Open Journal of Cell and Protein Science
Expression of PAR2 and NF-κB in human primary dental pulp odontoblasts during the progression of caries
Alisa Wichaidit1, Namthip Patinotham1, Kullanun Nukaeow1 and Aunwaya Kaewpitak2*
2Assistant Professor, Preventive Department, Faculty of Dentistry, Prince of Songkla University, HatYai, Thailand
Cite this as
Wichaidit A, Patinotham N, Nukaeow K, Kaewpitak A (2022) Expression of PAR2 and NF-κB in human primary dental pulp odontoblasts during the progression of caries. Open J Cell Protein Sci 5(1): 001-004. DOI: 10.17352/ojcps.000004Copyright
© 2022 Wichaidit A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Increased proteinase-activated receptor-2 (PAR2) expression is observed in various diseases related to inflammation. However, the expression of PAR2 in odontoblasts in response to dental caries has not been investigated. Therefore, to explore the functions of odontoblasts during the progression of carious infection, we measured PAR2 and NF-κB expression using immunofluorescence techniques in the odontoblast layer and pulpocytes in the sub-odontoblast region of 44 teeth extracted from children undergoing dental treatment (eight sound samples, 13 early carious samples, 16 advanced carious samples, and eight exposed pulp samples). PAR2 and NF-κB were expressed at moderate levels in sound teeth with non-carious pulp, and the expression levels changed as caries progressed. PAR2 was significantly upregulated in the odontoblast layer during early-stage and advanced-stage caries, and reduced below healthy levels in teeth with exposed pulp. NF-κB was significantly upregulated in early-stage caries and significantly downregulated in advanced-stage and late-stage caries. Moreover, in the sub-odontoblast region, NF-κB expression increased with the progression of caries. Overall, this study suggests PAR2 may represent a crucial cell signalling receptor in the dentine-pulp complex during dental inflammation, and that NF-κB may be one of the key pathways that regulate inflammatory immune responses in the dental pulp.
Inroduction
Odontoblasts are highly differentiated cells that align at the periphery of the dental pulp. These multifunctional cells can synthesize reparative dentine in response to pathological stimulations, and also function as neuronal transmitters for dental pain [1] Odontoblasts also possess various types of cellular receptors and act as immune effector cells.
When teeth undergo carious infection, cellular receptors on odontoblasts such as mechanosensitive ion channels play a role in the response of the dentin-pulp complex [2,3]. Additionally, specialized pattern recognition receptors (PRRs) on odontoblasts sense pathogen-associated molecular patterns (PAMPs) to detect invading microorganisms and thus contribute to the innate and adaptive immune responses. Proteinase-activated receptor-2 (PAR2) is also expressed on odontoblasts, and known to regulate both physiological and pathological processes in lung, respiratory tract and gastrointestinal tissues by acting as a sensor for extracellular proteases. However, our knowledge of the expression and function of PAR2 in dental pulp is largely limited to in vitro studies of odontoblast-like cells.
In addition to odontoblast cells, the pulpocytes beneath the odontoblast layer also function as immune effectors. Signalling networks within pulpocytes have been suggested to amplify inflammatory responses during carious infection [4,5]. Ritchie, et al. (2007) suggested that NF-κB and p38 MAP kinase regulate PAR2 and PAR4 expression in human endothelial cells during the pro-inflammatory challenge in vitro [6]. Moreover, Alvarez, et al. (2017) and Sun, et al. (2018) found that challenging MDPC-23 odontoblast-like cells with proinflammatory cytokines upregulated PAR-1 and PAR-2 [7,8].
In terms of the bacteriology of dental infection in shallow carious lesions, the dental pulp can detect caries-related Gram-positive aerobic pathogens in the biofilm, such as Streptococcus mutants, and subsequently trigger the innate immune system (9). In deeper carious lesions, the dominant pathogens transition to Gram-negative aerobic bacteria and the infection becomes more complex with high bacterial diversity [10].
In this study, we aimed to address the histomorphological modifications and, PAR2 and NF-κB p65 expression profiles in odontoblasts and pulpocytes under the odontoblast layer during different stages of carious infection.
Materials and methods
Sample collection
A total of 44 primary tooth pulp samples were analysed in this study, including eight sound samples, 13 early carious samples, 16 advanced carious samples, and eight exposed pulp samples. The teeth were acquired from children who required dental extractions under local or general anaesthesia. The mean age of the patients was 5.2 years (range: 4.3-7.2). Ethical approval for the study was granted by the Faculty of Dentistry, Prince of Songkhla University Ethics Committee (EC6004-10-P-LR).
After extraction, the whole teeth were washed and immediately immersed in 0.1 M phosphate-buffered saline (PBS), stored at 4 °C for 6-8 h, and then placed in fixative (0.1m phosphate buffer, pH 7.4, containing 4% paraformaldehyde) for 24 h at 4 °C. To remove the fixed pulp from the pulp chamber, a longitudinal groove was cut on the buccal aspect of each crown and each tooth was split open. The degree of caries was confirmed under a dissection microscope. Then, the pulpal tissues were carefully detached from the pulp chamber and maintained overnight in 0.1M PBS.
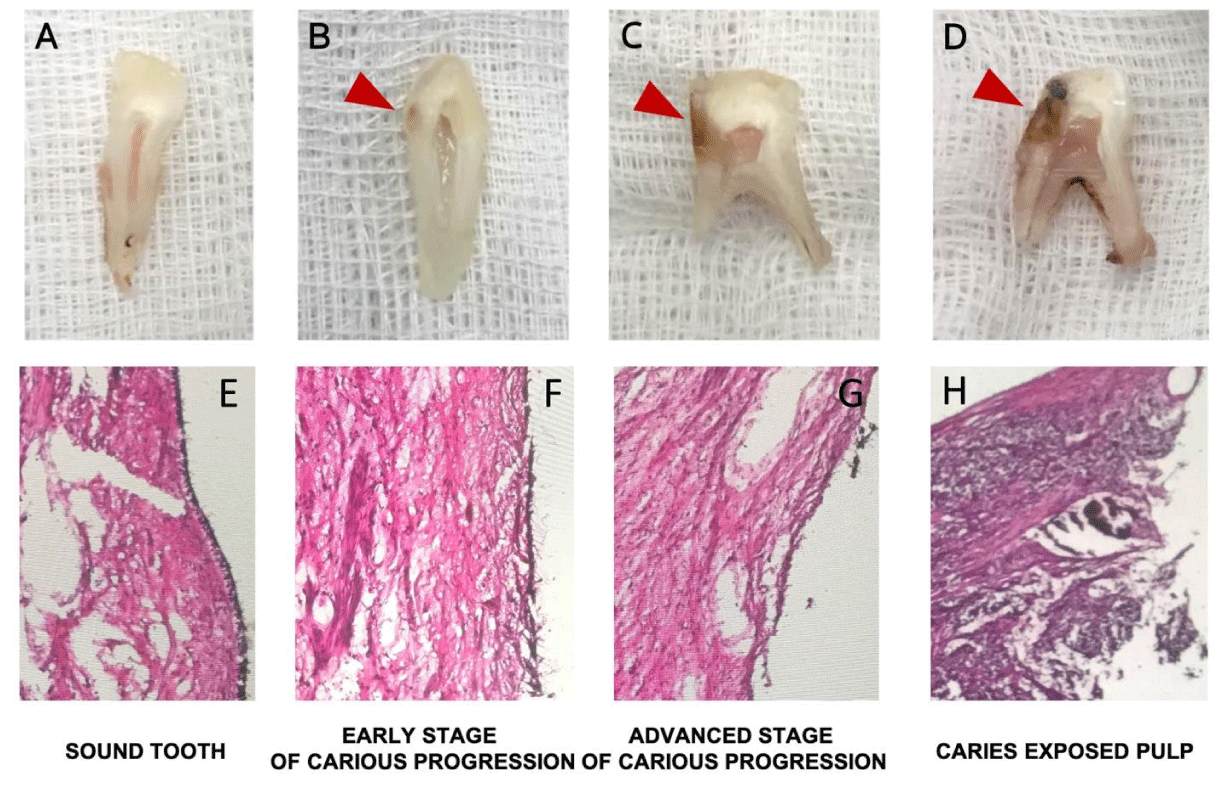
The degree of caries for each tooth was categorized into four stages as shown in Figure 1 [1] sound (no colour change within the dentine), [2] early-stage carious progression (colour changes within the dentine, but only within half of the dentine thickness), [3] advanced-stage carious progression (colour changes extending beyond half of the dentine thickness, but the barrier to the dental pulp was intact), or [4] caries-exposed pulp (colour changes extending beyond the dentinal thickness with no barrier to the dental pulp). Teeth in which the dental pulp exhibited obvious necrosis were excluded. The H&E stain provided an overall microanatomy of dental pulp tissues that inflammatory cells progressively infiltrate the pulp tissues in various stages of dental caries (Figure 1E-H).
The pulp samples were cryopreserved in 30% sucrose (in 0.1M PBS) for 5-8h at 4°C, embedded in OCT compound, and 14µm thick longitudinal sections were cut using a cryostat, collected on poly-D-lysine-coated glass slides, air-dried for 60min at room temperature, and kept at -70°C for long-term storage.
Immunofluorescence
The pulpal sections were washed twice with 0.1 M PBS, then incubated in 10% normal donkey serum (NDS) in 0.5% Triton X-100 PBS for 1h at room temperature to block non-specific antibody-binding sites. Next, the sections were incubated with primary monoclonal anti-mouse β-tubulin III (Abcam), polyclonal anti-rabbit PAR2 (Novus biological), or Anti-NF-κB p65 Antibody (Santa Cruz) antibodies diluted in 1% NDS containing 0.5% Triton X-100 for 1h at room temperature. The sections were washed twice in PBS with 0.5% Triton X-100 PBS, then incubated with goat anti-mouse conjugated CY3 (Thermo Fisher) and goat anti-rabbit conjugated FITC (Novus Biological) secondary antibodies in 1% NDS containing 0.5% Triton X-100 for 1h at room temperature. Finally, the sections were mounted with Vectashield containing DAPI and visualized by fluorescence microscopy. This Anti-NF-κB p65 Antibody is a mouse monoclonal IgG1 κ NFκB p65 antibody that is recommended for detection of NF-κB p65 of human origin by immunofluorescence.
Analysis of labelling
PAR2 and NF-KB immunofluorescent images were captured using 5×,20×,and 40×objective lenses on a Zeiss® fluorescence microscope with Zen 2.5 blue edition software (Zeiss). The exposure time of each channel was kept constant throughout the experiment, using the same exposure time for all samples.
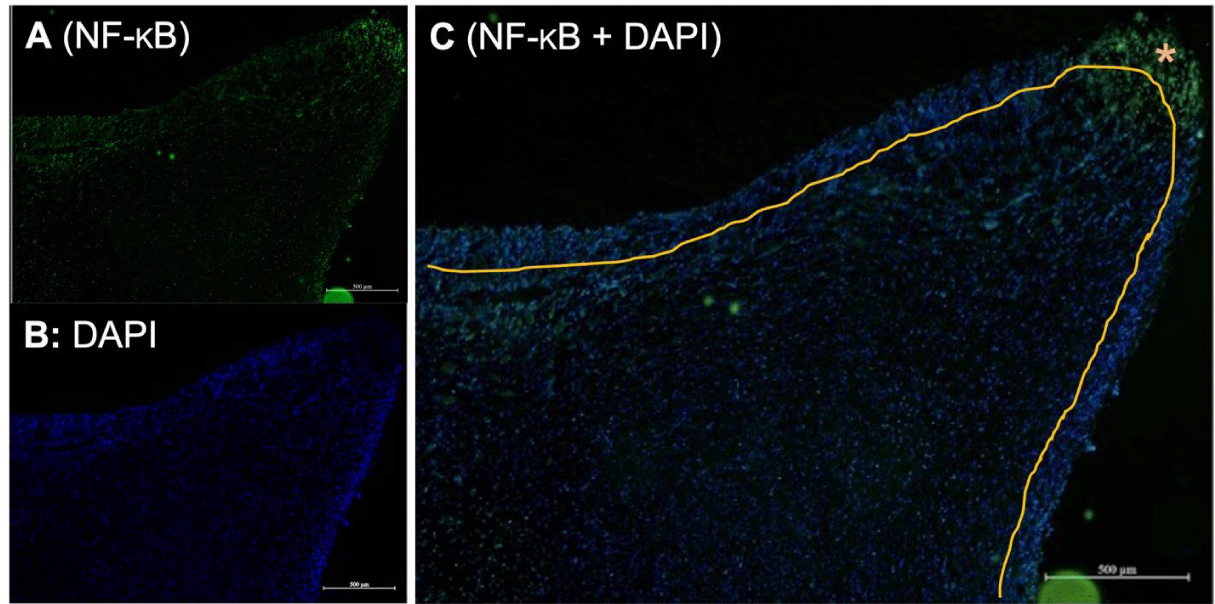
Odontoblasts are a layer of tall columnar cells located at the periphery of the dental pulp. The representative image in Figure 2 shows a cross-section of coronal pulp (the upper section of the dental pulp) immunostained for NF-κB and counterstained with DAPI. The picture in Figure 2 is zoomed in close to the pulpal horn area where carious injury usually occurs. DAPI staining revealed the nuclei of odontoblasts and pulpocytes distributed around the dental pulp. The area around the outer part of the pulp with a denser distribution of nuclei represents the odontoblast layer, which has a more densely packed structure than the pulpocytes underneath.
The area of interest (odontoblast layer) was selected by outlining the alignment of the layer of columnar cells on the outermost layer of the dental pulp. Then, the mean intensity PAR2 values were quantified within the area of interest. The fluorescence intensities of PAR2, NF-κB, and DAPI were determined using ZenBlue software.
In every NF-κB and PAR2 immunostaining image, we drew a line to differentiate the odontoblast layer from the pulpocytes underneath. We then measured the mean intensity value of both areas based on the difference in intensity value of the two areas to assess the changes in NF-κB and PAR2 expression. The same investigator repeated this entire process three times on each image to reduce subjective bias. We used the mean intensity value of the three measurements for further statistical analysis.
Statistical analysis
Data were analysed by one-way ANOVA, and individual groups were compared by Tukey’s post hoc test. Statistical significance is denoted as p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
Results
Odontoblast PAR2 expression during the progression of dental caries
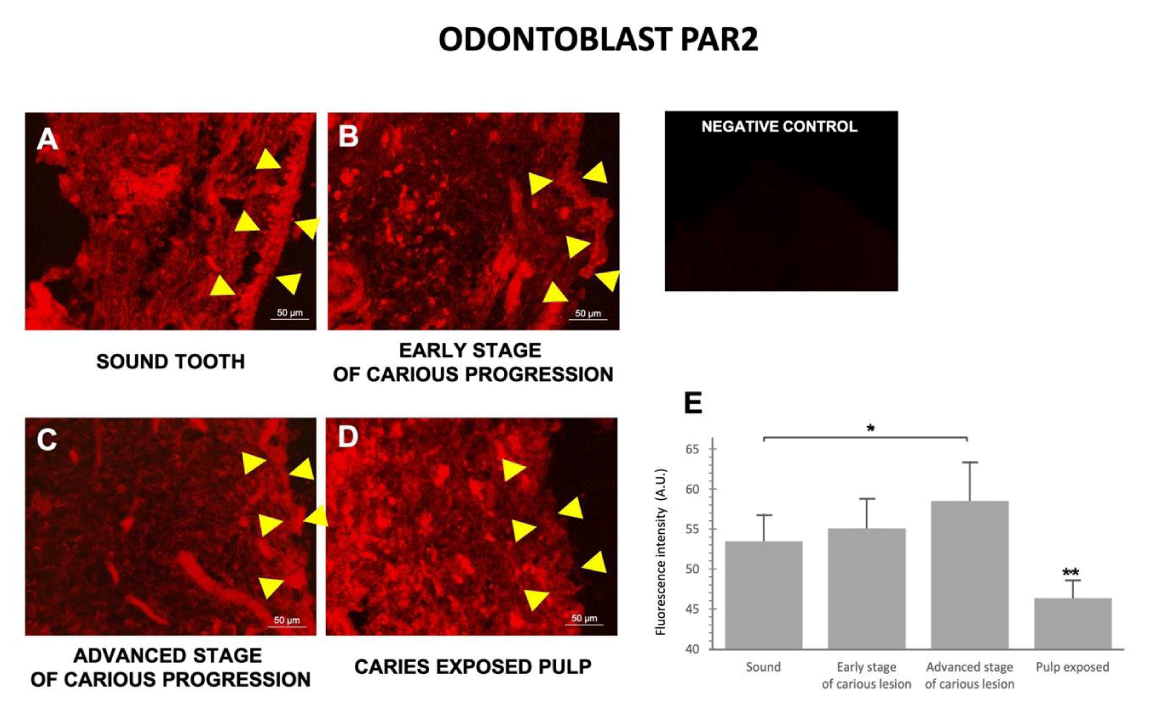
PAR2 immunostaining revealed the overall morphology of the odontoblast layer and pulpocytes. In non-carious pulp, the odontoblast cells were evenly distributed as a uniform layer (Figure 3A); however, as caries progressed the odontoblast layer became increasingly damaged. In teeth with early- and advanced-stage caries, the odontoblasts exhibited an increasingly uneven and irregular pattern (Figure 3B,C). In late-stage caries, the odontoblast layer almost completely disappeared, and could no longer be observed in some samples (Figure 3D).
Interestingly, although the odontoblast layer was damaged and lost its uniformity in early- and advanced-stage caries, the overall level of PAR2 expression in the odontoblast area did not decrease, and even seemed to slightly increase. Quantitative analysis (Figure 3E) confirmed the intensity of PAR2 expression in odontoblasts gradually increased from healthy teeth to the advanced stage of caries, then significantly decreased in late-stage caries.
NF-κB expression during the progression of dental caries
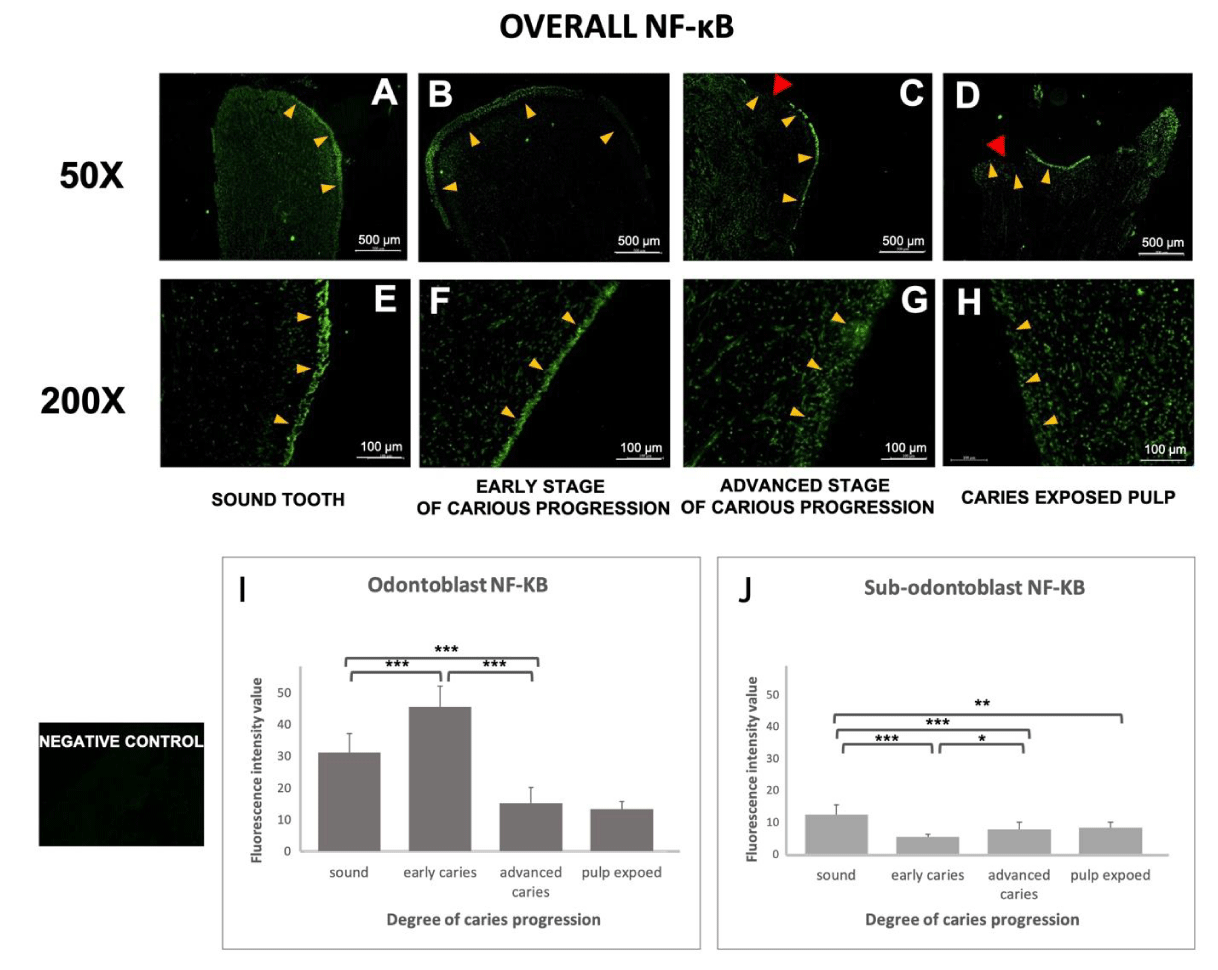
NF-κB is a central mediator of inflammation in various tissues, including dental pulp, and is involved in the odontogenesis of the dental pulp. Therefore, we measured the expression level of NF-κB in both the odontoblast layer and sub-odontoblasts (the area underneath odontoblasts). Immunostaining NF-κB and counterstaining for DAPI revealed the overall distribution of odontoblasts and pulpocytes. In agreement with the PAR2 results above, NF-κB staining confirmed the odontoblastic layers were evenly distributed along with the outer layer of the pulp in non-carious teeth and early-stage caries (Figures 4A,B,E,F). In advanced-stage caries, the odontoblast layers started to exhibit an uneven and irregular pattern and were more disputed at the injury site (indicated by the red arrow in Figures 4C and G). In the late stage of caries, the odontoblast layer could no longer be observed at the injury site, although some of the odontoblast layers in other areas of the teeth were intact (Figure 4D,H).
Quantitative analysis (Figure 4I) showed the mean odontoblast NF-κB staining intensity increased during the early stage of dental caries, and then dramatically decreased at the advanced stage of caries and in the caries-exposed pulp samples. Conversely, in the pulpocytes of the sub-odontoblast region, non-carious samples showed the highest expression of NF-κB. Expression of NF-κB in the sub-odontoblast layer decreased in the early stage of caries, and then gradually increased during the progression of caries, though remained lower than in non-carious teeth.
Discussion
Rationale for the study
Previous studies suggested PAR2 is upregulated in various diseases, such as arthritis [11], cancer [12] and lung disease [13]. These pathological processes are likely to involve increased activation of p38 MAP kinase and NF-κB. Ritchie, et al. (2007) reported that key proinflammatory cytokines such as tumour necrosis factor-α (TNF-α) could upregulate PAR2 [6]. Moreover, Alvarez, et al. (2017) demonstrated upregulation of PAR1 and PAR2 in odontoblast-like cells during inflammatory responses in vitro.
To date, PAR2 studies have been limited to cultured odontoblasts and/or odontoblast-like cells that do not mimic the dentin-pulp complex during dental pulp inflammation. PAR2 is the main mediator of pulpal inflammation induced by pro-inflammatory cytokines or even bacterial components such as Gingipain from P. gingivalis, which in turn cleave PAR2 and lead to internalization of the receptor into the cells [14].
We hypothesized that dental caries could affect odontoblast PAR2 during the progression of dental caries. It is possible that the dentine-pulp complex could promote the differentiation of pulpocytes via the NF-κB pathway. NF-κB is known to participate in the defensive mechanisms that provide innate immunity during carious infection to regulate cytokine production and promote cell differentiation via inflammatory processes [15]. However, odontoblasts and nearby cells may express different levels of the cell surface receptor PAR2 and NF-κB, and the cellular and molecular events may vary at each stage of dental inflammation.
The primary aim of this study was to investigate the effect of carious infection on the expression of PAR2 in odontoblasts. The secondary aim was to determine whether NF-κB may also be involved in inflammatory processes in the dental pulp.
We performed direct immunostaining for PAR2 and NF-κB on non-carious teeth and teeth with different stages of caries. The immunofluorescence approach was reproducible and enabled a comparative analysis of PAR2 and NF-κB expression in odontoblasts on the outermost layer of the dental pulp.
Empirical findings and theoretical implication
Immunostaining for PAR2 and NF-κB revealed the intact dental pulp maintained the primary odontoblast layer in the early stage of caries. However, once caries advanced into the inner half of the dentine, the primary odontoblast layer exhibited an uneven and irregular pattern, and the primary odontoblast layer was completely lost in the samples with exposed pulp.
Odontoblasts could be an initial target of bacterial pathogens. The early stage of carious infection may result in acute activation of the innate immune response, as our data showed PAR2 was upregulated in odontoblasts in early (mild infection) and advanced-stage caries (moderate infection) in which the dental pulp was not exposed. However, PAR2 expression sharply dropped once the pulp was exposed.
NF-κB is known to serve as a central inflammatory mediator in the dental pulp. As the first line of defence, we observed odontoblasts expressed higher levels of NF-κB than the sub-odontoblast region in all stages of carious infection. In early-stage caries, NF-κB was upregulated in the odontoblast layer but downregulated in the sub-odontoblast layer. In advanced- and late-stage caries where the odontoblast layer was severely damaged, NF-κB was downregulated in the odontoblast layer, and upregulated in the sub-odontoblast layer.
Moreover, many studies reported that cleavages of PAR2 resulted in a synergistic increase in NF-kB-mediated gene expression, especially regulated TLR2, TLR3, and TLR4 activation of NF-κB in various cells such as mast cells [16], renal cells [17].
PAR2 expression in response to various infection
We found the expression of PAR2 in odontoblasts was affected by the progression of caries. Similar correlations have been reported in other organs. For example, previous studies showed intestinal epithelial PAR2 was upregulated during intestinal inflammation and subsequently enhanced the process of protease sensing, cell proliferation and epithelial barrier function [18-21). Another study also reported higher PAR2 levels in the periodontal secretions of patients with chronic periodontitis [22]. Ritchie, et al. (2007) revealed that proinflammatory cytokines such as tumour necrosis factor-α (TNF-α) could enhance PAR2 expression [6]. Moreover, Alvarez, et al. (2017) also demonstrated that PAR1 and PAR2 are upregulated in odontoblast-like cells during the inflammatory response in vitro [6,7].
Notably, the levels of PAR2 in odontoblasts dramatically dropped after exposure to the dental pulp. This effect may be related to the role of PAR2 in periodontal disease, as Porphyromonas gingivalis-derived proteases can cleave PAR2, which leads to upregulation of pro-inflammatory cytokines. Similarly, an in vitro study by Giacaman, et al. (2009) found that P. gingivalis could activate and cleave PAR2 on oral keratinocytes in an Arg-(Rgp) gingipain-specific manner, which induced downstream signalling to upregulate pro-inflammatory cytokines to promote an innate immune response [21]. Therefore, we suggest that the expression of odontoblast PAR2 on the cell surface is downregulated as direct exposure of the dental pulp to Arg-(Rgp) gingipain leads to cleavage of Arg2.
NF-κB expression in response to various infection
To the best of our knowledge, this is the first prospective study suggesting that there were contrasting patterns of NF-κB expression between odontoblasts and the sub-odontoblast region during early-stage, advanced-stage and late-stage caries. The levels of NF-κB in the odontoblast region gradually decreased as caries progressed, whereas we observed a gradual increase in NF-κB expression in the sub-odontoblast region as caries progressed.
Firstly, the decrease in NF-κB expression in the odontoblast region may be because primary odontoblasts are lost and begin the regeneration of dentin. This is in agreement with the results of an in vitro study by Pei, et al. (2016), which showed that the expression of NF-κB in dental pulp stem cells was reduced in the presence of inflammatory cytokines, which subsequently enhanced odontoblastic differentiation and collagen matrix formation [23].
Secondly, the increase in the level of NF-κB in the sub-odontoblast region with the progression of caries could be related to the varied roles of NF-κB in inflammatory immune responses. NF-κB is involved in the regulation of cytokine production, and it is possible that upregulation of NF-κB by sub-odontoblast cells would regulate the production of cytokines by immune cells during the progression of caries. The upregulation of NF-κB might also be related to the odontogenic differentiation of pulpocytes. Pulpocytes, the cells in the sub-odontoblast region, including fibroblasts, immune cells and stem cells, are activated by NF-κB and form new odontoblast-like cells [24]; human dental pulp stem cells were shown to have a high potential for differentiation via the NF-κB pathway [25]. However, we could not determine the specific types of cells involved in odontogenic differentiation. A further cell-type-specific immunocytochemical analysis is required to identify the stem/precursor cells in the carious dental pulp.
However, it should be noted that the reductions in odontoblast PAR2 and NF-κB observed in this study may simply be due to the destruction of primary odontoblasts by inflammatory processes. However, if primary odontoblasts are not destroyed, the next step would be to further investigate how odontoblasts and the nearby cells respond to inflammatory processes. Further studies are required to directly explore the downstream effects of mild to severe inflammation on the odontoblast layer and pulp cells, to more precisely identify the interplay between odontoblasts and nearby cells and provide further insight into the inflammatory mechanisms involved in dental caries.
Conclusion
Our analysis of clinical samples extends the roles of PAR2 and NF-κB in the dentine-pulp complex and indicates the expression of these factors varies in the dentine-pulp complex and nearby cells during the progression of caries.
Limitations
Previously, many studies investigated the characteristics of dead odontoblasts. However, they were limited to only in vitro studies. In this study, we studied the destructions of primary odontoblasts by inflammatory processes, but we cannot directly detect cell death in the odontoblast layer. Which may lead to altering various protein expressions.
The facilities for this study were provided by the Oral neuroscience and molecular biology of dental pulp and bone cell research unit and the research centre of the Faculty of Dentistry, Prince of Songkla University.
Funding
This research was supported by the Oral neuroscience and molecular biology of dental pulp and bone cell research unit, Faculty of Dentistry, Prince of Songkla University.
Author contributions
Conceptualization: Aunwaya Kaewpitak. Formal analysis: Alisa Wichaidit, Namthip Patinotham, Kullanun Nukaeow. Investigation: Alisa Wichaidit, Namthip Patinotham, Kullanun Nukaeow. Data Curation: Alisa Wichaidit, Aunwaya Kaewpitak. Writing-Original draft preparation: Alisa Wichaidit. Writing-Reviewing and Editing: Aunwaya Kaewpitak. Visualization: Alisa Wichaidit, Namthip Patinotham, Kullanun Nukaeow. Funding acquisition: Aunwaya Kaewpitak.
- Shibukawa Y, Sato M, Kimura M, Sobhan U, Shimada M, Nishiyama A, Kawaguchi A, Soya M, Kuroda H, Katakura A, Ichinohe T, Tazaki M. Odontoblasts as sensory receptors: transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast-neuron signal transduction. Pflugers Arch. 2015 Apr;467(4):843-63. doi: 10.1007/s00424-014-1551-x. Epub 2014 Jun 18. PMID: 24939701.
- Okumura R, Shima K, Muramatsu T, Nakagawa K, Shimono M, Suzuki T, Magloire H, Shibukawa Y. The odontoblast as a sensory receptor cell? The expression of TRPV1 (VR-1) channels. Arch Histol Cytol. 2005 Dec;68(4):251-7. doi: 10.1679/aohc.68.251. PMID: 16477145.
- El Karim IA, Linden GJ, Curtis TM, About I, McGahon MK, Irwin CR, Lundy FT. Human odontoblasts express functional thermo-sensitive TRP channels: implications for dentin sensitivity. Pain. 2011 Oct;152(10):2211-2223. doi: 10.1016/j.pain.2010.10.016. Epub 2010 Dec 17. PMID: 21168271.
- Farges JC, Alliot-Licht B, Renard E, Ducret M, Gaudin A, Smith AJ, Cooper PR. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediators Inflamm. 2015;2015:230251.
- Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014 Nov;1843(11):2563-2582. doi: 10.1016/j.bbamcr.2014.05.014. Epub 2014 Jun 2. PMID: 24892271.
- Ritchie E, Saka M, Mackenzie C, Drummond R, Wheeler-Jones C, Kanke T, Plevin R. Cytokine upregulation of proteinase-activated-receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase beta in human endothelial cells. Br J Pharmacol. 2007 Apr;150(8):1044-54. doi: 10.1038/sj.bjp.0707150. Epub 2007 Mar 5. PMID: 17339845; PMCID: PMC2013917.
- Alvarez MMP, Moura GE, Machado MFM, Viana GM, de Souza Costa CA, Tjäderhane L, Nader HB, Tersariol ILS, Nascimento FD. PAR-1 and PAR-2 Expression Is Enhanced in Inflamed Odontoblast Cells. J Dent Res. 2017 Dec;96(13):1518-1525. doi: 10.1177/0022034517719415. Epub 2017 Jul 31. PMID: 28759300.
- Sun S, Wang GL, Huang Y, Diwu HL, Luo YC, Su J, Xiao YH. The effects of 2-hydroxyethyl methacrylate on matrix metalloproteinases 2 and 9 in human pulp cells and odontoblast-like cells in vitro. Int Endod J. 2018 Feb;51 Suppl 2:e157-e166. doi: 10.1111/iej.12812. Epub 2017 Aug 1. PMID: 28667765.
- Song Y, Na HS, Park E, Park MH, Lee HA, Chung J. Streptococcus mutans activates the AIM2, NLRP3 and NLRC4 inflammasomes in human THP-1 macrophages. Int J Oral Sci. 2018 Aug 6;10(3):23. doi: 10.1038/s41368-018-0024-z. PMID: 30078841; PMCID: PMC6080406.
- Struzycka I. The oral microbiome in dental caries. Pol J Microbiol. 2014;63(2):127-35. PMID: 25115106.
- Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, Smith AJ, Hunter GD, McLean JS, McGarry F, Ramage R, Jiang L, Kanke T, Kawagoe J. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003 Jan;111(1):35-41. doi: 10.1172/JCI16913. PMID: 12511586; PMCID: PMC151840.
- D'Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P. Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol. 2001 Jun;158(6):2031-41. doi: 10.1016/S0002-9440(10)64675-5. PMID: 11395381; PMCID: PMC1891970.
- Lan RS, Stewart GA, Goldie RG, Henry PJ. Altered expression and in vivo lung function of protease-activated receptors during influenza A virus infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004 Feb;286(2):L388-98. doi: 10.1152/ajplung.00286.2003. Epub 2003 Nov 21. PMID: 14633513.
- Nonaka S, Nakanishi H. Secreted gingipains from Porphyromonas gingivalis induce microglia migration through endosomal signaling by protease-activated receptor 2. Neurochem Int. 2020 Nov;140:104840. doi: 10.1016/j.neuint.2020.104840. Epub 2020 Aug 25. PMID: 32858090.
- Hahn CL, Liewehr FR. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. J Endod. 2007 Mar;33(3):213-9. doi: 10.1016/j.joen.2006.11.008. Epub 2007 Jan 4. PMID: 17320699.
- Ocak U, Eser Ocak P, Huang L, Xu W, Zuo Y, Li P, Gamdzyk M, Zuo G, Mo J, Zhang G, Zhang JH. Inhibition of mast cell tryptase attenuates neuroinflammation via PAR-2/p38/NFκB pathway following asphyxial cardiac arrest in rats. J Neuroinflammation. 2020 May 4;17(1):144. doi: 10.1186/s12974-020-01808-2. PMID: 32366312; PMCID: PMC7199326.
- Heuberger DM, Franchini AG, Madon J, Schuepbach RA. Thrombin cleaves and activates the protease-activated receptor 2 dependent on thrombomodulin co-receptor availability. Thromb Res. 2019 May;177:91-101. doi: 10.1016/j.thromres.2019.02.032. Epub 2019 Feb 28. PMID: 30861432.
- Mitsiadis TA, Rahiotis C. Parallels between tooth development and repair: conserved molecular mechanisms following carious and dental injury. J Dent Res. 2004 Dec;83(12):896-902. doi: 10.1177/154405910408301202. PMID: 15557394.
- Hyun E, Andrade-Gordon P, Steinhoff M, Vergnolle N. Protease-activated receptor-2 activation: a major actor in intestinal inflammation. Gut. 2008 Sep;57(9):1222-9. doi: 10.1136/gut.2008.150722. Epub 2008 May 6. PMID: 18460552.
- Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS, Coelho AM, Singh P, Grady EF, Perdue M, Bunnett NW. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005 Sep 9;280(36):31936-48. doi: 10.1074/jbc.M506338200. Epub 2005 Jul 18. PMID: 16027150.
- Giacaman RA, Asrani AC, Ross KF, Herzberg MC. Cleavage of protease-activated receptors on an immortalized oral epithelial cell line by Porphyromonas gingivalis gingipains. Microbiology (Reading). 2009 Oct;155(Pt 10):3238-3246. doi: 10.1099/mic.0.029132-0. Epub 2009 Jul 16. PMID: 19608609; PMCID: PMC2889418.
- Euzebio Alves VT, Bueno da Silva HA, de França BN, Eichler RS, Saraiva L, de Carvalho MH, Holzhausen M. Periodontal treatment downregulates protease-activated receptor 2 in human gingival crevicular fluid cells. Infect Immun. 2013 Dec;81(12):4399-407. doi: 10.1128/IAI.01107-13. Epub 2013 Sep 16. Erratum in: Infect Immun. 2014 Mar;82(3):1354. PMID: 24042113; PMCID: PMC3837994.
- Pei F, Wang HS, Chen Z, Zhang L. Autophagy regulates odontoblast differentiation by suppressing NF-κB activation in an inflammatory environment. Cell Death Dis. 2016 Mar 3;7(3):e2122. doi: 10.1038/cddis.2015.397. PMID: 26938294; PMCID: PMC4823923.
- Yumoto H, Hirao K, Hosokawa Y, Kuramoto H, Takegawa D, Nakanishi T, Matsuo T. The roles of odontoblasts in dental pulp innate immunity. Jpn Dent Sci Rev. 2018 Aug;54(3):105-117. doi: 10.1016/j.jdsr.2018.03.001. Epub 2018 Mar 27. PMID: 30128058; PMCID: PMC6094490.
- Gu S, Ran S, Qin F, Cao D, Wang J, Liu B, Liang J. Human Dental Pulp Stem Cells via the NF-κB Pathway. Cell Physiol Biochem. 2015;36(5):1725-34. doi: 10.1159/000430145. PMID: 26183640.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
