to continue research [31]. Further research has found many diagnostic markers. For example, aldehyde dehydrogenase (ALDH) is a group of is enzymes that protect cells from peroxide damage while oxidizing acetaldehyde to acetic acid. Hou et al. have explored that aldehyde dehydrogenase 1 (ALDH1) expression is associated with colon cancer invasion and metastasis. It can be used as a diagnostic marker for colon cancer and can reduce the growth and metastasis of colon cancer by inhibiting ALDH1 protein expression, thereby improving patient’s survival rate [4]. Serum oncostatin M (OSM) is an amino acid peptides can act as pro-inflammatory cytokines. Gurluler et al. have found that OSM can be used as a diagnostic marker for colon cancer patients and can be transferred to lymph nodes and distal organs [21]. Interleukin-8 (IL-8) is involved in many pathophysiological processes, and IL-8 is also involved in the proliferation and metastasis of other cancers such as colon cancer, together with family members of TNF, so we can use IL-8 together with other biomarkers as markers for colon cancer diagnosis [32].
Metastasis
Although colon cancer mortality is high, patients do not die directly from colon cancer. Colon cancer metastasis to vital organs such as the liver and lungs is the main cause of death of the patient [33,34], although colon cancer can be diagnosed in the early stages through screening, still more than 25% of patients are diagnosed with metastatic colon cancer [35]. Sinagra et al. have evidence that the John Cunningham virus (JC virus) in the polyoma family can infect humans and is associated with several human tumors, which can be infected with the JC virus and produce liver metastasis [36]. Ying et al. have noticed that the intestinal flora can produce lipopolysaccharide, which can transfer colon cancer to the liver. The expression of Toll-like receptor 4 (TLR4) promotes metastasis of colon cancer, while aspirin can inhibit its expression and inhibit colon cancer metastasis [37]. Colon cancer rarely occurs when it is transferred to bones. There was some data through previous research reports suggested that colon cancer also has cases of bone metastasis, and the probability of metastasis is 2%-24% [38]. Colon cancer rarely metastasizes to the spermatic cord or the scrotum, but there are cases where colon cancer can be transferred to the spermatic cord, but the mechanism of metastasis remains unclear and remains to be studied [39]. Colon cancer also metastasizes to the umbilicus, and Mary Joseph’s nodule (SMJN) is a rare umbilical nodule secondary to colon cancer [40]. Since colon cancer metastasis is a main factor of death in patients, we should study the mechanism of metastasis to prevent colon cancer metastasis. Hirai et al. have found that CCR1 and its metalloproteinase MMP9 and MMP2 produced in the microenvironment of colon cancer cells can promote cancer cell metastasis, but the results of this study are based on a single model that can be further studied. It can find effective measures to prevent cancer metastasis [41]. Thioredoxin-like protein 2 (Tx-2) is the target of colon cancer monoclonal antibody MC3. Lu et al. explored that Txl-2 expression is elevated in colon cancer, and Txl-2 subtype Txl-2b can interact with Ran and PI3K signaling pathways to promote cells transfer [42], we can use this study to adjust the relationship between the three to achieve the purpose of controlling colon cancer metastasis.
Therapy
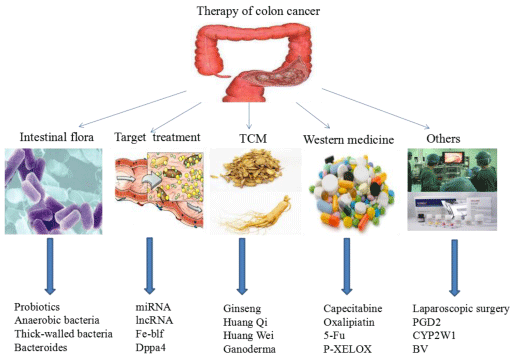
There are many methods for the treatment of colon cancer. This paper introduces the advantages and disadvantages of methods and methods for treating colon cancer from intestinal flora, targeting, traditional Chinese medicine, western medicine and other aspects. Intestinal flora, targeting, traditional Chinese medicine, western medicine and other treatment methods for colon cancer are shown in figure 2.
Intestinal flora
The gut microbiota, commonly referred to as the “intestinal flora”, is a complex, large microbial ecosystem. The intestinal flora can regulate some metabolic and physiological functions, including regulation of lumen pH, metabolism of undigested food, regulation of intestinal movement and stimulation of immune function, etc. It is regarded as a unique organ with metabolic, immune and inflammatory central functions in the human body, and the pathogenesis of colon cancer is related to the intestinal flora [43-47]. Under normal circumstances, in the human intestinal tract, the intestinal flora maintains a steady state, and the disorder of the intestinal flora triggers abnormal intestinal signaling pathways and epigenetic modifications, through the 16S rRNA gene or whole genome micro genomic sequencing analysis found that intestinal flora is associated with IBD, colon cancer and other diseases. Researchers also through the study of human patients and rodent cancer models, found that the intestinal flora changes will increase the risk of cancer such as colon cancer [48-52]. Similarly, the 16S rDNA sequencing platform was used to find differences in colony characteristics between colon cancer and adjacent normal mucosa tissues, and by genomic analysis of colon cancer flora and histological analysis of tumor tissues, it was found that Fusobacterium is a key factor in colon cancer [53-56]. The advantage of these studies is the use of the molecular pathology epidemiology database, but there are certain limitations. For example, depending on the number of tissue Fusobacterium nucleatum DNA, the distribution of chemotherapy use may not be significantly different, we need to continue to study [57,58]. Zhu et al. have noticed that probiotics include Bifid bacterium strains as beneficial bacteria that regulate intestinal flora and its metabolism, and tungstate treatment selectively inhibits molybdenum-cofactor-dependent microbial respiratory pathways that are only operable during the onset of inflammation to prevent probiotic expansion and thus beneficial for the treatment of colon cancer [59]. In addition, vitamin D receptor (VDR) and leptin receptor (LPR) can induce probiotics to produce anti-inflammatory effects, although there are still obstacles to the widespread use of probiotics in the clinic, it has been of great help in the treatment of colon cancer [60-62]. The anaerobic fungus Faecalibacterium prausnitzii is one of the main components of the intestinal flora and has been considered as a biological indicator of human health. The increase in the amount of Faecalibacterium prausnitzii can reduce the risk of colon cancer. The use of Faecalibacterium prausnitzii as a potential active ingredient in probiotic preparations is expected to be used to treat colon cancer [63]. Gao et al. have explored that histamine has a potential anti-tumor effect, and the intestinal flora can mediate inhibition of inflammation-related colon cancer through luminalamine histamine production [64]. Intestinal flora is also affected by many factors, such as genetic factors, intestinal inflammation, diet and environmental factors [65]. Bultman et al. have evidence that dietary fiber can produce butyrate under the action of intestinal flora, it can inhibit the survival and growth of colon cancer cell lines, so we can prevent and treat colon cancer in this way [66,67]. Dietary fiber can also increase the abundance of Prevo bacteria to improve glucose metabolism, and sugar metabolism can affect drug resistance. Therefore, controlling intestinal flora may be an effective strategy to reduce drug resistance [68]. By studying the dietary components of specific pathogen-free (SPF) and sterile (GF) mice, it has been found that complex nutrient mixtures such as proteins and fibers affect intestinal permeability and have a great influence on the development of colitis [69]. It has been found that adding walnuts to the diet can increase the number of thick-walled bacteria in the intestine and reduce the number of bacteria such as Bacteroides, which indicates that eating walnuts can change the intestinal flora and provide us with a new mechanism for health [70]. However, Wang et al. have noticed that inadequate characterization of the intestinal flora may contribute to the onset of colon cancer, as symbiotic infected macrophages activate Wnt/β-catenin signaling and dedifferentiation through the bystander effect (MIBE) caused by the flora. Reprogramming and pluripotency transcription factors associated with the development of colorectal cancer stem cells (CSCs), on the other hand, also provide a mechanism for colony-induced colon cancer, providing a new potential target for the prevention of colon cancer [71]. Metabolomics uses high-resolution mass spectrometry to provide chemical maps of metabolites in cells, tissues, or body fluids, and can study various disease states [72]. Metabolomics can be used to demonstrate the role of gut microbiota and to demonstrate that the relevant metabolites of the gut flora can serve as new preventive and chemical targets [73,74].
Targeted therapy
MicroRNAs (miRNAs) are non-coding small RNA molecules that are present in prokaryotes and eukaryotes that regulate translation and transcription to regulate gene expression, while microRNA21 (miR21) is involved in chronic inflammatory processes and colitis-associated colon cancer in the development of the disease, Yang et al. found that miR21 is a downstream target of F.nucleatum in the colon, so miRNA can be used as a new target for anticancer therapy, and miRNA-based therapies may be an effective method for individuals to treat colon cancer [75-77]. Luo et al. have explored that the expression of long-chain non-coding RNA (lncRNA) HOTAIR is closely related to tumor metastasis, and plays an important role in cancers such as colon cancer, gastric cancer, pancreatic cancer, esophageal cancer, especially in the occurrence and metastasis of colon cancer, can be used as a new target for the treatment of colon cancer [78-80]. TPX2 is a microtubule-associated protein encoded by a gene located on a human chromosome that promotes proliferation and metastasis of colon cancer cells and serves as a novel prognostic biomarker and therapeutic target for colon cancer [81]. Iron-saturated bovine lactoferrin (Fe-bLf) nanocarriers have anti-cancer effects and can be used to target colon cancer and other cancer stem cells [82]. The p38γ-activated ternary complex of Hsp90 and K-Ras can be used as a novel target for the treatment of K-Ras-dependent colon cancer [83]. The developmental pluripotency-related 4 (Dppa4) gene plays an important role in the self-renewal of embryonic stem cells. This gene can be re-expressed in many malignant tumors and has been shown to play a large role in the development of colon cancer. So it can be used as a new target for the treatment of colon cancer [84]. Morita et al. have found that DnaJ (Hsp40) homologous subfamily B member 8 (DNAJB8), a member of the heat shock protein (HSP) 40 family, have the effect of inhibiting the accumulation of erroneous folding toxic proteins, which may be related to the development and metastasis of cancer. Combining DNAJ88 targeted immunotherapy with other standard therapies may effectively cure cancer and will be a new target for colon cancer targeted immunotherapy [85].
Traditional medicine
Traditional Chinese medicine is widely used in various inflammation-related diseases. The main components of the medicinal plant Scutellaria baicalensisis are baicalin and scutellarin. The study using human intestinal bacteria group culture and HPLC analysis found that baicalin can be converted to baicalein. Another study shows that baicalin has a limited anti-proliferative effect on cancer cells, while baicalein has a significant anti-proliferative effect on cancer cells, especially on HCT-116 human colon cancer cells, therefore, baicalein is an effective anti-cancer metabolite and has a chemo preventive effect on colon cancer [86-88]. The saponin AST obtained from the medicinal plant Astragalus can play a role in anti-tumor and apoptosis promotion in colon cancer cells [89,90]. Araliaceae ginseng can prevent and treat many chronic diseases. Several studies have shown that ginsenosides Rg3 and Rh2 in ginseng have anticancer effects, which can reduce the incidence of colon cancer, while protosan diol (PPD) can increase the anticancer effect of the chemotherapeutic agent fluorouracil (5-FU), thereby improving the overall condition of patients [91-93]. Guan Chang Fu Fang (GCFF) is extracted from the three plants of Agrimonia pilosa Ledeb., Patrinia scabiosaefolia and Solanum nigrum L., which is similar to the original ginseng diol (PPD).It can also be combined with fluorouracil (5-FU) to treat colon cancer [94,95]. However, Wang et al. have indicated that 5-FU can cause intestinal flora disorder and colon damage, and the polysaccharide carboxymethylated sclerotium (CMP) isolated from Poria cocos can regulate the balance of intestinal flora and alleviate FU-induced colon injury [96-98]. The main active components of Sophora flavescens are alkaloids and brass, and its different components have a good inhibitory effect on the activities of cells such as SW1116, SW620 and SW480, and the ethanol extract of Sophora also inhibited the proliferation of colon cancer HT29 cells [99,100]. Yang et al. have explored that the consumption of Ganoderma lucidum mushroom can prevent the occurrence of colon cancer in rodents, so the mechanism of action was studied and it was found that Ganoderma lucidum extract regulates secondary bile acids, flora, mucin and propionate associated with colon cancer. It has an impact on colon health [101]. Some prescriptions such as Shiquan Dabu Decoction, Shenqi Decoction, Qihuang Decoction, Jiedu Sangen Decoction, etc. can also be used to treat colon cancer, and the mechanism of action can be applied to the method of metabolomics of traditional Chinese medicine. Metabolomics is based on the symptom as the entry point, using prescriptions as the research object, using metabolomics technology to discover and identify the biomarkers of the symptoms, and using the biomarkers of the symptoms as parameters to evaluate the overall efficacy of the prescription. Using this method we can understand the mechanism of action and provide effective help for the treatment of colon cancer [102-104].
Western medicine
The main means of treating colon cancer is surgery, and prevention of postoperative recurrence is also important, so effective postoperative adjuvant chemotherapy is needed. Sadahiro et al. have evidence that 5-fluorouracil (5-FU) monotherapy and 5-FU plus Oxali Platinum is an effective adjuvant chemotherapy program for colon cancer surgery, especially for adjuvant chemotherapy in patients with stage III colon cancer [105,106]. The new study shows that the combination of three active chemotherapeutic drugs, namely leucovorin, oxaliplatin (XELOX), 5-fluorouracil and irinotecan, can improve the safety and effectiveness of adjuvant chemotherapy and improves patient survival [107]. However, it has been reported that capecitabine and oxaliplatin may induce coronary vasospasm and acute coronary thromboembolism [108,109]. Taking bevacizumab may also form thrombosis, and there are differences between people, black patients are more likely to develop thrombosis than white patients, and are prone to occur with increasing age [110]. Panitumab (P-XELOX) can be combined with oxaliplatin and oral capecitabine to treat colon cancer and advanced liver metastases [111]. Matsuda et al. have found that after treatment with capecitabine, oxaliplatin and bevacizumab in patients with liver metastases with advanced colon cancer, symptoms of hand-foot syndrome (HFS) have emerged [112,113]. Simvastatin combined with irinotecan could overcome the resistance of irinotecan, and the combination of the two in a molar ratio of 2:1 can achieve the best effect to treat colon cancer [114].
Other therapies
In order to treat colon cancer, a colitis-associated colon cancer model of azomethane/dextran sulfate (AOM / DSS) was established in the laboratory, and this model was used to analyze the role of p38γ and p38δ in colon cancer associated with colitis, it can be found that p38γ / δ deletion can reduce tumor formation, but p38 γ / δ deficiency does not have much effect on advanced tumors [115]. Iwanaga et al. have evidence that prostaglandin D2 (PGD2) produced by mast cells can inhibit colitis and subsequent tumor formation, and can be used to prevent and treat colon cancer [116]. Canine uric acid (KYNA) acts as a tryptophan metabolite to inhibit the proliferation of cancer cells such as colon cancer and kidney cancer, and KYNA is considered to be a potential chemo preventive agent for colon cancer [117-119]. The small molecule compound sulfidic can prevent colon cancer from treating pre-stage adenoma, but it is toxic in the cardiovascular and renal systems. Lee et al. have explored that small doses of sulfidic can be used in combination with other chemo preventive agents to treat colon cancer, and can increase its effectiveness [120]. 5-Fu is the most commonly used chemoprevention therapy for the therapy of colon cancer. Fu et al. have noticed that when combined with 5-Fu, antioxidants sometimes do not work for colon cancer patients, but antioxidants can pass regulation of Src-dependent caspase-7 phosphorylation, thereby reducing the apoptosis of 5-Fu in colon cancer, and improving the therapeutic effect [121]. Cytochrome P450 2W1 (CYP2W1) is a monooxygenase that can be detected in 30% of colon cancers, but it is not expressed in non-transformed adult tissues. It can be used as a new treatment for colon cancer [122,123]. Bee venom (BV) has anticancer activity and is a traditional medicine for treating skin diseases, cancerous tumors and rheumatism. Recently, Zhenget al. have evidence that BV can induce apoptosis by activating DR4 and DR5 and inhibiting NF-kB, thereby inhibiting the growth of colon cancer cells [124]. Vitamin D can participate in various physiological functions including immune response in the human body, it can regulate intestinal barrier function and antibacterial peptide synthesis. Epidemiological studies have found that vitamin D supplementation can alleviate the symptoms of colitis and have a protective and therapeutic effect on colon cancer [125].
Xiaet al. have found that laparoscopic surgery has become an alternative treatment for colon-related diseases in recent years, and laparoscopic colectomy has many advantages in postoperative recovery compared with conventional surgery, because inflammation can promote colon cancer recurrence and metastasis, and laparoscopic surgery may also cause postoperative complications. Therefore, doctors should minimize their postoperative complications and improve their survival rate through their own efforts [126,127]. Postoperative intestinal obstruction (POI) is the most common complication after intestinal surgery and is associated with dendritic cells (DCs) and macrophages. Pohl et al. have explored that modifying the intestinal flora can prevent inappropriate activation of these cells. It can be used as a new method to prevent POI [128]. During the patient’s hospitalization, for patients with stage III colon cancer, the communication and cooperation between the surgeon and the oncologist can promptly identify postoperative and chemotherapy-related complications, reduce some unnecessary errors and improve patient care strategies in a timely manner [129], and appropriate individual treatment strategies can be used to improve patient survival [130]. Modified FOLFOX is a widely accepted standard of treatment for colon cancer removal. Kotaka et al. have noticed that modified FOLFOX6 can be used as adjunctive therapy for patients with stage II or III colon cancer radical resection, but further research is needed [131]. In addition, elderly patients with locally advanced and metastatic colon cancer need to consider the pharmacokinetics and pharmacodynamics of the drug during treatment, as well as the performance status and assessment of activities of daily living (ADL) or instrumental ADL, and tailored treatment options for each patient and timely return visits [132].
Prevention
People with colon cancer are the result of many factors interacting with obesity, sedentary, diet, alcohol, smoking, low-fiber intake, high-fat diet, sleep, and lack of exercise are all related to the incidence of colon cancer [133-136]. Colon cancer can be prevented to a large extent. By establishing a corresponding mouse model, it is found that dietary adjustment and nutrient intake can prevent colon cancer very well. An important mechanism of action is by regulating the concentration of micronutrients in the target tissue [137], for example, vitamin E is a micronutrient that has the characteristics of preventing colon cancer, and can appropriately increase the intake of vitamin E in the diet, but it cannot be overdose because high alpha-tocopherol intakes from vitamin E supplements can result in decreased blood gamma-tocopherol concentrations, while gamma-tocopherol has a higher ability to prevent cancer than alpha-tocopherol [138,139]. Pomegranate juice and citrus juice can also be added to the diet, and the polyphenols in the juice have chemo preventive properties against colon cancer [140]. The main ingredient of hot-processed ginger is Shogaols, which has proven to be a very effective anti-cancer agent, and studies have shown that the coupled metabolite of Shogaols and cysteine can be used as a novel dietary preventive agent for colon cancer [141]. Liu et al. have evidence that the main triggers of colon cancer are inflammation and intestinal flora, and regular consumption of broccoli not only has great benefits for our health, but also reduces the risk of cancer and inflammatory diseases, thereby preventing colon cancer [142]. Del Pino-García et al. have found that red wine pomace seasoning has anti-proliferative and antigenic toxic effects, and has the potential to prevent colon cancer [143]. “Mediterranean” and vegetarian diets including oils, olive oil and vegetables and oily fish, have anti-inflammatory effects, prevent intestinal flora imbalance and inflammatory bowel disease, and can prevent the occurrence of colon cancer [144]. In addition, in the diet, a high animal protein diet promotes the binding of bile acids to taurine, and the combination of the two will produce a toxic compound hydrogen sulfide and tumor promoter deoxycholic acid through intestinal flora metabolism, which will promote colon cancer [145,146], so in the daily diet, should avoid high animal protein and other foods. In addition, Zelenskiy et al. have explored that the risk of colon cancer increases with age, and high dietary glucose load (GL) also increases the risk of colon cancer, while the high GL diet in the elderly will triple the risk of cancer, so people should take a low GL diet, especially for the elderly, which can reduce the risk of colon cancer and achieve the purpose of prevention [147]. Fat intake should also be reduced in the daily diet because a high-fat diet promotes inflammation and exacerbates the severity of colitis [148]. In addition, Nurdin et al. have found that dietary fiber supplementation can play a role in preventing colon cancer, and dietary fiber mixtures such as a traditional Indonesian dietary fiber may have a more pronounced effect on colon cancer than single dietary fiber [149]. Dietary emulsifier is a ubiquitous processed food ingredient that can change the composition of the intestinal flora and promote low-grade inflammation in the intestine. Therefore, reducing dietary emulsifier intake can prevent inflammatory bowel disease and colon cancer [150]. In today’s society, sedentary behavior is ubiquitous due to work. Cong et al. have evidence that the risk of colon cancer in a sedentary population increases by 30%, therefore, reducing sedentary behavior in daily life and work can prevent colon cancer [151]. When people have symptoms of colitis such as diarrhea and abdominal pain, they should pay attention and go to the hospital for examination [152]. In addition, HAMLET (human alpha-lactalbumin lethal to tumor cells) can be used as a new oral drug for the prevention and therapy of colon cancer, especially those with colon cancers carrying APC mutations [153,154]. The RNA-binding protein HuR, a small molecular target, can be used to prevent colon cancer in high-risk populations with familial adenoma polyposis (FAP) or inflammatory bowel disease (IBD) [155]. At the same time, researchers have found that long-term exposure to antibiotics can thin the intestinal protective mucus layer and increase the risk of colon cancer, so reduce the exposure of antibiotics such as vancomycin and streptomycin can effectively prevent colon cancer [156]. Colon cancer is a heterogeneous disease caused by at least two precursors, conventional adenoma (CA) and serrated polyps, and Peters et al. have explored that the intestinal flora may play through the development of CAs in the early stages of colon cancer. Therefore, it can further study the early driving factors of intestinal flora in colon cancer and effectively prevent colon cancer [157].
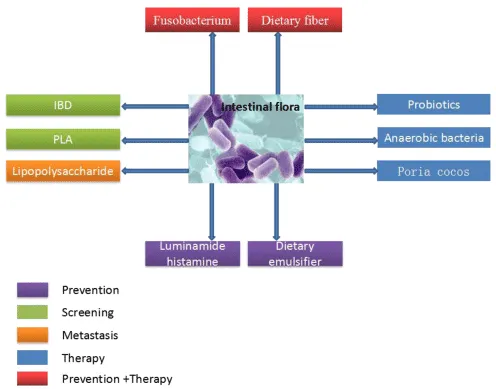
It can be seen from the above that the intestinal flora plays an important role in the disease of colon cancer, which is not only related to the screening diagnosis of colon cancer, but also related to the metastasis, treatment and prevention of colon cancer. The multiple effects of intestinal flora on colon cancer are shown in figure 3. We should constantly study the multiple mechanisms of action of the intestinal flora, which can better control colon cancer and reduce the harm of colon cancer to human health.
Future prospects
Continuous research on colon cancer has found that left and right colon cancers have different clinical and biological characteristics and require continuous efforts to provide personalized treatments. Recent studies have found that intestinal flora can regulate immunity, improve inflammation, and inhibit the growth of cancer cells. Intestinal flora has also been shown to be useful in the treatment of pancreatic cancer, breast cancer, liver cancer, lung cancer, bladder cancer, kidney stones, autoimmune diseases, etc. In order to better utilize the intestinal flora and understand its mechanism of action, further research is needed.
Conclusion
This article systematically introduces the latest advances in the study of colon cancer and its treatment, and summarizes the advantages and disadvantages of effective methods and methods for screening, diagnosis, treatment and prevention of colon cancer. The methods for treating colon cancer are introduced from the intestinal flora, targeting, Chinese medicine, western medicine and other aspects, respectively, which gives us a more comprehensive understanding of colon cancer. As people’s living conditions continue to improve, they are also more concerned about the health of themselves and their families, but the preventive measures in daily life may not completely curb the occurrence of colon cancer. Therefore, effective treatment is particularly important, and prognostic care should also be taken seriously. In recent years, studies have found that intestinal flora not only has a good effect on the treatment of colon cancer, but also plays an important role in the screening, diagnosis, metastasis and prevention of colon cancer. So we can continue and focus on research to achieve better results, better to provide protection for human health.
This work was supported by grants from the Key Program of Natural Science Foundation of State (Grant No. 81830110, 81430093, 81373930, 81673586, 81703685, 81302905, 81503386), National Key Subject of Drug Innovation (Grant No. 2015ZX09101043-005, 2015ZX09101043-011), TCM State Administration Subject of Public Welfare of (Grant No. 2015468004), Major Projects of Application Technology Research and Development Plan in Heilongjiang Province (GX16C003), TCM State Administration Subject of Public Welfare (Grant No. 2015468004), Specialized Research Fund for the Doctoral Program of Higher Education (20132327130001, 20122327120006), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2015118, UNPYSCT-2016213), Young Talent Lift Engineering Project of China Association of Traditional Chinese Medicine (QNRC2-B06), Outstanding Talents Foundation of Heilongjiang University of Chinese Medicine (2018jc01), Doctoral Innovation Fund of Heilongjiang University of Chinese Medicine (2018bs02), Foundation of Heilongjiang University of Chinese Medicine (Grant no. 201209), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2015118, UNPYSCT-2016213, UNPYSCT-2016212), Application Technology and Development of Youth Talents Project in Harbin (2014RFQXJ116), Chinese Postdoctoral Science Foundation (2017M621319b), Returned Oversea Scholars Program of Heilongjiang Province (2017QD0025), Natural Science Foundation of Heilongjiang Province of China (QC2018117).
- Liu C, Huang Z, Jiang H, Shi F (2014) The sirtuin 3 expression profile is associated with pathological and clinical outcomes in colon cancer patients. Biomed Res Int 2014: 871263. Link: https://goo.gl/MM8XG9
- Kang M, Martin A (2017) Microbiome and colorectal cancer: Unraveling host-microbiota interactions in colitis-associated colorectal cancer development. Semin. Immunol 32: 3-13. Link: https://goo.gl/q24C2r
- Liu X, Duan B, Dong Y, He C, Zhou H, et al. (2014) MicroRNA-139-3p indicates a poor prognosis of colon cancer. Int J Clin Exp Pathol 7: 8046-8052. Link: https://goo.gl/JEQARx
- Hou Y, Liu YY, Zhao XK (2013) Expression of aldehyde dehydrogenase 1 in colon cancer. Asian Pac J Trop Med 6: 574-577. Link: https://goo.gl/RkQjPo
- Zhou E, Huang Q, Wang J, Fang C, Yang L, et al. (2015) Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int J Clin Exp Pathol 8: 8018-8027. Link: https://goo.gl/PrcNcL
- Hong J, Sun J, Huang T (2013) Increased expression of TRPS1 affects tumor progression and correlates with patients' prognosis of colon cancer. Biomed Res Int 2013: 454085. Link: https://goo.gl/h2kRMe
- Siegel R, Desantis C, Jemal A (2014) Colorectal cancer statistics, 2014. CA Cancer J Clin 64: 104-117. Link: https://goo.gl/4NYyJf
- Bhattacharjee PK, Halder S (2013) Combined adenocarcinoma-carcinoid tumor of transverse colon. J Cancer Res Ther 9: 746-747. Link: https://goo.gl/d64x6y
- Winner M, Mooney SJ, Hershman DL, Feingold DL, Allendorf JD, et al. (2013) Management and outcomes of bowel obstruction in patients with stage IV colon cancer: a population-based cohort study. Dis Colon Rectum 56: 834-843. Link: https://goo.gl/BiQs6E
- Zhang A, Sun H, Wang X (2014) Potentiating therapeutic effects by enhancing synergism based on active constituents from traditional medicine. Phytother Res 28: 526–533. Link: https://goo.gl/at5rfE
- Hale VL, Chen J, Johnson S, Harrington SC, Yab TC, et al. (2017) Shifts in the Fecal Microbiota Associated with Adenomatous Polyps. Cancer Epidemiol. Biomarkers Prev 26: 85-94. Link: https://goo.gl/MqL45h
- Zhang K, Yan G, Zhang A, Sun H, Wang X (2017) Recent advances in pharmacokinetics approach for herbal medicine. RSC Advances 7: 28876-28888. Link: https://goo.gl/xu7QEN
- Ojidu H, Palmer H, Lewandowski J, Hampton J, Blakeborough T, et al. (2018) Patient tolerance and acceptance of different colonic imaging modalities: an observational cohort study. Eur J Gastroenterol Hepatol 30: 520-525. Link: https://goo.gl/ctF5oX
- Sun H, Wu F, Zhang A, Wei W, Han Y, et al. (2013) Profiling and identification of the absorbed constituents and metabolites of Schisandra lignans by ultra-performance liquid chromatography coupled to mass spectrometry. Biomed Chromatogr 27: 1511–1519. Link: https://goo.gl/dv5RsR
- Xu H, Lai W, Zhang Y, Liu L, Luo X, et al. (2014) Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer 14: 330. Link: https://goo.gl/AXhXdC
- Zhou L, Yu L, Zhu B, Wu S, Song W, et al. (2016) Metastasis-associated in colon cancer-1 and aldehyde dehydrogenase 1 are metastatic and prognostic biomarker for non-small cell lung cancer. BMC Cancer 16: 876. Link: https://goo.gl/ti5nki
- Zhang A, Sun H, Qiu S (2013) Advancing drug discovery and development from active constituents of yinchenhao tang, a famous traditional chinese medicine formula. Evidence-Based Complementray and Alternative Medicine 2013: 257909. Link: https://goo.gl/jZz2eT
- Bai Y, Qiu J, Shang X, Liu P, Zhang Y, et al. (2015) Differential diagnosis and cancer staging of a unique case with multiple nodules in the lung - lung adenocarcinoma, metastasis of colon adenocarcinoma, and colon adenocarcinoma metastasizing to lung adenocarcinoma. Thorac Cancer6: 363-367. Link: https://goo.gl/iRWhQt
- Xie MJ, Ma YH, Miao L, Wang Y, Wang HZ, et al. (2014) Emodin-provoked oxidative stress induces apoptosis in human colon cancer HCT116 cells through a p53-mitochondrial apoptotic pathway. Asian Pac J Cancer Prev 15: 5201-5205. Link: https://goo.gl/XnKuFS
- Sun H, Liu J, Zhang A (2016) Characterization of the multiple components of AcanthopanaxSenticosus stem by ultra-high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. Journal of Separation Science 39: 496-502. Link: https://goo.gl/UH4WGp
- Gurluler E, Tumay LV, Guner OS, Kucukmetin NT, Hizli B, et al. (2014) Oncostatin-M as a novel biomarker in colon cancer patients and its association with clinicopathologic variables. Eur Rev Med Pharmacol Sci 18: 2042-2047. Link: https://goo.gl/yGpRji
- Bostanci O, Kemik O, Kemik A, Battal M, Demir U, et al. (2014) Preoperative serum levels of mesothelin in patients with colon cancer. Dis Markers 2014: 161954. Link: https://goo.gl/mi2Rp2
- Zhang Y, Zhang A, Zhang Y, Sun H, Meng X, et al. (2016) Application of Ultra-performance Liquid Chromatography with Time-of-Flight Mass Spectrometry for the Rapid Analysis of Constituents and Metabolites from the Extracts of Acanthopanaxsenticosus Harms Leaf. Pharmacogn Mag 12: 145-152. Link: https://goo.gl/NbxskN
- Teng CL, Yu JT, Chen YH, Lin CH, Hwang WL (2014) Early colonoscopy confers survival benefits on colon cancer patients with pre-existing iron deficiency anemia: a nationwide population-based study. PLoS One 9: e86714. Link: https://goo.gl/fvon5M
- Chen J, Pitmon E, Wang K (2017) Microbiome, inflammation and colorectal cancer. Semin. Immunol 32: 43-53. Link: https://goo.gl/S1M5iZ
- Bamola VD, Ghosh A, Kapardar RK, Lal B, Cheema S, et al. (2017) Gut microbial diversity in health and disease: experience of healthy Indian subjects, and colon carcinoma and inflammatory bowel disease patients. Microb. Ecol. Health Dis 28: 1322447. Link: https://goo.gl/yKjvQF
- Zhang A, Sun H, Wang X (2014) Urinary metabolic profiling of rat models revealed protective function of scoparone against alcohol induced hepatotoxicity. Sci Rep 4: 6768. Link: https://goo.gl/DjQogh
- Piper MS, Saad RJ (2017) Diabetes Mellitus and the Colon. Curr Treat Options Gastroenterol 15: 460-474. Link: https://goo.gl/ujGkoj
- Heo NY, Hong YM, Kim TO, Moon YS, Yang SY, et al. (2016) The Prevalence of Colonic Neoplasm in Cryptogenic Pyogenic Liver Abscess: A Prospectively Enrolled Cross-sectional Study. Korean J Gastroenterol 68: 195-201. Link: https://goo.gl/kNpoj4
- Wang X, Lv H, Zhang A (2014) Metabolite profiling and pathway analysis of acute hepatitis rats by UPLC-ESI MS combined with pattern recognition methods. Liver Int 34: 759–770. Link: https://goo.gl/rnfsLK
- Kongkam P, Linlawan S, Aniwan S, Lakananurak N, Khemnark S, et al. (2014) Forward-viewing radial-array echoendoscope for staging of colon cancer beyond the rectum. World J Gastroenterol 20: 2681-2687. Link: https://goo.gl/yYsrZ4
- Wang X, Zhang A, Yan G (2014) UHPLC-MS for the analytical characterization of traditional Chinese me-dicines. Trac Trends in Analytical Chemistry 63: 180-187. Link: https://goo.gl/aLyHcb
- Wang J, Du Y, Liu X, Cho WC, Yang Y (2015) MicroRNAs as Regulator of Signaling Networks in Metastatic Colon Cancer. Biomed Res Int 2015: 823620. Link: https://goo.gl/MxwkCG
- Zhang A, Sun H, Wang X (2018) Mass spectrometry-driven drug discovery for development of herbal medicine. Mass Spectrom Rev 37: 307-320. Link: https://goo.gl/FoWBXD
- Sinagra E, Raimondo D, Gallo E, Stella M, Cottone M, et al. (2014) Could JC virus provoke metastasis in colon cancer? World J Gastroenterol 20: 15745-15749. Link: https://goo.gl/NbnBT8
- Sinagra E, Raimondo D, Gallo E, Stella M, Cottone M, et al. (2014) Could JC virus provoke metastasis in colon cancer? World J Gastroenterol 20: 15745-15749. Link: https://goo.gl/NbnBT8
- Xue C, Zhang A, Sun H (2014) An improved ultra-performance liquid chromatography-electro spray ioniza-tion/quadrupole-time-of-flight high-definition mass spectrometry method for determining ingredients of herbal Fructus corni in blood samples. Pharmacognosy Magazine 10: 422-429. Link: https://goo.gl/PEo9oc
- Jain T, Williams R, Liechty B, Ann Chen L (2016) Vertebral Metastasis as the Initial Manifestation of Colon Cancer. ACG Case Rep J 3: e122. Link: https://goo.gl/VM4hhm
- Sun H, Wu F, Zhang A, Wei W, Han Y, et al. (2013) Pharmacokinetic study of schisandrin, schisandrol B, schisantherin A, deoxyschisandrin, and schisandrin B in rat plasma after oral administration of S hengmaisan formula by UPLC‐MS. Journal of separation science 36: 485-491. Link: https://goo.gl/xpo2cy
- Hori T, Okada N, Nakauchi M, Hiramoto S, Kikuchi-Mizota A, et al. (2013) Hematogenous umbilical metastasis from colon cancer treated by palliative single-incision laparoscopic surgery. World J Gastrointest Surg 5: 272-277. Link: https://goo.gl/TwZmRt
- Sun H, Zhang Hl (2018) Network pharmacology combined with functional metabolomics discover bile acid metabolism as a promising target for mirabilite against colorectal cancer. RSC Adv 8: 30061-30070. Link: https://goo.gl/mfbcVS
- Lu Y, Zhao X, Li K, Luo G, Nie Y, et al. (2013) Thioredoxin-like protein 2 is overexpressed in colon cancer and promotes cancer cell metastasis by interaction with ran. Antioxid Redox Signal 19: 899-911. Link: https://goo.gl/eVyDph
- Morita R, Nishizawa S, Torigoe T, Takahashi A, Tamura Y, et al. (2014) Heat shock protein DNAJB8 is a novel target for immunotherapy of colon cancer-initiating cells. Cancer Sci 105: 389-395. Link: https://goo.gl/tYXSbz
- Wang X, Zhang A, Yan G, Sun W, Han Y, (2013) Metabolomics and proteomics annotate therapeutic properties of geniposide: targeting and regulating multiple perturbed pathways. PLoS One 8: e71403. Link: https://goo.gl/8WdZnj
- Hullar MA, Fu BC (2014) Diet, the gut microbiome, and epigenetics. Cancer J 20: 170-175. Link: https://goo.gl/g97geC
- Boursi B, Werner TJ, Gholami S, Houshmand S, Mamtani R, et al. (2018) Functional imaging of the interaction between gut microbiota and the human host: A proof-of-concept clinical study evaluating novel use for 18F-FDG PET-CT. PLoS One 13: e0192747. Link: https://goo.gl/DA6uLv
- Wang X, Zhang A, Hui S (2016) Discovery and development of innovative drug from traditional medicine by integrated chinmedomics strategies in the post-genomic era. Trends in Analytical Chemistry 76: 86-94. Link: https://goo.gl/YTvFjL
- Wroblewski LE, Peek RJ, Coburn LA (2016) The Role of the Microbiome in Gastrointestinal Cancer. Gastroenterol Clin North Am 45: 543-556. Link: https://goo.gl/zNmpwF
- Zhu Q, Jin Z, Wu W, Gao R, Guo B, et al. (2014) Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PLoS One 9: e90849. Link: https://goo.gl/77aVCS
- Rezasoltani S, Asadzadeh-Aghdaei H, Nazemalhosseini-Mojarad E, Dabiri H, Ghanbari R, et al. (2017) Gut microbiota, epigenetic modification and colorectal cancer. Iran J Microbiol 9: 55-63. Link: https://goo.gl/c3hexp
- Zhang A, Zou D, Yan G, Tan Y, Sun H, et al. (2014) Identification and characterization of the chemical constituents of Simiao Wan by ultra high performance liquid chromatography with mass spectrometry coupled to an automated multiple data processing method. Journal of separation science 37: 1742-1747. Link: https://goo.gl/HmEEQq
- Tomkovich S, Yang Y, Winglee K, Gauthier J, Mühlbauer M, et al. (2017) Locoregional Effects of Microbiota in a Preclinical Model of Colon Carcinogenesis. Cancer Res 77: 2620-2632. Link: https://goo.gl/GbYP29
- Zhang T, Zhang A, Qiu S (2016) Current Trends and Innovations in Bioanalytical Techniques of Metabolomics. Critical Reviews in Analytical Chemistry 46: 342-351. Link: https://goo.gl/wPjPFU
- Mehta RS, Nishihara R, Cao Y, Song M, Mima K, et al. (2017) Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol 3: 921-927. Link: https://goo.gl/JoetjL
- Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, et al. (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22: 292-298. Link: https://goo.gl/iXDQem
- Ye X, Wang R, Bhattacharya R, Boulbes DR, Fan F, et al. (2017) Fusobacterium Nucleatum Subspecies Animalis Influences Proinflammatory Cytokine Expression and Monocyte Activation in Human Colorectal Tumors. Cancer Prev Res (Phila) 10: 398-409. Link: https://goo.gl/CCvvco
- Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, et al. (2016) Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65: 1973-1980. Link: https://goo.gl/UQzmCD
- Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, et al. (2015) Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol 1: 653-661. Link: https://goo.gl/ijZeUu
- Zhu W, Winter MG, Byndloss MX, Spiga L, Duerkop BA, et al. (2018) Precision editing of the gut microbiota ameliorates colitis. Nature 553: 208-211. Link: https://goo.gl/vDCizn
- Ranji P, Akbarzadeh A, Rahmati-Yamchi M (2015) Associations of Probiotics with Vitamin D and Leptin Receptors and their Effects on Colon Cancer. Asian Pac J Cancer Prev 16: 3621-3627. Link: https://goo.gl/wyGi1Z
- Tojo R, Suárez A, Clemente MG, de los Reyes-Gavilán CG, Margolles A (2014) Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol 20: 15163-5176. Link: https://goo.gl/UwCQSE
- Shang M, Sun J (2017) Vitamin D/VDR, Probiotics, and Gastrointestinal Diseases. Curr. Med. Chem 24: 876-887. Link: https://goo.gl/RWGSoH
- Liu Q, Zhang A, Wang L (2016) High-throughput chinmedomics-based prediction of effective components and targets from herbal medicine AS1350. Scientific Reports 6: 38437. Link: https://goo.gl/jzvtxX
- Gao C, Ganesh BP, Shi Z, Shah RR, Fultz R, et al. (2017) Gut Microbe-Mediated Suppression of Inflammation-Associated Colon Carcinogenesis by Luminal Histamine Production. Am. J. Pathol 187: 2323-2336. Link: https://goo.gl/i83Fm5
- Fang H, Zhang A, Yu J (2016) Insight into the metabolic mechanism of scoparone on biomarkers for inhibiting Yanghuang syndrome. Scientific Reports 6: 37519. Link: https://goo.gl/DMw5KZ
- Bultman SJ (2016) The microbiome and its potential as a cancer preventive intervention. Semin Oncol 43: 97-106. Link: https://goo.gl/3MUTLi
- Ericsson AC, Akter S, Hanson MM, Busi SB, Parker TW, et al. (2015) Differential susceptibility to colorectal cancer due to naturally occurring gut microbiota. Oncotarget 6: 33689-33704. Link: https://goo.gl/N6nzjF
- Zou S, Fang L, Lee MH (2018) Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep (Oxf) 6: 1-12. Link: https://goo.gl/TfcYBR
- Llewellyn SR, Britton GJ, Contijoch EJ, Vennaro OH, Mortha A (2018) Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 154: 1037-1046. Link: https://goo.gl/pvMtbV
- Byerley LO, Samuelson D, Luo M, Lorenzen BN, Banks S, et al. (2017) Changes in the gut microbial communities following addition of walnuts to the diet. J. Nutr. Biochem 48: 94-102. Link: https://goo.gl/T9AsXH
- Wang X, Yang Y, Huycke MM (2017) Commensal-infected macrophages induce dedifferentiation and reprogramming of epithelial cells during colorectal carcinogenesis. Oncotarget 8: 102176-102190. Link: https://goo.gl/6Zf761
- Wang H, Sun H, Zhang A, Li Y, Wang L, et al. (2013) Rapid identification and comparative analysis of the chemical constituents and metabolites of P hellodendri amurensis cortex and Z hibai dihuang pill by ultra‐performance liquid chromatography with quadrupole TOF‐MS. Journal of separation science 36: 3874-3882. Link: https://goo.gl/c1Ttfr
- Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, et al. (2013) Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One 8: e70803. Link: https://goo.gl/AUnM41
- Klaassen CD, Cui JY (2015) Review: Mechanisms of How the Intestinal Microbiota Alters the Effects of Drugs and Bile Acids. Drug Metab Dispos 43: 1505-1521. Link: https://goo.gl/BPyDb3
- Wang J, Du Y, Liu X, Cho WC, Yang Y (2015) MicroRNAs as Regulator of Signaling Networks in Metastatic Colon Cancer. Biomed Res Int, 2015: 823620. Link: https://goo.gl/wDDLmn
- Yang Y, Weng W, Peng J, Hong L, Yang L, et al. (2017) Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-kappaB, and Up-regulating Expression of MicroRNA-21. Gastroenterology 152: 851-866. Link: https://goo.gl/37VXvJ
- Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, et al. (2016) Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol 22: 557-566. Link: https://goo.gl/V7Swu4
- Luo ZF, Zhao D, Li XQ, Cui YX, Ma N, et al. (2016) Clinical significance of HOTAIR expression in colon cancer. World J Gastroenterol 22: 5254-5259. Link: https://goo.gl/NyyY7K
- Wu ZH, Wang XL, Tang HM, Jiang T, Chen J, et al. (2014) Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol Rep 32: 395-402. Link: https://goo.gl/bK3qMe
- Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, et al. (2013) HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 32: 1616-1625. Link: https://goo.gl/RvsfJt
- Wei P, Zhang N, Xu Y, Li X, Shi D, et al. (2013) TPX2 is a novel prognostic marker for the growth and metastasis of colon cancer. J Transl Med 11: 313. Link: https://goo.gl/R75zXf
- Kanwar JR, Mahidhara G, Roy K, Sasidharan S, Krishnakumar S, et al. (2015) Fe-bLf nanoformulation targets survivin to kill colon cancer stem cells and maintains absorption of iron, calcium and zinc. Nanomedicine (Lond) 10: 35-55. Link: https://goo.gl/5W1wtN
- Qi X, Xie C, Hou S, Li G, Yin N, et al. (2014) Identification of a ternary protein-complex as a therapeutic target for K-Ras-dependent colon cancer. Oncotarget 5: 4269-4282. Link: https://goo.gl/baWaoi
- Zhang M, Cui F, Lu S, Lu H, Xue Y, et al. (2015) Developmental pluripotency-associated 4: a novel predictor for prognosis and a potential therapeutic target for colon cancer. J Exp Clin Cancer Res 34: 60. Link: https://goo.gl/saQDZQ
- Morita R, Nishizawa S, Torigoe T, Takahashi A, Tamura Y, et al. (2014) Heat shock protein DNAJB8 is a novel target for immunotherapy of colon cancer-initiating cells. Cancer Sci 105: 389-395. Link: https://goo.gl/K1TozB
- Wang CZ, Zhang CF, Chen L, Anderson S, Lu F, et al. (2015) Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int J Oncol 47: 1749-1758. Link: https://goo.gl/HFpdH8
- Wang X, Zhang A, Zhou X, Liu Q, Nan Y, et al. (2016) An integrated chinmedomics strategy for discovery of effective constituents from traditionalherbal medicine. Sci Rep 6: 18997. Link: https://goo.gl/3vJ42m
- Wang X, Zhang A, Zhou X, Liu Q, Nan Y, et al. (2016) An integrated chinmedomics strategy for discovery of effective constituents from traditional herbal medicine. Sci Rep 6: 18997. Link: https://goo.gl/3vJ42m
- Wang Y, Auyeung KK, Zhang X, Ko JK (2014) Astragalus saponins modulates colon cancer development by regulating calpain-mediated glucose-regulated protein expression. BMC Complement Altern Med 14: 401. Link: https://goo.gl/BAuMyz
- Liu Q, Zhang A, Wang L, Yan G, Zhao H, et al. (2016) High-throughput chinmedomics-based prediction of effective components and targets from herbal medicine AS1350. Sci Rep 6: 38437. Link: https://goo.gl/w9RNdQ
- Vayghan HJ, Ghadimi SS, Nourazarian AR (2014) Preventive and therapeutic roles of ginseng - focus on colon cancer. Asian Pac J Cancer Prev 15: 585-588. Link: https://goo.gl/xDrHhj
- Wang CZ, Zhang Z, Wan JY, Zhang CF, Anderson S, et al. (2015) Protopanaxadiol, an active ginseng metabolite, significantly enhances the effects of fluorouracil on colon cancer. Nutrients 7: 799-814. Link: https://goo.gl/c9Ziaq
- Zhang Y, Zhang A, Zhang Y, Sun H, Meng X, et al. (2016) Application of Ultra-performance Liquid Chromatography with Time-of-Flight Mass Spectrometry for the Rapid Analysis of Constituents and Metabolites from the Extracts of Acanthopanax senticosus Harms Leaf. Pharmacogn Mag 12: 145-152. Link: https://goo.gl/mvoF5L
- Yu C, Liu SL, Qi MH, Zou X, Wu J, et al. (2015) Herbal medicine Guan Chang Fu Fang enhances 5-fluorouracil cytotoxicity and affects drug-associated genes in human colorectal carcinoma cells. Oncol Lett 9: 701-708. Link: https://goo.gl/7fpfTo
- Zhang A, Sun H, Wang X (2018) Mass spectrometry-driven drug discovery for development of herbal medicine. Mass Spectrom Rev 37: 307-320. Link: https://goo.gl/K6qSh5
- Wang C, Yang S, Gao L, Wang L, Cao L (2018) Carboxymethyl pachyman (CMP) reduces intestinal mucositis and regulates the intestinal microflora in 5-fluorouracil-treated CT26 tumour-bearing mice. Food Funct 9: 2695-2704. Link: https://goo.gl/2SH627
- Zhang A, Sun H, Wang X (2018) Mass spectrometrydriven drug discovery for development of herbal medicine.Mass Spectrom Rev 37: 307-320. Link: https://goo.gl/K6qSh5
- Liu Q, Zhang A, Wang L, Yan G, Zhao H, et al. (2016) High-throughput chinmedomics-based prediction of effective components and targets from herbal medicine AS1350.Sci Rep 6: 38437. Link: https://goo.gl/kerNaU
- Xiao ZM, Wang AM, Wang XY, Shen SR (2013) Effects of ethanol extract of Radix Sophorae Flavescentis on activity of colon cancer HT29 cells. Afr J Tradit Complement Altern Med 10: 352-355. Link: https://goo.gl/h1U75r
- Nan Y, Zhou X, Liu Q, Zhang A, Guan Y, et al. (2016) Serum metabolomics strategy for understanding pharmacological effects of ShenQi pill acting on kidney yang deficiency syndrome. J Chromatogr B Analyt Technol Biomed Life Sci 1026: 217-226. Link: https://goo.gl/ULXMKW
- Yang Y, Nirmagustina DE, Kumrungsee T, Okazaki Y, Tomotake H, et al. (2017) Feeding of the water extract from Ganoderma lingzhi to rats modulates secondary bile acids, intestinal microflora, mucins, and propionate important to colon cancer. Biosci. Biotechnol. Biochem 81: 1796-1804. Link: https://goo.gl/9hKP8s
- Zhang T, Zhang A, Qiu S, Yang S, Wang X (2016) Current Trends and Innovations in Bioanalytical Techniques of Metabolomics.Crit Rev Anal Chem 46: 342-351. Link: https://goo.gl/qeaESy
- Zhang AH, Yu JB, Sun H, Kong L, Wang XQ, et al. (2018) Identifying quality-markers from Shengmai San protects against transgenic mouse model of Alzheimer's disease using chinmedomics approach. Phytomedicine 45: 84-92. Link: https://goo.gl/i4EGwt
- Zhang AH, Sun H, Yan GL, Wang P, Han Y, et al. (2015) Chinmedomics: a new strategy for research of traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 40: 569-576. Link: https://goo.gl/cWKezW
- Sadahiro S, Tsuchiya T, Sasaki K, Kondo K, Katsumata K, et al. (2015) Randomized phase III trial of treatment duration for oral uracil and tegafur plus leucovorin as adjuvant chemotherapy for patients with stage IIB/III colon cancer: final results of JFMC33-0502. Ann Oncol 26: 2274-2280. Link: https://goo.gl/v3oCLB
- Schmoll HJ, Twelves C, Sun W, O'Connell MJ, Cartwright T, et al. (2014) Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol 15: 1481-1492. Link: https://goo.gl/15yT3Z
- Zhou H, Song Y, Jiang J, Niu H, Zhao H, et al. (2016) A pilot phase II study of neoadjuvant triplet chemotherapy regimen in patients with locally advanced resectable colon cancer. Chin J Cancer Res 28: 598-605. Link: https://goo.gl/AgAmF2
- A DO, Cleator S, Nihoyannopoulos P (2014) Acute coronary artery thrombosis and vasospasm following capecitabine in conjunction with oxaliplatin treatment for cancer. BMJ Case Rep 2014. Link: https://goo.gl/kYBJuX
- Sun H, Liu J, Zhang A, Zhang Y, Meng X, et al. (2016) Characterization of the multiple components of Acanthopanax Senticosus stem by ultra high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry.J Sep Sci 39: 496-502. Link: https://goo.gl/4BzDoH
- Hershman DL, Wright JD, Lim E, Buono DL, Tsai WY, et al. (2013) Contraindicated use of bevacizumab and toxicity in elderly patients with cancer. J Clin Oncol 31: 3592-3599. Link: https://goo.gl/nXA48C
- Leone F, Artale S, Marino D, Cagnazzo C, Cascinu S, et al. (2013) Panitumumab in combination with infusional oxaliplatin and oral capecitabine for conversion therapy in patients with colon cancer and advance liver metastases. The MetaPan study. Cancer 119: 3429-3435. Link: https://goo.gl/Jg58xE
- Matsuda S, Koketsu H, Hayakawa M, Nagata N (2015) Unilateral Capecitabine-related Hand-foot Syndrome. Intern Med 54: 2779. Link: https://goo.gl/q51scx
- Li XN, Zhang A, Sun H, Song Y, Zou D (2016) Rapid discovery of absorbed constituents and metabolites in rat plasma after the oraladministration of Zi Shen Wan using high-throughput UHPLC-MS with a multivariate analysisapproach.J Sep Sci 39: 4700-4711. Link: https://goo.gl/xYAFbV
- Jang HJ, Hong EM, Jang J, Choi JE, Park SW, et al. (2016) Synergistic Effects of Simvastatin and Irinotecan against Colon Cancer Cells with or without Irinotecan Resistance. Gastroenterol Res Pract 2016: 7891374. Link: https://goo.gl/uG7qjL
- Del RP, Alsina-Beauchamp D, Escós A, Cerezo-Guisado MI, Risco A (2014) Pro-oncogenic role of alternative p38 mitogen-activated protein kinases p38gamma and p38delta, linking inflammation and cancer in colitis-associated colon cancer. Cancer Res 74: 6150-6160. Link: https://goo.gl/5NKQWF
- Iwanaga K, Nakamura T, Maeda S, Aritake K, Hori M, et al. (2014) Mast cell-derived prostaglandin D2 inhibits colitis and colitis-associated colon cancer in mice. Cancer Res 74: 3011-3019. Link: https://goo.gl/7BuQLt
- Walczak K, Turski WA, Rajtar G (2014) Kynurenic acid inhibits colon cancer proliferation in vitro: effects on signaling pathways. Amino Acids 46: 2393-2401. Link: https://goo.gl/Kw31Xq
- Liu C, Zhang A, Yan GL, Shi H, Sun H, et al. (2017) High-throughput ultra high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry method forthe rapid analysis and characterization of multipleconstituents of Radix Polygalae.J Sep Sci 40: 663-670. Link: https://goo.gl/qXW4Qu
- Zhang A, Zhou X, Zhao H, Zou S, Ma CW, et al. (2017) Metabolomics and proteomics technologies to explore the herbal preparation affecting metabolic disorders using high resolution mass spectrometry. Mol Biosyst 13: 320-329. Link: https://goo.gl/f9SCha
- Lee YS, Choi D, Kim NY, Yang S, Jung E, et al. (2014) CXCR2 inhibition enhances sulindac-mediated suppression of colon cancer development. Int J Cancer 135: 232-237. Link: https://goo.gl/2EfcfA
- Fu Y, Yang G, Zhu F, Peng C, Li W, et al. (2014) Antioxidants decrease the apoptotic effect of 5-Fu in colon cancer by regulating Src-dependent caspase-7 phosphorylation. Cell Death Dis 5: e983. Link: https://goo.gl/1k5NUf
- Travica S, Pors K, Loadman PM, Shnyder SD, Johansson I, et al. (2013) Colon cancer-specific cytochrome P450 2W1 converts duocarmycin analogues into potent tumor cytotoxins. Clin Cancer Res 19: 2952-2961. Link: https://goo.gl/PLfLV9
- Chu H, Zhang A, Han Y, Lu S, Kong L, et al. (2016) Metabolomics approach to explore the effects of Kai-Xin-San on Alzheimer's disease using UPLC/ESI-Q-TOF mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci 15; 1015-1016: 50-61. Link: https://goo.gl/eWkSmj
- Zheng J, Lee HL, Ham YW, Song HS, Song MJ, et al. (2015) Anti-cancer effect of bee venom on colon cancer cell growth by tivation of death receptors and inhibition of nuclear factor kappa B. Oncotarget 6: 44437-4451. Link: https://goo.gl/TLjYZ3
- Meeker S, Seamons A, Maggio-Price L, Paik J (2016) Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J Gastroenterol 22: 933-948. Link: https://goo.gl/7adi83
- Xia X, Wu W, Zhang K, Cen G, Jiang T, et al. (2014) Prognostic significance of complications after laparoscopic colectomy for colon cancer. PLoS One 9: e108348. Link: https://goo.gl/8qMHPZ
- Zhao LY, Chi P, Ding WX, Huang SR, Zhang SF, et al. (2014) Laparoscopic vs open extended right hemicolectomy for colon cancer. World J Gastroenterol 20: 7926-7932. Link: https://goo.gl/U8NEC3
- Pohl JM, Gutweiler S, Thiebes S, Volke JK, Klein-Hitpass L, et al. (2017) Irf4-dependent CD103CD11b dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut 66: 2110-2120. Link: https://goo.gl/rBxx4E
- Hussain T, Chang HY, Veenstra CM, Pollack CE (2015) Collaboration Between Surgeons and Medical Oncologists and Outcomes for Patients With Stage III Colon Cancer. J Oncol Pract 11: e388-397. Link: https://goo.gl/XohvaQ
- Lu CS, Chen YG, Chen JH, Wu YY, Ho CL, et al. (2015) Stage III Colon Cancer: The Individualized Strategy of Adjuvant Chemotherapy for Aged Under and Over 70. PLoS One 10: e0138632. Link: https://goo.gl/fkx1gE
- Kotaka M, Yoshino T, Oba K, Shinozaki K, Touyama T, et al. (2015) Initial safety report on the tolerability of modified FOLFOX6 as adjuvant therapy in patients with curatively resected stage II or III colon cancer (JFMC41-1001-C2: JOIN trial). Cancer Chemother Pharmacol 76: 75-84. Link: https://goo.gl/Ssz4ij
- Kurniali PC, Hrinczenko B, Al-Janadi A (2014) Management of locally advanced and metastatic colon cancer in elderly patients. World J Gastroenterol 20: 1910-1922. Link: https://goo.gl/bzRC68
- Liu C, Huang Z, Jiang H, Shi F (2014) The sirtuin 3 expression profile is associated with pathological and clinical outcomes in colon cancer patients. Biomed Res Int 2014: 871263. Link: https://goo.gl/ChwB1d
- Zheng J, Lee HL, Ham YW, Song HS, Song MJ, et al. (2015) Anti-cancer effect of bee venom on colon cancer cell growth by activation of death receptors and inhibition of nuclear factor kappa B. Oncotarget 6: 44437-44451. Link: https://goo.gl/b6tAz6
- Hollis M, Nair K, Vyas A, Chaturvedi LS, Gambhir S, et al. (2015) Vyas D, MicroRNAs potential utility in colon cancer: Early detection, prognosis, and chemosensitivity. World J Gastroenterol 21: 8284-8292. Link: https://goo.gl/2P6d9U
- Neuman MG, French SW, Zakhari S, Malnick S, Seitz HK, et al. (2017) Alcohol, microbiome, life style influence alcohol and non-alcoholic organ damage. Exp. Mol. Pathol 102: 162-180. Link: https://goo.gl/YpoB6n
- Llewellyn SR, Britton GJ, Contijoch EJ, Vennaro OH, Mortha A, et al. (2018) Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 154: 1037-1046. Link: https://goo.gl/pqEDs7
- Li Y, Sen A, Ren J, Askew LM, Sidahmed E, et al. (2015) Effects of vitamin E from supplements and diet on colonic alpha- and gamma-tocopherol concentrations in persons at increased colon cancer risk. Nutr Cancer 67: 73-81. Link: https://goo.gl/6A6Es9
- Yang CS, Suh N (2013) Cancer prevention by different forms of tocopherols. Top Curr Chem 329: 21-33. Link: https://goo.gl/vfkb5h
- Jaganathan SK, Vellayappan MV, Narasimhan G, Supriyanto E (2014) Role of pomegranate and citrus fruit juices in colon cancer prevention. World J Gastroenterol 20: 4618-4625. Link: https://goo.gl/fdcpBL
- Fu J, Chen H, Soroka DN, Warin RF, Sang S (2014) Cysteine-conjugated metabolites of ginger components, shogaols, induce apoptosis through oxidative stress-mediated p53 pathway in human colon cancer cells. J Agric Food Chem 62: 4632-4642. Link: https://goo.gl/ibn3sh
- Liu X, Wang Y, Hoeflinger JL, Neme BP, Jeffery EH, et al. (2017) Miller MJ, Dietary Broccoli Alters Rat Cecal Microbiota to Improve Glucoraphanin Hydrolysis to Bioactive Isothiocyanates. Nutrients 9: E262 Link: https://goo.gl/JyL6jA
- Del Pino-García R, Rivero-Pérez MD, González-SanJosé ML, Ortega-Heras M, García Lomillo J, et al. (2017) Chemopreventive Potential of Powdered Red Wine Pomace Seasonings against Colorectal Cancer in HT-29 Cells. J. Agric. Food Chem 65: 66-73. Link: https://goo.gl/sfsH1L
- Tomasello G, Mazzola M, Leone A, Sinagra E, Zummo G, et al. (2016) Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 160: 461-466. Link: https://goo.gl/MXH1rJ
- Zhang A, Sun H, Yan G, Wang P, Wang X (2016) Mass spectrometry‐based metabolomics: applications to biomarker and metabolic pathway research. Biomedical Chromatography 30: 7-12. Link: https://goo.gl/F1TbY3
- Ma N, Tian Y, Wu Y, Ma X (2017) Contributions of the Interaction Between Dietary Protein and Gut Microbiota to Intestinal Health. Curr. Protein Pept. Sci 18: 795-808. Link: https://goo.gl/vpZB2r
- Sun H, Zhang A, Yan G, Han Y, Sun W, et al. (2013) Proteomics study on the hepatoprotective effects of traditional Chinese medicine formulae Yin-Chen-Hao-Tang by a combination of two-dimensional polyacrylamide gel electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis 75: 173-179. Link: https://goo.gl/QmYm8i
- Cheng L, Jin H, Qiang Y, Wu S, Yan C, et al. (2016) High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int. Immunopharmacol 40: 1-10. Link: https://goo.gl/5vHc3E
- Cao H, Zhang A, Zhang H, Sun H, Wang X (2015) The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytotherapy research 29: 159-166. Link: https://goo.gl/xhLahV
- Viennois E, Merlin D, Gewirtz AT, Chassaing B (2017) Dietary Emulsifier-Induced Low-Grade Inflammation Promotes Colon Carcinogenesis. Cancer Res 77: 27-40. Link: https://goo.gl/yBaUJD
- Cong YJ, Gan Y, Sun HL, Deng J, Cao SY, et al. (2014) Association of sedentary behaviour with colon and rectal cancer: a meta-analysis of observational studies. Br J Cancer 110: 817-826. Link: https://goo.gl/WsjUJX
- Sun H, Wang M, Zhang A, Ni B, Dong H, et al (2013) UPLC–Q‐TOF–HDMS Analysis of Constituents in the Root of Two Kinds of Aconitum Using a Metabolomics Approach. Phytochemical Analysis 24: 263-276. Link: https://goo.gl/CY9kXo
- Puthia M, Storm P, Nadeem A, Hsiung S, Svanborg C (2014) Prevention and treatment of colon cancer by peroral administration of HAMLET (human alpha-lactalbumin made lethal to tumour cells). Gut 63: 131-142. Link: https://goo.gl/Fpzp2m
- Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, et al. (2018) Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359: 592-597. Link: https://goo.gl/Dm9YrW
- Lang M, Berry D, Passecker K, Mesteri I, Bhuju S, et al. (2017) HuR Small-Molecule Inhibitor Elicits Differential Effects in Adenomatosis Polyposis and Colorectal Carcinogenesis. Cancer Res 77: 2424-2438. Link: https://goo.gl/3HmNhp
- Kaur K, Saxena A, Debnath I, O'Brien JL, Ajami NJ, et al. (2018) Antibiotic-mediated bacteriome depletion in Apc(Min/+) mice is associated with reduction in mucus-producing goblet cells and increased colorectal cancer progression. Cancer Med 7: 2003-2012. Link: https://goo.gl/igao2r
- Peters BA, Dominianni C, Shapiro JA, Church TR, Wu J, et al. (2016) The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome 4: 69. Link: https://goo.gl/M9ow3r
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
