Open Journal of Proteomics and Genomics
Exploring the detoxification effects and mechanism of Caowu in prescription using liquid chromatography-high-resolution mass spectrometry-based metabolomics
Li Wang, Hui Dong, Ai-hua Zhang, Ying Han, Tai-ping Li, Le Yang, Hui Sun, Xiang-cai Meng and Xi-jun Wang*
Cite this as
Wang L, Dong H, Zhang AH, Han Y, Li TP, et al. (2018) Exploring the detoxification effects and mechanism of Caowu in prescription using liquid chromatography-high-resolution mass spectrometry-based metabolomics. Open J Proteom Genom 3(1): 011-023. DOI: 10.17352/ojpg.000006Liquid chromatography/mass spectrometry-based metabolomics analyses have been used in the evaluation of drug toxicity and finding detoxification methods. Yunnan Baiyao (YNBY) is a famous prescription for its effects on traumatic blood stasis in China. However, the side effects of YNBY including anaphylactic shock, arrhythmia and renal failure hindered its further development due to Aconite kusnezoffii Radix. (caowu) in YNBY. The purpose of our research is to study the safety of YNBY compatibility of caowu for 4 weeks on SD rats by oral administration. In this study, we determined the toxic effects on rats by conducting examinations of histopathological and clinical physiological and biochemical indicators. Next, we prospectively analyzed known metabolites using an untargeted approach in serum of SD rats administrated with YNBY and NCY (caowu replaced by excipient from YNBY) respectively for four weeks by mass spectrometry analysis. As a result, we found caowu in YNBY had no toxicity on rats according to general toxicological evaluations. It was no obvious difference between administration and control groups on AKP, GOT, GPT, BUN, CK, MDA and LDH, except Na+/K+-ATPase (ATP) and SOD index. NCY had a greater influence on rats than YNBY according to metabolism. In this study, we found 77 biomarkers in YNBY and 110 biomarker of NCY, involved 12 and 20 metabolic pathways respectively. L-arginine, palmitic acid, oleic acid and stearic acid both in YNBY and NCY could participated in liver necrosis/cell death including apoptosis of hepatocytes, cell death of hepatocytes and lipoapoptosis of hepatocytes, which predicted by Ingenuity pathway Analysis (IPA). Of note, betaine produced by YNBY and cholic acid produced by NCY merely contributed to cell death of hepatocytes by prediction. Our findings based on metabolite profiles were that, caowu is safe existed in YNBY during four weeks. Caowu promoted the function of energy metabolism in heart and protecting the heart from oxygen free radicals. Betaine produced by YNBY and cholic acid produced by NCY may play a role in cell death of hepatocytes.
Introduction
Yunnan Baiyao (YNBY), a famous Chinese patent drug for its excellent efficacy clinically and special production process, formulated in 1902, has been designated as class one protected prescription by Chinese government. The major of traditional Chinese medicine are Panax notoginseng, Aconite kusnezoffii Radix. and so on according to the prescription compositions. The reputation of YNBY is characterized with the effects on hemostasis [1,2], promoting blood circulation, detumescence, analgesia and antibacterial [3] for more than one hundred years. Historically, YNBY made a great contribution to the Chinese soldiers in word II and later by the Viet Cong for control of hemorrhage. YNBY has been suggested that effect of hemostasis achieved by improving the release of alpha granules in plasma, facilitating platelet activation and wound sealing. In addition, YNBY is used for relieving the pain and inflammation caused by kinds of trauma even rheumatoid arthritis [4].
However, a few adverse drug reaction of anaphylactic shock, arrhythmia, renal failure and cardiac toxicity from YNBY in clinical limited its application and further development due to caowu included in recent years. Caowu, one of aconitum herbs, has been widely applied to clinical for anti-inflammatory, cardiotonic, antipyretic and analgesic effects about 2000 years. Studies have shown that, intrathecal processed Aconitum jaluense showed antiallodynic activity on neuropathic pain through inhibiting P2X7R production and expression and decreasing microglial activation in the spinal cord. In addition, aconitum carmichaelii had the protective effect against acetaminophen induced by hepatotoxicity was probably through inhibiting CYP2E1 activity, GSH depletion, and mitochondrial dysfunction. Of note, aconitine extracted from the aconitum plant is both toxic and active ingredient [5-10]. The main chemical constituents of caowu are alkaloids including C20-, C19-, C18- diterpenoid alkaloids. Among them, C19- diterpenoid alkaloids are the most toxic on cardiovascular and nervous system, especially cardiotoxicity by inducing cardiovascular symptoms both atrial and ventricular dysrhythmias. Studies have shown that the toxicity of the diester alkaloid decreases to monoester and alcohol amine alkaloid. [11-15]. Clinically, caowu is usually processed to products [16,17] or used in combination with other drugs [18] to reduce its toxic side effects on the heart, liver, kidneys and nerves.
In the recent years, a wide range of metabolomic technologies as a systems biology approach are widely used in the modern research of TCM [19-25] with the most prevailing methods such as UPLC-MS with instrument analysis and data processing [26-33]. Metabolomics monitor the endogenous metabolites [34] of biofluid including serum, plasma [35], urine [36,37] and organism for evaluating the drug efficacy and safety, identifying new mechanisms [38] and targets of diseases development [39,40] and drugs treatment [41] via biomarker discovery and metabolic pathway research [42,43], discovering functional biomarkers for clinically accurate diagnosis and treatment. For TCM, UPLC-MS can characterize the fingerprint [44,45] and full-component analysis of single- Chinese medicines [46-53], formula compatibility [54-61] and absorbed bioactive components [62-67], which help us to understand the “black box” more clearly. On this basis, chinmedomics [68-72] as a powerful approach integrating metabolomics with serum pharmacochemistry to evaluate the efficacy of TCM, confirme the mechanism of syndrome and drug interaction [73] to treat the diseases and further seeking new drugs [74-80]. Prior to this, other members of our team conducted a systematic study of the chemical composition [8,81], toxicity [82] and attenuation mechanisms [5,83,84], of Aconitum by metabolomics. In the present study, in order to evaluate the role of caowu in YNBY compatibility, we analyzed the survival status of rats after administrating with YNBY and NCY by histopathological and clinical physiological and biochemical indicators respectively. In addition, we investigated the effects of YNBY and NCY on serum metabolism of rats through non-target metabolomics techniques, so as to look for the effects of caowu in YNBY and predict the toxicity by IPA.
Methods
Drugs and chemical reagents
YNBY pulvis and NCY pulvis were provided by Yunnan Baiyao Group Limited by Share Ltd. (Kunming, China). Carboxy methyl cellulose sodium (CMCNa) come from Kermel Chemical Reagent Co., Ltd (Tianjin, China). Acetonitrible and methanol (HPLC grade) was purchased from Merck (Darmstadt, Germany). Formic acid was purchased from Dikma Technologies Inc. (DIKMA, USA). Leucine enkephalin was purchased from Sigma-Aldrich (MO, USA). GOT, GPT, AKP, BUN, CK, ATP, LDH, SOD and MDA kits were charged from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Animals and study designs
SD rats, 120 ± 10g, from Liaoning changsheng biotechnology co. Ltd. (Benxi, China), were housed in Heilongjiang Traditional Chinese Medicine University with SPF conditions. The center with a controlled environmental conditions: a 12:12-h lighted-dark cycle with free access to food and water, a temperature with 24 ± 2 ◦C and the relative humidity with 55 ± 5%. All rats were randomly split into 3 groups of 12 rats each, control group, YNBY group and NCY group, sex in half. The dose of medication administration teams was 205.8 mg/kg/day keeping feeding for four weeks, rats of control group were administered with 0.5% CMCNa solution according to body weight at the same time. The body weight of the rats and the fodder given to rats was recorded during the experiment. All experiments were performed in accordance with approved animal protocols and guidelines established by Heilongjiang University of Chinese Medicine.
Serum preparation
Blood of rats was collected from the hepatic portal vein with vacuum negative pressure tubes after the rats anaesthetized by injecting of 3% pentobarbital sodium in abdomen (0.3mL/100g body weight) when the treatment finished. Then, serum were obtained by means of centrifugation at 4000 rpm, 4◦C retaining 15min. The obtained serum was dispensed and frozen at -80◦C immediately. Frozen serum samples were placed into ice water for reconstitution and mixed by vortex mixer before use. We added 800μL methanol into 200μL serum sample and mixed by vortex mixer for 30s, then the suspensions centrifuged at 13,000 rpm, 4◦C retaining 15min for removing macromolecular compounds such proteins and extracting metabolites. After that, the supernate liquid acquired was transferred into a new tube and dried by nitrogen gas at 40◦C. The dried samples were added with 200μL methanol for ultrasonic dissolution and centrifuging at 13,000 rpm, 4◦C retaining 15min. The supernatant was filtrated by 0.22μm filter membrane before transferred into glass bottles for UPLC-MS analysis. In addition, 50μL of each serum sample was mixed as QC sample with the same method for processing. The QC sample contained entire information of all the samples that would be prepared to evaluate the stability of instrument and method. Analysis of a QC sample interspersed with every six sample using the same method.
Tissue weighting and histopathological examination
Extract the heart, liver, spleen, lungs and kidneys of rats immediately after anesthesia and blood collection. After rinsing the organs with physiological saline, the water was sucked with filter paper and weighed. About 200mg of myocardium was removed from the left ventricle of the heart and weighed accurate for the determination of heart tissue index. The remaining tissues were fixed by putting into formalin solution immediately for HE staining.
Biochemical assay test
Part of the collected serum was used to determine AKP, GOT, GPT, BUN and CK. Before the rats’ heart were fixed, approximately 200 mg of myocardium was removed from the left ventricle of the heart, added with 9 fold physiological saline, homogenized at a low temperature of 13,000rpm for 1 min. A part of the obtained homogenate was centrifuged at 2500rpm, 4◦C retaining 10min, then the supernate was drawed for activity test of Na-K-ATPase assay. The remaining myocardial homogenate was centrifuged 5000rpm, 4◦C for 20min, and the supernatant was collected for activity of SOD, MDA and LDH. SOD, MDA, BUN, CK and Na-K-ATPase were detected by UV spectrophotometer, while AKP, GOT, GPT and LDH were detected by microplate reader.
Chromatography and mass conditions
An ACQUITY UPLC system from Waters (Milford, MA, USA) combined with an ACQUITY UPLC BEH C18 column (50 mm × 2.1 mm, 1.7μm, Waters Corporation, Milford, USA) were used for chromatographic separation. We used optimized conditions including the velocity was 0.4 mL min-1, while the column temperature was 40◦C. we adopt the optimal mobile phase containing 0.1% formic acid in acetonitrile (A) and 0.1% formic acid in water (B). The UPLC performed method for instrument analysis with a linear gradient elution procedure as follows: 0-2min, 1-32% A; 2-5min, 20-80% A; 5-10min, 80-99%; 10-11min, 99% A; 11-12min, 99-1%; 12-15.5min, 1% A.
A SYNAPT-G2-Si high definition mass spectrometer (HDMS) (Waters Milford, MA, USA) processed with a mass spectrometer system with an assembly of electrospray ionzation source (ESI) were used for detecting serum metabolites by MassLynx software on version 4.1 (Waters Milford, MA, USA). We adopted the MS parameters conditions including an ion voltage of 3.0 KV in ESI+ and ESI- mode, the flow rate of cone gas and desolvation gas were 50 L h-1 and 800 L h-1 respectively, the temperature of desolvation gas and ion sourcat at 350◦C and 110◦C respectively. The rate of MS data acquired was set at 0.2s per scan with a delay of 0.1s between scans from 50 to 1200Da in centroid mode. A lock mass of leucine enkephalin with a concentration of 4 ng μL-1 was utilized for acquiring accurate mass data with a flow rate of 10μL min-1 in ESI+ and ESI- mode ( [M+H]+ = 556.2771, [M-H]- = 554.2615).
Statistical analysis
The raw MS-data files acquired by UPLC-MS of serum samples from all rats were uploaded on to Progenesis QI software (Nonlinear Dynamics, version 2.0) for peak detection, normalization, visualization and multi-dimensional processing and analysis. The results of the above process were imported into Ezinfo 3.0 software for unsupervised principal components analysis (PCA) combined with orthogonal partial least square– discriminant analysis (OPLS-DA) and verified through analysis of variance (ANOVA) simultaneously. On this basis, we collected MS/MS information of metabolite peaks via the Mss FragmentTM (Waters corp., Milford, USA), ChemSpider (https://www.chemspider.com/) and Human Metabolism Database (HMDB) (https://www.hmdb.ca/) by chemically intelligent peak-matching algorithms for determining the compound structure preliminary. In addition, we combined KEGG (https://www.genome.jp/kegg) and MetPA (https://metpa.metabolomics.ca./MetPa/faces/Home.Jsp) for the analysis of biological pathways for determined the potential biomarkers meet the requirements of p-value of 0.05 or less by t-test and P-value threshold set at 1.0. All the identified biomarkers of YNBY and NCY were introduced to IPA (https://www.ingenuity.com) to focus on metabolic pathways that have been obtained resulting in more specific functional and disease-associated metabolic pathways. Simultaneously, IPA help us predict the toxic biomarkers and targets produced by drug.
Results
Effects of YNBY and NCY on weight and food intake in rats
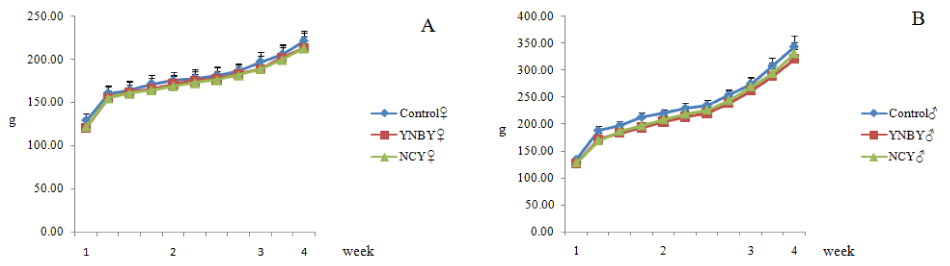
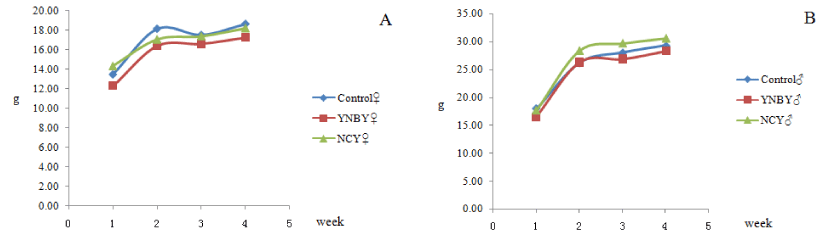
Using weight and food intake assessment to evaluate the effects of YNBY and NCY. The body weight of YNBY and NCY group had little difference with the control group. There were no significant fluctuations in feed intake between the YNBY group and NCY group compared to the control group (Figures 1,2).
Effects of YNBY and NCY on histopathology and clinical biochemical index
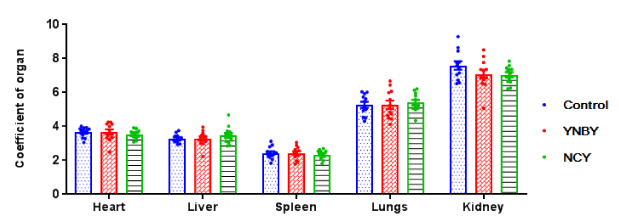
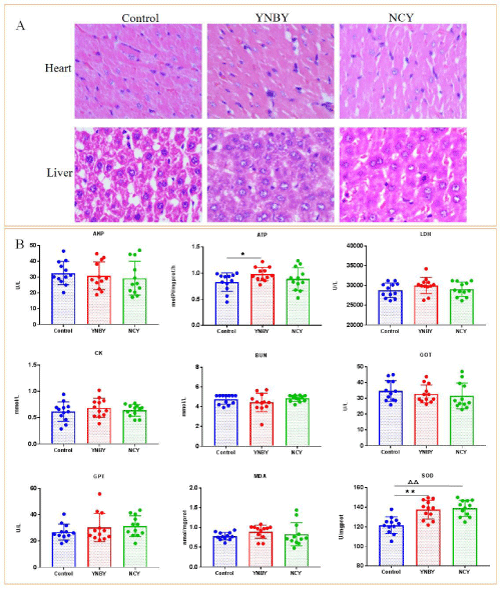
There were no significant difference in the coefficient of heart, liver, spleen, lungs and kidneys both YNBY and NCY compared with control group (Figure 3). Cardiomyocytes are morphologically regular with clear horizontal stripes, a nuclear center, abundant interstitial capillaries and a small amount of connective tissue. Liver cells are polygonal, centered on the central vein and regularly arranged into a single row of plate-like structures with hepatic sinusoids between hepatocytes (Figure 4A). There were no significant difference both YNBY group and NCY group compared to control group in AKP, GOT, GPT, BUN, CK, MDA and LDH index. The ATP index of YNBY group increased than control group with P<0.05. Of note, there was no such result in NCY group. The SOD index of YNBY group and NCY increased than control group with P <0.01. ATP and SOD indicators from cardiac tissue differ in groups (Figure 4B).
Effects of YNBY and NCY on serum metabolic profiling
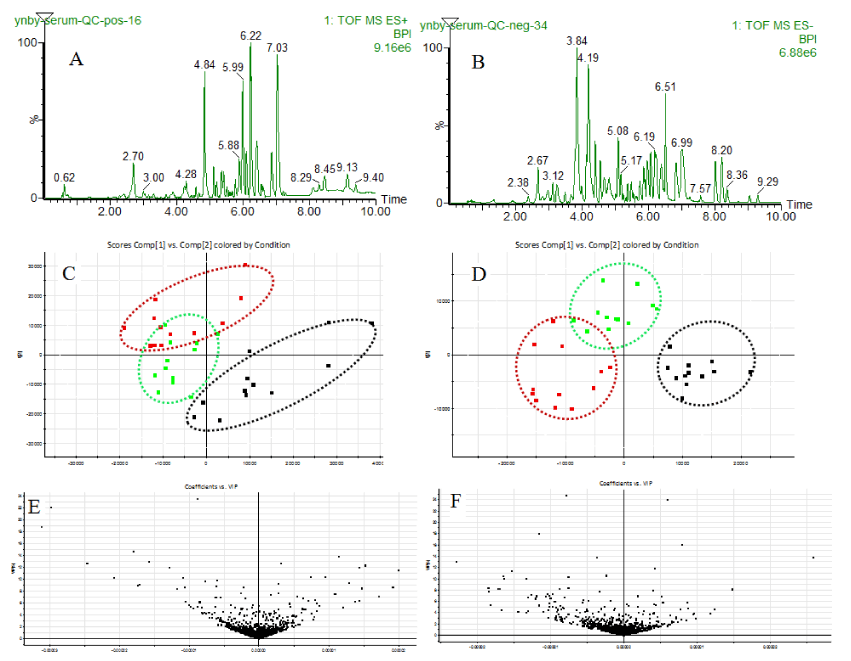
In this study, we obtained the fingerprints (Figure 5A,B) of all the serum samples analyzed by UPLC-MS with the optimal conditions in positive and negative ionization modes. After QI software processing and analisis, we detected about 22631 peaks in positive ions and 24525 peaks in negative ions. The PLS-DA plots in positive ionization mode (Figure 5C) and negative ionization mode (Figure 5D) can reflect a clear distinction between YNBY and NCY group, which suggest that caowu made a great contribution to other medicines from YNBY by interfering body metabolism.
Identification and relative intensity of metabolites influenced by YNBY and NCY
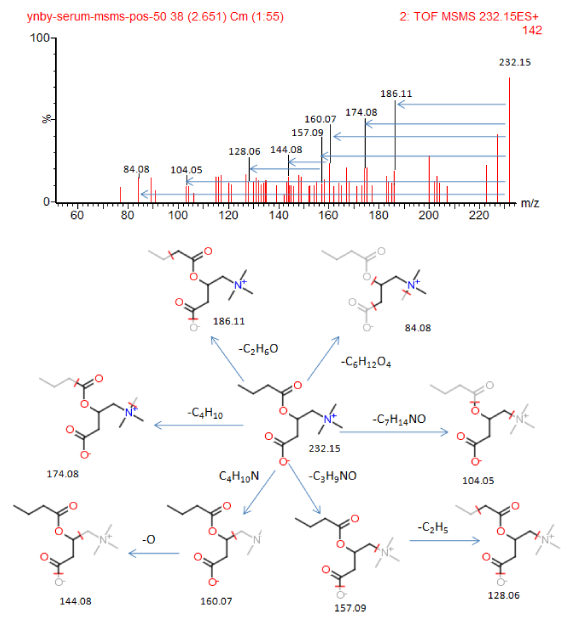
VIP plots (Figure 5E,F) from PLS-DA help us analyze feature ions. The potential biomarkers met requirements used to pick out and identify chemical structure further. The candidate potential biomarkers used MassFragment TM application manager combined with ChemSpider to match the MSMS information carefully, for example, the compound of butyrylcarnitine with chemical structure and cracked fragments information in positive ion mode (Figure 6). In the present study, we identified 77 potential biomarkers in serum of YNBY group, while 110 potential biomarkers in serum of NCY group.
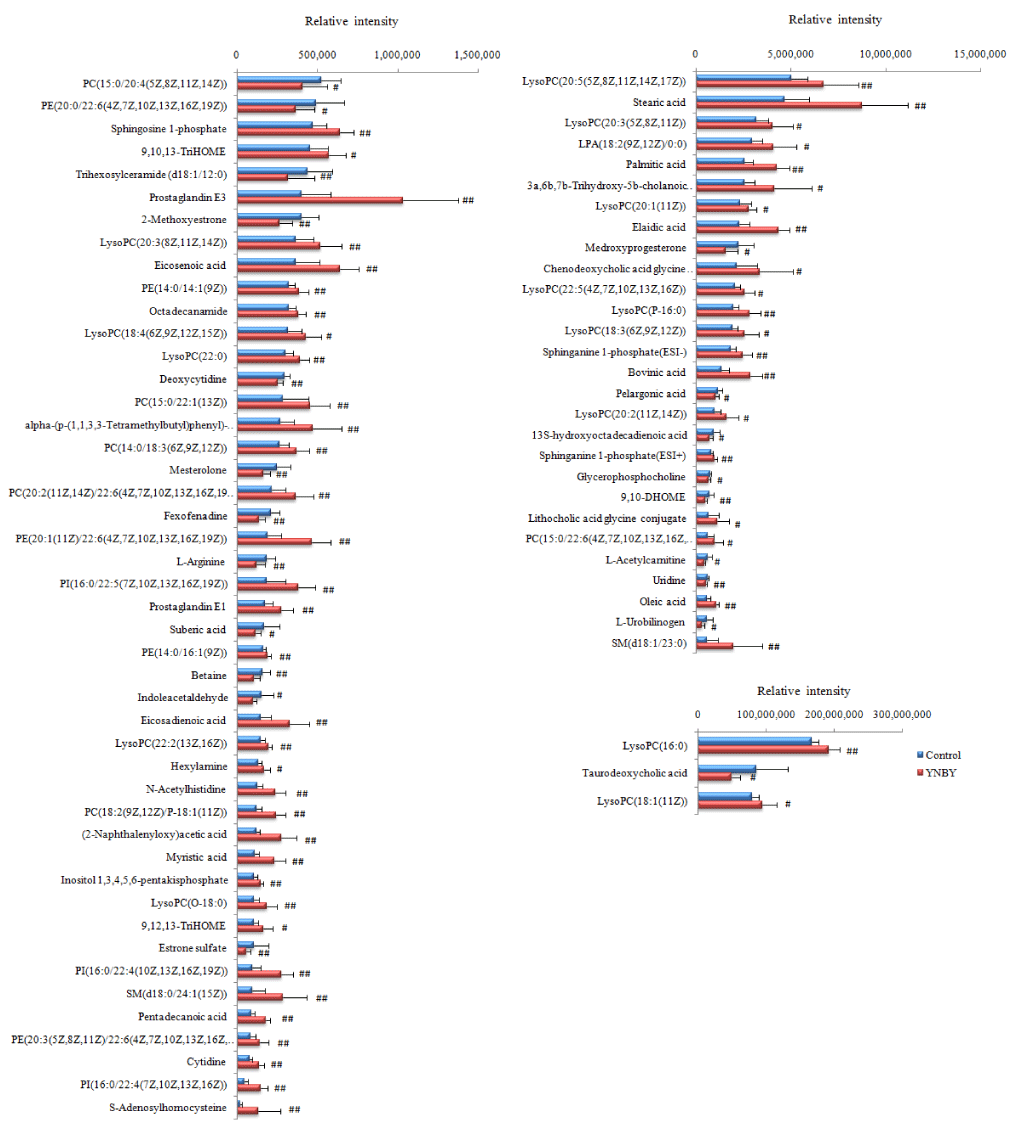
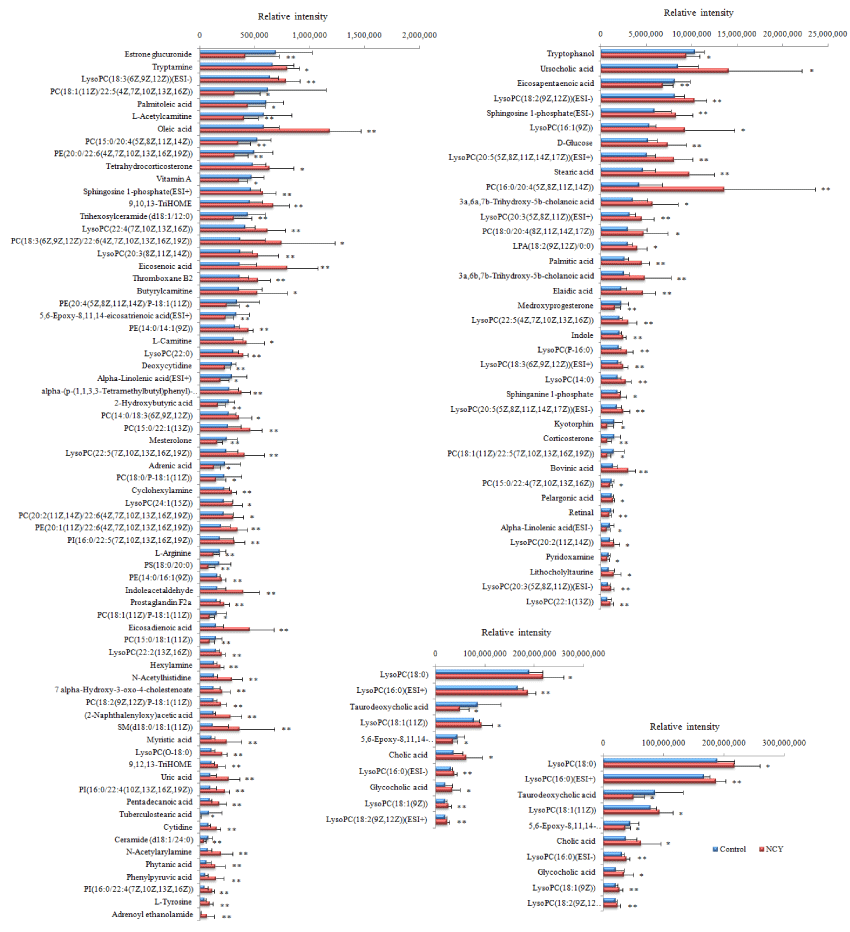
The information of the identified results of the potential biomarkers of YNBY and NCY in ESI+ sand ESI- mode in Table S1 and S2 including retention time, molecular weight, name and MSMS fragments, etc. The results of comparison of relative intensity of peaks after standardization of potential biomarkers produced by YNBY and NCY in figures 7,8 respectively.
Metabolic pathways influenced by YNBY and NCY
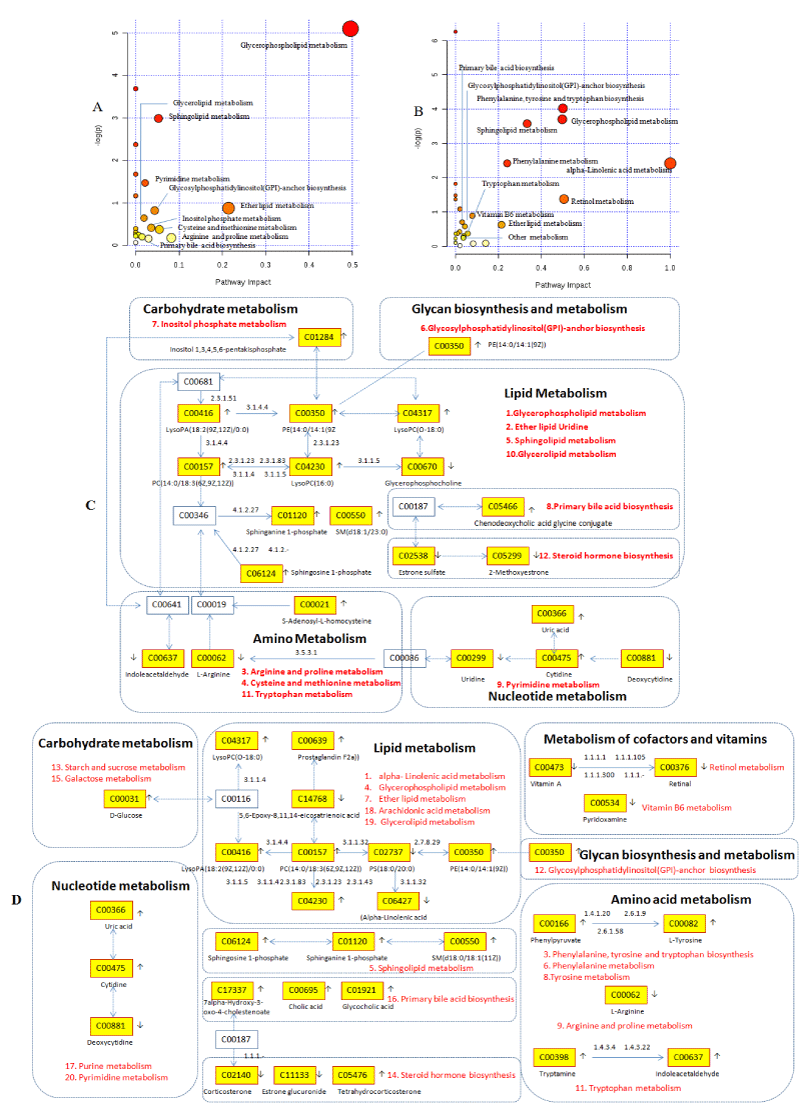
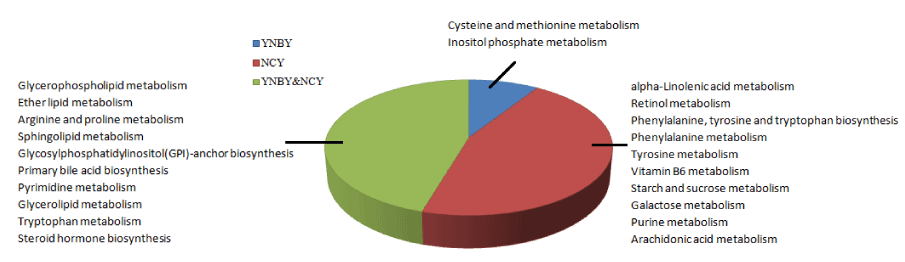
To gain insight to the role of caowu in YNBY, metabolic pathways of YNBY (Figure 9A) and NCY (Figure 9B) were constructed respectively by importing biomarkers identified to MetaboAnalyst website and the KEGG. It turned out that the most relevant pathways (impact value > 0) involved by YNBY mainly involved five classes of pathway for lipid, nucleotide, amino acid, carbohydrate metabolism and glycan biosynthesis and metabolism. However, NCY group added metabolism of cofactors and vitamins than YNBY group for retinol metabolism and vitamin B6 metabolism due to the lack of caowu. Based on this results, we constructed the network of pathways produced by YNBY (Figure 9C) and NCY (Figure 9D), which indicate that caowu made a great importance to the integrity of metabolic network on rats for four weeks administration. The details comparison information of MetPA pathways produced by YNBY and NCY in Table S3. Then we known that the number of YNBY influenced the metabolic pathways was 10 and 20 metabolic pathways influenced by NCY was more than YNBY attached by 10 metabolic pathways, which indicated YNBY may be safer than NCY due to lower degree of influence (Figure 10).
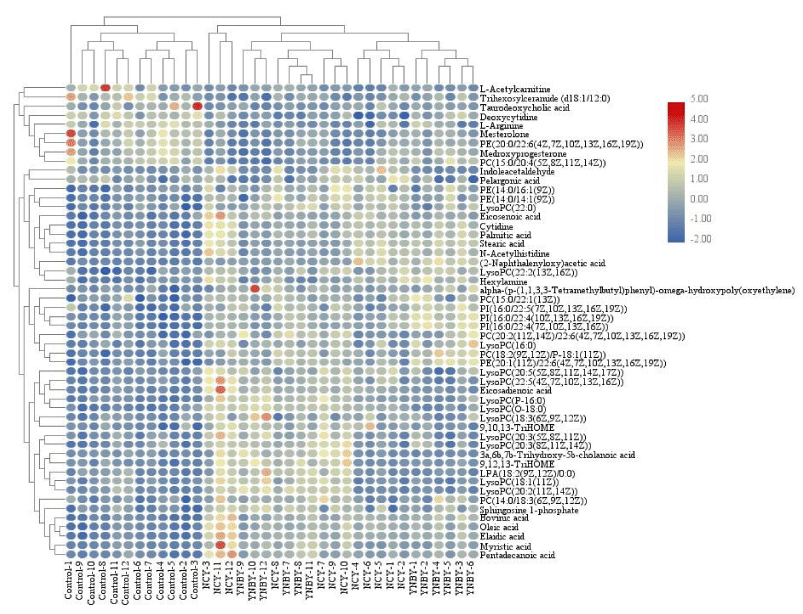
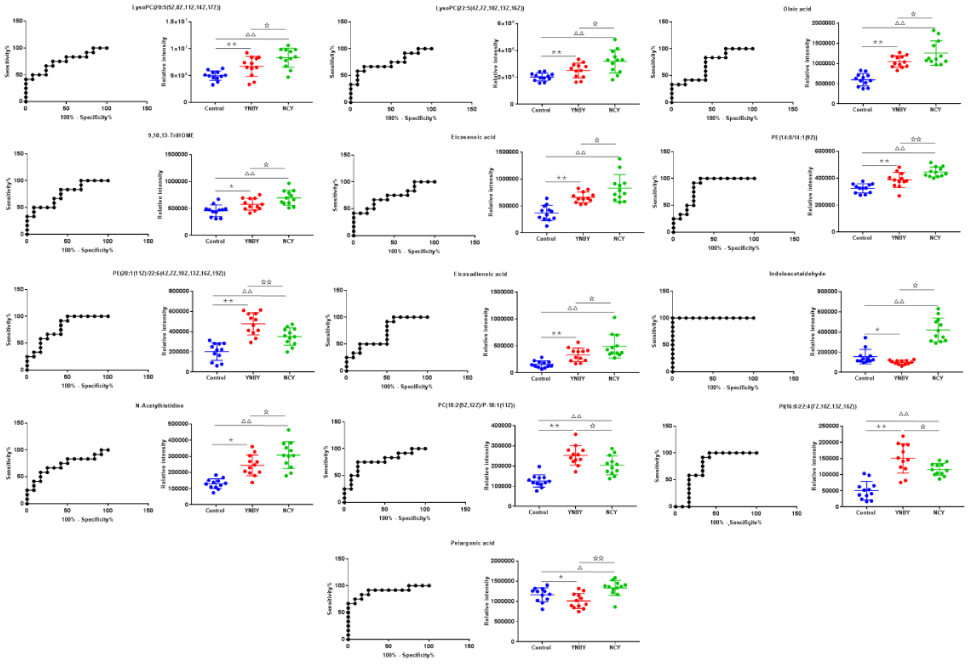
In order to analysis the difference of the 53 common biomarkers form YNBY group and NCY group, a clustering heat map (Figure 11) was constructed. Based on the above, we analyzed 13 biomarkers from YNBY and NCY on ROC curve and quantitative analysis (Figure 12), the significant difference of these biomarkers included LysoPC (20:5(5Z,8Z,11Z,14Z,17Z)), oleic acid, 9,10,13-TriHOME, eicosenoic acid, PE(14:0/14:1(9Z)), PI(16:0/22:4(7Z,10Z,13Z,16Z)), PE(20:1(11Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)), pelargonic acid, eicosadienoic acid, indoleacetaldehyde, N-acetylhistidine, PC(18:2(9Z,12Z)/P-18:1(11Z)), LysoPC(22:5(4Z,7Z,10Z, 13Z,16Z)) between YNBY and NCY group indicate that caowu may act on other medicines of YNBY to influence the metabolism.
The canonical pathways and GO enrichment of biological processes of YNBY and NCY
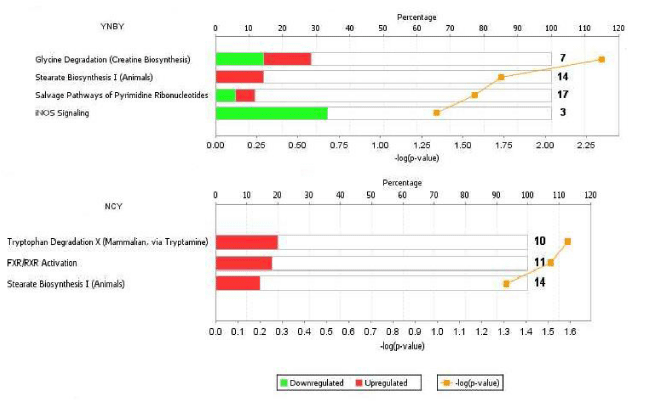
In order to gain the most important message of canonical pathways, IPA software was used to help us analyze the network interactions between dysregulated biomarkers identified in metabolism analysis by IPA database. The analysis of canonical pathway showed that related three pathways in YNBY and four related pathways in NCY (Figure 13). The four pathways in YNBY comprised glycine degradation, stearate biosynthesis and salvage pathways of pyrimidine ribonucleotides and iNOS signaling. The three pathways in NCY comprised tryptophan degradation, FXR/RXR activation and stearate biosynthesis.
The biomarkers produced by YNBY including 1D-myo-inositol 1,3,4,5,6-pentakisphosphate, 2-methoxyestrone, beta-muricholic acid, cytidine, deoxycytidine, dihydrosphingosine 1-phosphate, glycolithocholic acid, pelargonic acid, pentadecanoic acid, prostaglandin E3, stearic acid participated in DNA replication, recombination and repair, nucleic acid metabolism, small molecule biochemistry (Figure 14A). The biomarkers produced by YNBY including 15:0/20:4(5Z,8Z,11Z,14Z) phosphatidylcholine, betaine, dihydrosphingosine 1-phosphate, elaidic acid, L-arginine, oleic acid, palmitic acid, stearic acid participated in cell death and survival, immunological disease, inflammatory disease (Figure 14B). The biomarkers produced by YNBY including betaine, myristic acid, S-adenosylhomocysteine, sn-glycero-3-phosphocholine, suberic acid, taurodeoxycholic acid, uridine participated in Lipid metabolism, molecular transport, small molecule biochemistry (Figure 14C). SOD AKT and ALT were the key predictors by YNBY from IPA results.
Toxic targets produced by YNBY and NCY predicted by IPA
In addition, IPA help us to further analyze detailed functional changes and predict system toxicity by YNBY and NCY on rats. L-arginine, palmitic acid, oleic acid and stearic acid both in YNBY and NCY could participated in liver necrosis/cell death including apoptosis of hepatocytes, cell death of hepatocytes and lipoapoptosis of hepatocytes, which predicted by IPA. Of note, betaine produced by YNBY and cholic acid produced by NCY merely contributed to Cell death of hepatocytes.
Discussion
YNBY is famous for exact curative effect of hemostasis, blood circulation, anti-inflammatory and analgesia clinically, even without the prescription compositions. However, caowu participated in YNBY impeded its applications due to its cardiotoxicity and neurotoxicity. Recent work has highlighted the safety and effectiveness of caowu on active ingredients, toxic components, effect target and mechanism. Yet, it largely has remained unknown what is the role of caowu in YNBY on the influence on other medicines from YNBY and what is the aftereffects if remove caowu from YNBY. Thus, we designed experiment to explore the difference with untargeted serum metabolism between YNBY and NCY on rats after four administration. Experimental results showed that YNBY and NCY had no effect on intake, body weight and organ coefficient included heart, liver, spleen, lung and kidney of rats, which indicated that, YNBY and NCY had no significant effect on the above indicators during the administration period. The compatibility of caowu in YNBY would not have toxic effect on rats during the experiment. This indicate and liver functions that it is safety for rats with YNBY for four weeks with clinical equivalent dose even participated with caowu.
In addition, we analyzed the effects of compatibility with caowu in YNBY on the liver, heart and kidney with clinical biochemical indicators and hematological detections. The serum biochemical index and hematological detections are widely applied to diagnosis of the diseases, evaluation of the prognosis and the access and monitor the body metabolic status. As far as we, aconitum plants have a great influence on the functions of heart, liver and kidneys as target organs. Thus, we detected the levels of ATP, SOD, MDA and LDH from myocardium and CK from serum, which represent the severity of myocardial injury [85]. The serum level of AKP, GOT and GPT applied to our research, represented the severity of liver injury [86], due to several enzymes leak out into the blood from the cytosol of hepatocytes [87]. The level of BUN from serum represented the severity of renal damage by YNBY participated with caowu [88]. Cardiac toxicity of aconitum plants performed with severe arrhythmias and even leading to death [89], which could be caused by inhibiting ATP activity remarkably, then increase Na+ influx and the Ca2+ concentration in intracellular, which leading to damage of the heart tissue [90]. Several studies proved that ATP been known as a membrane protein plays a important role for the active transport of Na+ and K+ across over the cell membrane by converting the energy of ATP [85,89,92]. The present study revealed YNBY increased the activity of ATP in the heart significantly, which was lack of NCY groups. SOD plays a crucial role on the balance of oxidative and antioxidant function in the body by scavenging superoxide anion free radicals and protecting cells from damage. The present studies shown that both YNBY and NCY could increase the level of SOD significantly. The studies showed that caowu could assist other drugs in YNBY to enhance the protection effect of the heart function. However, there was no difference between YNBY and NCY. Histopathological results show that, heart and liver were not observed for lesions and injures after administration with YNBY and NCY, which implying that they were safe for rats during the experiments.
Based on the above findings, the significance of YNBY compatibility of caowu was reserved. Therefore, we detected the all serum metabolites of rats influenced by YNBY and NCY based on UPLC-MS method. We found the degree of NCY disturbed the normal serum metabolic profiles of rats than YNBY greatly according to the score of PCA, the number of biomarkers, metabolic pathways, classes of metabolism.
Lipid metabolism. Phospholipids consisted of LysoPCs, PCs and PEs is a kind of important ingredients of the lipid bilayer of cells in biosphere, involving in various metabolisms and signaling process. In the present studies, we found 13 LysoPCs, 5 PCs, 4 PEs and 3 PIs were the major biomakers expressed with significant difference between administration groups to control group by serum metabolism, which involved in Lipid metabolism including glycerophospholipid, ether lipid, glyceroLipid metabolisms and glycosylphosphatidylinositol (GPI) -anchor biosynthesis. The contents of LysoPCs both in YNBY and NCY were increased than control group, especially the levels of LysoPC (20:5(5Z,8Z,11Z,14Z,17Z)), LysoPC (22:5(4Z,7Z,10Z,13Z,16Z)) and oleic acid in NCY were higher than YNBY significantly. Many studies have shown that blood status is associated with LysoPCs, linoleate and alphalinolenic acid height participated in Lipid metabolism [93], which affected the expression of choline substances [94]. Phosphatidylcholine produced LysoPCs with the action of phospholipase A2 attaching the effects on regulating vascular tension and producing endothelial dysfunction. The mechanism of the procedure is the expression of nitric oxide synthase which means NOS decreased with the level of mRNA to produce vascular endothelium injury due to the expression of NO disturbed by LysoPC [95,96]. The mechanism is consistent with the canonical pathways expressed by IPA. In addition, studies proved that LysoPC performs on κB binding sites to reduce the transcriptional activity and promote the level of cAMP in mononuclear cells in order to regulate the production of tissue factors participating in thrombosis syndrome during precipitation [97]. SM(d18:0/18:1(11Z)), sphingosine 1-phosphate and sphinganine 1-phosphate both produced by YNBY and NCY involved sphingoLipid metabolism from Lipid metabolism class and had effects of potential biological activity in the sphingoLipid metabolism, calcium signal transduction procedure and neuroactive ligand-receptor interaction. Cholic acid, glycocholic acid, 7 alpha-hydroxy-3-oxo 4-cholestenoate, ursocholic acid, 3a,6a,7b-trihydroxy-5b-cholanoic acid and lithocholyltaurine induced by NCY, chenodeoxycholic acid glycine conjugate and lithocholic acid glycine conjugate induced by YNBY, and 3a,6b,7b-Trihydroxy-5b-cholanoic acid from administration groups involved primary bile acid biosynthesis. The abnormal secretion of cholic acid in the NCY has brought hidden troubles on the normal metabolism of rats, which explains that the disorder of cholic acid affects the vitamin metabolism only occurs in NCY. In abnormal, bile acids as a kind of hepatotoxic substance circulating through the liver repeatedly have proved the potent toxic properties if sustained accumulation in blood and tissues result of membrane disruption. Bile acids accumulated in liver may damage liver and lead to hepatocyte apoptosis and deterioration of other severe liver diseases such as cholestatic [98]. The mechanisms of liver apoptosis was induced the transcriptional activity of AP-1 (activation protein-1) by lithocholyltaurine [99,100]. Chenodeoxycholic acid glycine conjugate from YNBY and lithocholyltaurine from NCY increased the risks of liver apoptosis by drugs, which is consistent with the results of IPA for toxic predicition. Estrone sulfate and 2-methoxyestrone produced from YNBY involved steroid hormone and biosynthesis. Mesterolone form two administration groups is a potential doping agent in equine sports by modulating the autonomic innervation of the vesical musculatureand has the effects of decreasing the cholesterol in human serum [101,102]. Prostaglandin E1 and prostaglandin E3 increased only from YNBY, as the cycloxygenase metabolism of eicosapentaenoic acid, are potent endogenous vasodilator agent, which can promote peripheral blood flow by inhibiting platelet aggregation for achieving various vascular protective functions. There are several mechanisms of PGE1 hepatic cytoprotection: inhibiting cytotoxicity induced by T-cell, enhancing DNA replication, recombination, and repair of the injured liver after partial hepatectomy by stimulating cyclic AMP function which is consistent with GO enrichment of biological processes predicted by IPA, promoting the level of ATP in liver to accelerate the recovery of mitochondrial respiratory function after reperfusion, and maintaining membrane microviscosity [103,104].
Amino acid metabolism. L-arginine used to create NO, creatine, glutamate and proline, even glucose and glycogen if necessary for body decreased in YNBY and NCY. L-arginine plays a important role in anmino and energy metabolisms due to promote the secretion of insulin by stimulating skeletal muscle consuming glucose with the help of AMP kinase activated by L-arginine [105]. Betaine decreased in YNBY plays an important role in protecting normal cardiac and kidneys functions from damage [105]. In addition, several studies showed that betaine had the effect of inhibiting the release of NO in activated microglial cells which may benefit to control neurological disorders [106,107]. In addition, researches have shown that it is associated with amino acid and lipid metabilism, which may have a role in cardiovascular [108-111]. Of note, the level of betaine increased may damage heart, liver, and kidneys of healthy individuals [112]. Thus, we confirmed that caowu promote the protective functions of rats through decreasing the level of betaine. The present study results indicated caowu participated in the YNBY would not have a toxic effect on heart.
Nucleotide metabolism. Cytidine increased and deoxycytidine decreased were included both in YNBY and NCY. A study proved that cytidine may have a effect on neurodegenerative diseases [113]. Simultaneously, uric acid increased only coming from NCY, is a heterocyclic purine derivative, which may lead to a type of arthritis known due to gout with excess accumulation. The pathways we obtained showed that cytidine effected uric acid and deoxycytidine at the same time, which involved purine and pyrimidine metabolism.
Energy Metabolism. L-acetylcarnitine decreased in YNBY and NCY, which may promote the process of acetyl-CoA into the mammalian mitochondria matrix during the fatty acids oxidation process. In addition, L-acetylcarnitine plays a role in neuroprotective, neuromodulatory, and neurotrophic properties for against numerous disease processes [114].
Conclusions
To our knowledge, this is the first study using metabolism approach for the comprehensive assessment of endogenous metabolites, which provide a method to solve the phenomenon and mechanism that clinical physiological and biochemical indicators and histopathology cannot. On the basis of intake, body weight and organ coefficient results, we can conclude that The compatibility of caowu in YNBY would not have toxic effects on rats during the four weeks experiment with clinical equivalent dose. In addition, 77 biomarkers produced by YNBY , which participated in 12 metabolic pathways, 110 biomarkers produced by NCY, which participated in 22 metabolic pathways, which indicates that YNBY may be safer than NCY due to lower degree of influence. In addition, 13 biomarkers from YNBY and NCY on ROC curve and quantitative analysis results suggest that caowu may act on other medicines of YNBY to influence the metabolism. The results of canonical pathways and GO enrichment of biological processes of YNBY and NCY illustrate that four pathways in YNBY comprised glycine degradation, stearate biosynthesis and salvage pathways of pyrimidine ribonucleotides and iNOS signaling, while three pathways in NCY comprised tryptophan degradation, FXR/RXR activation and stearate biosynthesis. Of note, rats administrated YNBY and NCY for longer may produce liver necrosis/cell death including apoptosis of hepatocytes, cell death of hepatocytes and lipoapoptosis of hepatocytes.
https://www.peertechzpublications.org/uploads/art_addfiles_1986.rar" class="btn btn-success">Table S1-S3
This work was supported by grants from the Key Program of Natural Science Foundation of State (Grant No. 81830110, 81430093, 81373930, 81673586, 81703685, 81302905, 81503386), National Key Subject of Drug Innovation (Grant No. 2015ZX09101043-005, 2015ZX09101043-011), TCM State Administration Subject of Public Welfare of (Grant No. 2015468004), Major Projects of Application Technology Research and Development Plan in Heilongjiang Province (GX16C003), TCM State Administration Subject of Public Welfare (Grant No. 2015468004), Specialized Research Fund for the Doctoral Program of Higher Education (20132327130001, 20122327120006), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2015118, UNPYSCT-2016213), Young Talent Lift Engineering Project of China Association of Traditional Chinese Medicine (QNRC2-B06), Outstanding Talents Foundation of Heilongjiang University of Chinese Medicine (2018jc01), Doctoral Innovation Fund of Heilongjiang University of Chinese Medicine (2018bs02), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2015118, UNPYSCT-2016213, UNPYSCT-2016212), Application Technology and Development of Youth Talents Project in Harbin (2014RFQXJ116), Chinese Postdoctoral Science Foundation (2017M621319b), Returned Oversea Scholars Program of Heilongjiang Province (2017QD0025), Natural Science Foundation of Heilongjiang Province of China (QC2018117).
- Ladas EJ, Karlik JB, Rooney D, Taromina K, Ndao DH, et al (2012) Topical Yunnan Baiyao administration as an adjunctive therapy for bleeding complications in adolescents with advanced cancer. Support Care Cancer 20: 3379-3383. Link: https://goo.gl/gDcTCG
- Ness SL, Frye AH, Divers TJ, Rishniw M, Erb HN, et al (2017) Randomized placebo-controlled study of the effects of Yunnan Baiyao on hemostasis in horses. Am J Vet Res 78: 969-976. Link: https://goo.gl/kGZR47
- Zhao ZG, Yan SS, Yu YM, Mi N, Zhang LX, et al. (2013) An Aqueous Extract of Yunnan Baiyao Inhibits the Quorum-Sensing-Related Virulence of Pseudomonas aeruginosa. J Microbiol 51: 207-212. Link: https://goo.gl/DJKmDS
- He HB, Ren XB, Wang XY, Shi XZ, Wang XL, et al. (2012) Therapeutic effect of Yunnan Baiyao on rheumatoid arthritis was partially due to regulating arachidonic acid metabolism in osteoblasts. J Pharm Biomed Anal 59: 130-137. Link: https://goo.gl/9Qzs9M
- Dong H, Zhang AH, Sun H, Wang HY, Lu X, et al. (2012) Ingenuity pathways analysis of urine metabolomics phenotypes toxicity of Chuanwu in Wistar rats by UPLC-Q-TOF-HDMS coupled with pattern recognition methods. Mol BioSyst 8: 1206-1221. Link: https://goo.gl/Tkvmkw
- Sun H, Ni B, Zhang AH, Wang M, Dong H, et al. (2012) Metabolomics study on Fuzi and its processed products using ultra-performance liquid-chromatography/electrospray- ionization synapt high-definition mass spectrometry coupled with pattern recognition analysis. Analyst 137: 170-185. Link: https://goo.gl/6Hvi2G
- Wang XJ, Wang HY, Zhang AH, Lu X, Sun H, et al. (2012) Metabolomics Study on the Toxicity of Aconite Root and Its Processed Products Using Ultraperformance Liquid-Chromatography/ Electrospray-Ionization Synapt High-Definition Mass Spectrometry Coupled with Pattern Recognition Approach and Ingenuity Pathways Analysis. J Proteome Res 11: 1284-1301. Link: https://goo.gl/NH412G
- Sun H, Wang M, Zhang AH, Ni B, Dong H, et al. (2013) UPLC-Q-TOF-HDMS Analysis of Constituents in the Root of Two Kinds of Aconitum Using a Metabolomics Approach. Phytochem Anal 24: 263-276. Link: https://goo.gl/UjcGgt
- Sun B, Zhang M, Zhang Q, Ma KP, Li HJ, et al. (2014) Metabonomics study of the effects of pretreatment with glycyrrhetinic acid on mesaconitine-induced toxicity in rats. J Ethnopharmacol 154: 839-846. Link: https://goo.gl/HTCrnw
- Tan Y, Liu XR, Lu C, He XJ, Li J, et al. (2014) Metabolic profiling reveals therapeutic biomarkers of processed Aconitum Carmichaeli Debx in treating hydrocortisone induced Kidney-Yang deficiency syndrome rats. J Ethnopharmacol 152: 585-593. Link: https://goo.gl/CiybNy
- Liu S, Li Y, Li WF, Xu JK, Li F, Du H (2016) Advances in studies on toxicity and modern toxicology of species in Aconitum L. Chinese Traditional and Herbal Drugs 47: 4095-4102. Link: https://goo.gl/tqtsGQ
- Li XP, He J, He SL, Meng J (2017) Research Progress of Aconitum vilmorinianum. Journal of West China Forestry Science 46: 1-7.
- Li JY, Hong SJ, Huangshu. BQ, Liu QQ, Ren GY, et al. (2017) Determination of Mainly Alkaloid Components in Human Liver of Aconitum Vilmorimianum Kom Poisoning by UPLC-MS / MS Method. J Henan Univ Sci Tech 35: 306-309.
- Li Q, Sun SD, Wang MY, Li CF, Yuan D, et al, (2018) Chemical constituents and analgesic activity of Aconitum kusnezoffii Reichb. J Chin Pharm Sci 27: 855-863.
- Wang D, Jia DX, Li ZZ, Yao JK, Zhang L, et al. (2018) Safety evaluation and risk control measures for Aconiti Kusnezoffii Radix. China Journal of Chinese Meteria medica 43: 3093-3100. Link: https://goo.gl/JhjZ5b
- Li SY, Liu XY, Tang CP, Chen YF, Wang LP, et al. (2018) Study on content of alkaloid and cardiac toxicity before and after preparation of Aconitum vilmorinianum. Chinese Traditional and Herbal Drugs 49: 5588-5593.
- Wang LP, Chen QW, Shen ZB, Jiang T, Tang CP. (2018) Toxic Effects and Analgesis Effects of Aconitm Vilmoriniani Radix and Its Processed Products. Journal of Chinese Medicinal Materials: 41:1864-1868.
- Li JL, Liang H, Cai SZ, Li ZY, Tu Y (2018) Mechanism of detoxification of Chebulae Fructus against Aconiti kusnezoffii radix toxicity based on network pharmacology. Acta Pharmaceutica Sinica 53: 1670-1679. Link: https://goo.gl/PLHexG
- Wang XJ, Zhang AH, Sun H, Wang P (2012) Systems biology technologies enable personalized traditional Chinese medicine: a systematic review. Am J Chin Med 40: 1109-1122. Link: https://goo.gl/H6CX7N
- Zhang AH, Sun H, Wang P, Han Y, Wang XJ (2012) Future perspectives of personalized medicine in traditional Chinese medicine: a systems biology approach. Complement Ther Med 20: 93-99. Link: https://goo.gl/vohBWe
- Zhang AH, Sun H, Wang Z, Sun W, Wang P, et al. (2010) Metabolomics: towards understanding traditional Chinese medicine. Planta Med 76: 2026-2035. Link: https://goo.gl/oVRiZ5
- Wang X, Sun H, Zhang A, Sun W, Wang P, et al. (2011) Potential role of metabolomics apporoaches in the area of traditional Chinese medicine: as pillars of the bridge between Chinese and Western medicine. J Pharm Biomed Anal 55: 859-868. Link: https://goo.gl/EbWHNn
- Qiu S, Zhang AH, Sun H, Yan GL, Wang XJ (2014) Overview on metabolomics in traditional Chinese medicine. World J Pharmacol 3: 33-38. Link: https://goo.gl/8JSXic
- Cao H, Zhang A, Zhang H, Sun H, Wang X (2015) The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytother Res 29: 159-166. Link: https://goo.gl/azNx4y
- Zhang A, Zhou X, Zhao H, Zou S, Ma CW, et al. (2016) Metabolomics and proteomics technologies to explore herbal preparation affecting metabolic disorders using high resolution mass spectrometry. Mol Biosyst. 12: 262-273.
- Wang X, Sun H, Zhang A, Wang P, Han Y (2011) Ultra-performance liquid chromatography coupled to mass spectrometry as a sensitive and powerful technology for metabolomic studies.J Sep Sci 34: 3451-3459. Link: https://goo.gl/ewGohu
- Zhang A, Sun H, Wang P, Han Y, Wang X (2012) Modern analytical techniques in metabolomics analysis. Analyst 137: 293-300. Link: https://goo.gl/JpEkim
- Wang X, Yang B, Sun H, Zhang A (2012) Pattern recognition approaches and computational systems tools for ultra performance liquid chromatography-mass spectrometry-based comprehensive metabolomic profiling and pathways analysis of biological data sets. Anal Chem 84: 428-439. Link: https://goo.gl/v7DGSS
- Zhang AH, Sun H, Han Y, Yan GL, Yuan Y, et al (2013) Ultraperformance liquid chromatography-mass spectrometry based comprehensive metabolomics combined with pattern recognition and network analysis methods for characterization of metabolites and metabolic pathways from biological data sets. Anal Chem 85: 7606-7612. Link: https://goo.gl/YEisgE
- Zhang AH, Wang P, Sun H, Yan GL, Han Y, et al (2013) High-throughput ultra-performance liquid chromatography-mass spectrometry characterization of metabolites guided by a bioinformatics program. Mol Biosyst 9: 2259-2265. Link: https://goo.gl/RKhGyK
- Zhang T, Zhang A, Qiu S, Yang S, Wang X (2015) Current Trends and Innovations in Bio-Analytical Techniques of Metabolomics. Crit Rev Anal Chem.
- Zhang T, Zhang A, Qiu S, Yang S, Wang X (2016) Current Trends and Innovations in Bioanalytical Techniques of Metabolomics. Crit Rev Anal Chem. 46: 342-351. Link: https://goo.gl/JqeA7a
- Ren JL, Zhang AH, Kong L, Wang XJ (2018) Advances in mass spectrometry-based metabolomics for investigation of metabolites. RSC Advances 8: 22335-22350. Link: https://goo.gl/T2BACn
- Zhang A, Sun H, Wang P, Han Y, Wang X (2012) Recent and potential developments of biofluid analyses in metabolomics.J Proteomics 75: 1079-1088. Link: https://goo.gl/L6DYtM
- Zhang A, Sun H, Wang X (2012) Serum metabolomics as a novel diagnostic approach for disease: a systematic review. Anal Bioanal Chem 404: 1239-1245. Link: https://goo.gl/5tFBUR
- Zhang A, Sun H, Wu X, Wang X (2012) Urine metabolomics.Clin Chim Acta 414: 65-69. Link: https://goo.gl/Spgqdo
- Zhang A, Sun H, Han Y, Yuan Y, Wang P, et al. (2012) Exploratory urinary metabolic biomarkers and pathways using UPLC-Q-TOF-HDMS coupled with pattern recognition approach. Analyst 137: 4200-4208. Link: https://goo.gl/bhxChg
- Ma WW, Zhang AH, Sun H, Meng XC, Wang XJ (2016) Metabolomics Deciphering Therapeutic Effects and Mechanism of Traditional Chinese Medicines. World J Tradit Chin Med 2: 17-24. Link: https://goo.gl/urqRyi
- Wang X, Zhang A, Wang P, Sun H, Wu G, et al. (2013) Mol Cell Proteomics. 12: 1226-1238. Link: https://goo.gl/Yd7R1k
- Zhang A, Fang H, Wang Y, Yan G, Sun H, et al. (2017) Discovery and verification of the potential targets from bioactive molecules by network pharmacology-based target prediction combined with high-throughput metabolomics. RSC Adv 7: 51069-51078. Link: https://goo.gl/tFwcgZ
- Song Q, Zhang AH, Yan GL, Liu L, Wang XJ (2017) Technological advances in current metabolomics and its application in herbal medicine. RSC Adv 7, 53516-53524. Link: https://goo.gl/1ymvL6
- Zhang A, Sun H, Yan G, Wang P, Wang X (2015) Metabolomics for Biomarker Discovery: Moving to the Clinic. Biomed Res Int 354671. Link: https://goo.gl/4HwG13
- Zhang A, Sun H, Yan G, Wang P, Wang X (2016) Mass spectrometry-based metabolomics: applications to biomarker and metabolic pathway research. Biomed Chromatogr. 30: 7-12. Link: https://goo.gl/3tQnvq
- Sun H, Chen X, Zhang A, Sakurai T, Jiang J, et al. (2014) Chromatographic fingerprinting analysis of Zhizhu Wan preparation by high-performance liquid chromatography coupled with photodiode array detector. Pharmacogn Mag. 10: 470-476. Link: https://goo.gl/YPMR8i
- Yan G, Zou D, Zhang A, Tan Y, Sun H, et al. (2015) UPLC-Q-TOF-MS/MS fingerpriting for rapid identification of the chemical constituents of Ermiao Wan. Anal. Methods 7: 846-862. Link: https://goo.gl/P8jced
- Sun H, Wu F, Zhang A, Wei W, Han Y, et al. (2013) Profiling and identification of the absorbed constituents and metabolites of schisandra lignans by ultra-performance liquid chromatography coupled to mass spectrometry. Biomed Chromatogr 27: 1511-1519. Link: https://goo.gl/FFRKNj
- Wang H, Yan G, Zhang A, Li Y, Wang Y, et al. (2013) Rapid discovery and global characterization of chemical constituents and rats metabolites of Phellodendri amurensis cortex by ultra-performance liquid chromatography-electrospray ionization/ quadrupole-time-of- flight mass spectrometry coupled with pattern recognition approach. Analyst 138: 3303-3312. Link: https://goo.gl/8WSFzd
- Wang H, Sun H, Zhang A, Li Y, Wang L, et al. (2013) Rapid identification and comparative analysis of the chemical constituents and metabolites of Phellodendri amurensis cortex and Zhibai dihuang pill by ultra-performance liquid chromatography with quadrupole TOF-MS. J Sep Sci 36: 3874-3882. Link: https://goo.gl/yNuZ4E
- Xue C, Zhang A, Sun H, Han Y, Zou D, et al. (2014) An improved ultra-performance liquid chromatography-electrospray ionization/quadrupole-time-of-flight high- definition mass spectrometry method for determining ingredients of herbal Fructus corni in blood samples. Pharmacogn Mag 10: 422-429. Link: https://goo.gl/jgdv8c
- Zhang Y, Zhang A, Zhang Y, Sun H, Meng X, et al. (2016) Application of Ultra-performance Liquid Chromatography with Time-of-Flight Mass Spectrometry for the Rapid Analysis of Constituents and Metabolites from the Extracts of Acanthopanax senticosus Harms Leaf. Pharmacogn Mag 12: 145-152. Link: https://goo.gl/r5818q
- Sun H, Liu J, Zhang A, Zhang Y, Meng X, et al. (2016) Characterization of the multiple components of Acanthopanax Senticosus stem by ultra high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J Sep Sci 39: 496-502. Link: https://goo.gl/tptW1N
- Han Y, Zhang A, Sun H, Zhang Y, Meng X, et al. (2017) High-throughput ultra high performance liquid chromatography combined with mass spectrometry approach for the rapid analysis and characterization of multiple constituents of the fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms. J Sep Sci. 40: 2178-2187. Link: https://goo.gl/k5pYiN
- Zhang AH, Sun H, Yan GL, Wang XJ (2017) Recent developments and emerging trends of mass spectrometry for herbal ingredients analysis. TrAC Trends in Analytical Chemistry 94: 70-76. Link: https://goo.gl/WmQctc
- Yang B, Dong W, Zhang A, Sun H, Wu F, et al. (2011) Ultra-performance liquid chromatography coupled with electrospray ionization/quadrupole-time-of-flight mass spectrometry for rapid analysis of constituents of Suanzaoren decoction.J Sep Sci 34: 3208-3215. Link: https://goo.gl/ueJdPu
- Wang P, Lv HT, Zhang AH, Sun H, Yan G, et al. (2013) Improved ultra-performance liquid chromatography with electrospray ionization quadrupole-time-of- flight high-definition mass spectrometry method for the rapid analysis of the chemical constituents of a typical medical formula: Liuwei Dihuang Wan. J Sep Sci 36: 3511-3516. Link: https://goo.gl/tcNDC6
- Yin Q, Wang P, Zhang A, Sun H, Wu X, et al. (2013) Ultra-performance LC-ESI/quadrupole-TOF MS for rapid analysis of chemical constituents of Shaoyao-Gancao decoction. J Sep Sci 36: 1238-1246. Link: https://goo.gl/k1WYM5
- Wang X, Zhang A, Yan G, Han Y, Sun H (2014) UHPLC-MS for the analytical characterization of traditional Chinese medicines. TrAC Trends in Analytical Chemistry 63: 180-187. Link: https://goo.gl/43djHs
- Zou D, Zhang AH, Yan GL, Tan YL, Sun H, et al. (2014) UPLC-MS coupled with a dynamic multiple data processing method for the comprehensive detection of the chemical constituents of the herbal formula San-Miao-Wan. Analytical Methods 6: 2848-2854. Link: https://goo.gl/Ahj7YP
- Zhang A, Zou D, Yan G, Tan Y, Sun H, et al. (2014) Identification and characterization of the chemical constituents of Simiao Wan by ultra high performance liquid chromatography with mass spectrometry coupled to an automated multiple data processing method. J Sep Sci 37: 1742-1747. Link: https://goo.gl/wp6x7a
- Sun H, Liu C, Zhang A, Han Y, Yan G, et al. (2015) Rapid discovery and global characterization of multiple constituents from Kai-Xin-San with an integrated MSE data acquisition mode strategy based on ultra-performance liquid chromatography coupled to electrospray ionization/quadrupole-time-of-flight mass spectrometry. Anal. Methods 7: 279-286. Link: https://goo.gl/PMrbVB
- Li X, Sun H, Zhang A, Liu Z, Zou D, et al. (2017) High-throughput LC-MS method for rapid characterization of multiple chemical constituents and metabolites of Da-Bu-Yin-Wan. J Sep Sci. J Sep Sci 40: 4102-4112. Link: https://goo.gl/EZM2kj
- Yan GL, Zhang AH, Sun H, Han Y, Shi H, et al. (2013) An effective method for determining the ingredients of Shuanghuanglian formula in blood samples using high-resolution LC-MS coupled with background subtraction and a multiple data processing approach. J Sep Sci 36: 3191-3199. Link: https://goo.gl/tzH3NL
- Wang P, Yin QW, Zhang AH, Sun H, Wu XH, et al. (2014) Preliminary identification of the absorbed bioactive components and metabolites in rat plasma after oral administration of Shaoyao-Gancao decoction by ultra-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Pharmacogn Mag. 10: 497-502. Link: https://goo.gl/qAXvEr
- Cao H, Zhang A, Zhang FM, Wang QQ, Zhang H, et al. (2014) Ultra-performance liquid chromatography tandem mass spectrometry combined with automated MetaboLynx analysis approach to screen the bioactive components and their metabolites in Wen-Xin-Formula. Biomed Chromatogr. 28: 1774-1781. Link: https://goo.gl/WNRKoS
- Han Y, Wu F, Zhang A, Sun H, Wei W, et al. (2015) Characterization of multiple constituents in rat plasma after oral administration of Shengmai San using ultra-performance liquid chromatography coupled with electrospray ionization/quadrupole-time-of-flight high- definition mass spectrometry. Analytical Methods 7: 830-837. Link: https://goo.gl/HWvaPG
- Liu C, Zhang A, Han Y, Lu S, Sun H, et al. (2015) Ultra-high performance liquid chromatography coupled with time-of-flight mass spectrometry screening and analysis of potential bioactive compounds from traditional chinese medicine Kai-Xin-San, using a multivariate data processing approach and the MetaboLynx tool. RSC Adv 5: 85-92. Link: https://goo.gl/YdcJC3
- Li XN, Zhang A, Sun H, Song Y, Zou D, et al. (2016) Rapid discovery of absorbed constituents and metabolites in rat plasma after the oral administration of Zi Shen Wan using high-throughput UHPLC-MS with a multivariate analysis approach. J Sep Sci. 39: 4700-4711. Link: https://goo.gl/pXyo7H
- Wang XJ, Zhang AH, Sun H (2012) Future perspectives of Chinese medical formulae: chinmedomics as an effector. OMICS 16: 414-421. Link: https://goo.gl/h2MPmU
- Zhang AH, Sun H, Qiu S, Wang XJ (2013) Recent highlights of metabolomics in chinese medicine syndrome research. Evid Based Complement Alternat Med 402159. Link: https://goo.gl/MKV2WG
- Wang XJ, Zhang AH, Zhou XH, Liu Q, Nan Y, et al. (2016) An integrated chinmedomics strategy for discovery of effective constituents from traditional herbal medicine. Sci Rep 6: 18997. Link: https://goo.gl/JVz6Pd
- Wang XJ, Zhang AH, Sun H, Yan GL (2016) Chinmedomics: Newer Theory and Application. Chinese Herbal Medicines 8: 299-307. Link: https://goo.gl/Wi2FXr
- Zhang AiH, Sun H, Yan GL, Han Y, Zhao QQ, etal. (2018) Chinmedomics: A Powerful Approach Integrating Metabolomics with Serum Pharmacochemistry to Evaluate the Efficacy of Traditional Chinese Medicine. Engineering. Link: https://goo.gl/8ZvEEy
- Zhang A, Sun H, Wang XJ (2014) Potentiating therapeutic effects by enhancing synergism based on active constituents from traditional medicine. Phytother Res. 28: 526-533. Link: https://goo.gl/bk5vxu
- Zhang A, Sun H, Qiu S, Wang X (2013) Advancing drug discovery and development from active constituents of yinchenhao tang, a famous traditional chinese medicine formula. Evid Based Complement Alternat Med 257909. Link: https://goo.gl/1H9Tdo
- Song YH, Sun H, Zhang AH, Wang XJ (2014) Plant-derived natural products as leads to anticancer drugs. J Med Plant Herb 2: 6-15. Link: https://goo.gl/keHx5t
- Chu H, Zhang A, Han Y, Wang X (2015) Metabolomics and its potential in drug discovery and development from TCM. World J Tradit Chin Med, 1: 26-32. Link: https://goo.gl/R7Q9G9
- Zhou Y, Zhang AH, Sun H, Yan GL, Wang XJ (2014) Plant-derived natural products as leads to antitumor drugs. Plant Sci Today 1: 46-61. Link: https://goo.gl/Q9koiH
- Zhang A, Sun H, Wang XJ (2016) Mass spectrometry-driven drug discovery for development of herbal medicine. Mass Spec Rev 9999: B-14. Link: https://goo.gl/UsLCrq
- Wang X, Zhang A, Sun H, Han Y, Yan G (2016) Discovery and development of innovative drug from traditional medicine by integrated chinmedomics strategies in the post-genomic era. TrAC Trends in Analytical Chemistry 76: 86-94. Link: https://goo.gl/KKj1wi
- Xie J, Zhang AH, Sun H, Yan GL, Wang XJ (2018) Recent advances and effective strategies in the discovery and applications of natural products. RSC Advances 2: 812-824. Link: https://goo.gl/UE1qKu
- Sun H, Ni B, Zhang A, Wang M, Dong H, et al. (2012) Metabolomics study on Fuzi and its processed products using ultra-performance liquid-chromatography/electrospray-ionization synapt high-definition mass spectrometry coupled with pattern recognition analysis.Analyst 137: 170-185. Link: https://goo.gl/DZLnUR
- Wang XJ, Wang HY, Zhang AH, Lu X, Sun H, et al. (2012) Metabolomics study on the toxicity of aconite root and its processed products using ultraperformance liquid-chromatography/electrospray-ionization synapt high-definition mass spectrometry coupled with pattern recognition approach and ingenuity pathways analysis. J Proteome Res 11: 1284-1301. Link: https://goo.gl/jMVcWX
- Yan Y, Zhang A, Dong H, Yan G, Sun H, et al. (2017) Toxicity and detoxification effects of herbal Caowu via ultra performance liquid chromatography/mass spectrometry metabolomics analyzed using pattern recognition method. Phcog Mag 13: 683-692. Link: https://goo.gl/Z3iS2q
- Dong H, Yan GL, Han Y, Sun H, Zhang AH, et al. (2015) UPLC-Q-TOF/MS-based metabolomic studies on the toxicity mechanisms of traditional Chinese medicine Chuanwu and the detoxification mechanisms of Gancao, Baishao, and Ganjiang. Chin J Nat Med 13: 687-698. Link: https://goo.gl/yzp7mB
- Obradovic M, Obradovic AJ, Stewart SJ, Pitt M, Labudovic-Borovic M, et al. (2014) In vivo effects of 17β-estradiol on cardiac Na+/K+-ATPase expression and activity in rat heart. Mol Cell Endocrinol 388: 58-68. Link: https://goo.gl/Mg6bDa
- Celik I, Suzek H (2008) The hematological effects of methyl parathion in rats. J. Hazard. Mater 153: 1117-1121. Link: https://goo.gl/Cph8Mi
- Kaysen GA, Dubin JA, Müller HG, Mitch WE, Rosales LM, et al. (2002) Relationships among inflammation nutrition and physiologic mechanisms establishing albumin levels in hemodialysis patients. Kidney Int 61: 2240-2249. Link: https://goo.gl/4AjBft
- Zou YX, Zhang XD, Mao Y, Lu GC, Huang M, et al. (2010) Acute toxicity of a single dose DATR, recombinant soluble human TRAIL mutant, in rodents and crab-eating macaques. Hum Exp Toxicol 29: 645-652. Link: https://goo.gl/kqQGrJ
- Singhuber J , Zhu M , Prinz S, Kopp B (2009) Aconitum in Traditional Chinese Medicine-A valuable drug or an unpredictable risk? J Ethnopharmacol 126: 18-30. Link: https://goo.gl/DQavoJ
- Ge YB, Jiang Y, Zhou H, Zheng M, Li J, et al. (2016) Antitoxic effect of Veratrilla baillonii on the acute toxicity in mice induced by Aconitum brachypodum, one of the genus Aconitum. J Ethnopharmacol 179: 27-37. Link: https://goo.gl/GSKwFM
- Greeb J, Shull GE (1989) Molecular cloning of a third isoform of the calmodulin-sensitive plasma membrane Ca2+-transporting ATPase that is expressed predominantly in brain and skeletal muscle. J Biol Chem 264: 18569-18576. Link: https://goo.gl/FeR6q5
- Kurihara K, Nakanishi N, Amano O, Tonosaki K (2008) Expression of Na(+)/K(+)-ATPase alpha subunit isoforms in rat salivary glands: occurrence of sense and antisense RNAs of the alpha3 isoform in the sublingual gland. Arch Oral Biol 53: 593-604. Link: https://goo.gl/EviNJU
- Zhang XS, Li PL, Hua YL, Ji P, Yao WL, et al. (2018) Urinary metabolomics study the mechanism of Taohong Siwu Decoction intervention in acute blood stasis model rats based on liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Journal of Chromatography B: 1074-1075. Link: https://goo.gl/JqXzo4
- Zhao X, Zhang Y, Meng X, Yin P, Deng C, et al. (2008) Effect of a traditional Chinese medicine preparation Xindi soft capsule on rat model of acute blood stasis: a urinary metabonomics study based on liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 873: 151-158. Link: https://goo.gl/qDNESc
- Mangin EL, Kugiyama K, Nguy JH, Kerns SA, Henry PD (1993) Effects of lysolipids and oxidatively modified low density lipoprotein on endothelium-dependent relaxation of rabbit aorta. Circ Res 72: 161-166. Link: https://goo.gl/nKDETL
- Engelmann B, Zieseniss S, Brand K, Page S, Lentschat A, et al. (1999) Tissue factor expression of human monocytes is suppressed by lysophosphatidylcholine. Arterioscler Thromb Vasc Biol 19: 47-53. Link: https://goo.gl/XrWqAL
- Horiuchi M, Mogi M (2011) Role of angiotensin II receptor subtype activation in cognitive function and ischaemic brain damage. Br J Pharmacol 163: 1122-1130. Link: https://goo.gl/8f9X6Q
- Crocenzi FA, Basiglio CL, Pe´rez LM, Portesio MS, Pozzi EJ, et al. (2005) Silibinin prevents cholestasis-associated retrieval of the bile salt export pump, Bsep, in isolated rat hepatocyte couplets: Possible involvement of cAMP. Biochem Pharmacol 69: 1113-1120. Link: https://goo.gl/tPy9Hs
- Hayashi H, Takada T, Suzuki H, Onuki R, Hofmann AF, et al. (2005) Transport by vesicles of glycine- and taurine-conjugated bile salts and taurolithocholate 3-sulfate: A comparison of human BSEP with rat Bsep. Biochim Biophys Acta 1738: 54-62. Link: https://goo.gl/QptxCm
- Pusl T, Vennegeerts T, Wimmer R, Denk GU, Beuers U, et al. (2008) Tauroursodeoxycholic acid reduces bile acid-induced apoptosis by modulation of AP-1. Biochem Biophys Res Commun 367: 208-212. Link: https://goo.gl/y29Jcu
- Ho EM, Leung DK, Leung GN, Wan TS, Wong HN, et al. (2007) Metabolic studies of mesterolone in horses. Analytica Chimica Acta 596: 149-155. Link: https://goo.gl/pZJPjN
- Itil TM (1983) The discovery of antidepressant drugs by computer-analyzed human cerebral bio-electrical potentials (CEEG). Prog Neurobiol 20: 185-249. Link: https://goo.gl/nhxumE
- Liu XL, Fan DM (2000) Protective effects of prostaglandin E1 on hepatocytes. World J Gastroenterol 6: 326-329. Link: https://goo.gl/B9EAKJ
- Masoodi M, Nicolaou A (2006) Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom 20: 3023-3029. Link: https://goo.gl/1HxmiH
- Lever M, Slow S (2010) The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem 43: 732-744. Link: https://goo.gl/762kHK
- Amiraslani B, Sabouni F, Abbasi S, Nazem H., Sabet M (2012) Recognition of betaine as an inhibitor of lipopolysaccharide-induced nitric oxide production in activated microglial cells. Iran Biomed J 16: 84-89. Link: https://goo.gl/6YLpqh
- Lever M, George PM, Elmslie JL, Atkinson W, Slow S, et al. (2012) Betaine and secondary events in an acute coronary syndrome cohort. PloS One 7: e37883. Link: https://goo.gl/9TGE4M
- House JD, Hall BN, Brosnan JT (2001) Threonine metabolism in isolated rat hepatocytes. Am J Physiol Endocrinol Metab 281: e1300-1307. Link: https://goo.gl/EAyqeM
- Kohno M, Fujii T, Hirayama C (1990) [15N] glycine metabolism in normal and cirrhotic subjects. Biochem Med Metab Biol 43: 201-213. Link: https://goo.gl/z8sRmr
- Lever M, George PM, Atkinson W, Molyneux SL, Elmslie JL, et al. (2011) Plasma lipids and betaine are related in an acute coronary syndrome cohort. PloS One 6: e21666. Link: https://goo.gl/bPwSQ5
- Snell K, Weber G (1986) Enzymic imbalance in serine metabolism in rat hepatomas. Biochem J 233: 617-620. Link: https://goo.gl/vxGSA2
- Grunewald RW, Eckstein A (1995) Osmotic regulation of the betaine metabolism in immortalized renal cells. Kidney Int 48: 1714-1720. Link: https://goo.gl/urj1VA
- Cansev M (2006) Uridine and cytidine in the brain: Their transport and utilization. Brain Res Rev 52: 389-397. Link: https://goo.gl/KEPVwB
- Virmani A, Binienda Z (2004) Role of carnitine esters in brain neuropathology. Mol Aspects Med 25: 533-549. Link: https://goo.gl/pESdLs
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
