Open Journal of Cell and Protein Science
Response to first-line erlotinib in a false EGFR mutation-negative patient with non-small-cell lung cancer: Make no assumptions
Caixia Deng, Hu Luo and Xiangdong Zhou*
Cite this as
Deng C, Luo H, Zhou X (2018) Response to first-line erlotinib in a false EGFR mutation-negative patient with non-small-cell lung cancer: Make no assumptions. Open J Cell Protein Sci 1(1): 001-005. DOI: 10.17352/ojcps.000001So far, in the advanced non-small cell lung cancer (NSCLC) with clear epidermal growth factor receptor (EGFR) gene status, the treatment remmendations has reached an agreement: for patients with EGFR mutation-positive, epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) is the first choice, which can maximize the benefit from the treatment; while for the patients with wild-type EGFR gene, we should give priority to chemotherapy whether in the first-line or second-line therapy. However, about 70% of the patients were diagnosed at the late stage, so the pathological diagnosis and EGFR gene mutation detection depend on small specimens. Due to the limitations of small specimens, it may lead false EGFR mutation-negative, which results in these patients losing the opportunity to receive EGFR-TKI. Therefore, more simple and accessible predictors, in order to discover these potential false negatives of EGFR mutation, are urgently warranted.
Here, we report a case showing a positive response to erlotinib treatment in the first-line setting. The patient, an middle-aged male smoker with stage IV NSCLC, had a tumor that was EGFR mutation-negative (wild-type EGFR). Based on this clinical case, we will discuss how to distinguish the false negatives of EGFR mutation and explore the predictors of EGFR mutation-positive , response and survival to EGFR-TKIs in first-line treatment.
Case Report
A 42-year-old Asian man presented with progressive cough, expectoration and chest pain admitted to our department on January 28, 2013. His past medical history was notable for a 40 pack–year history of smoking.
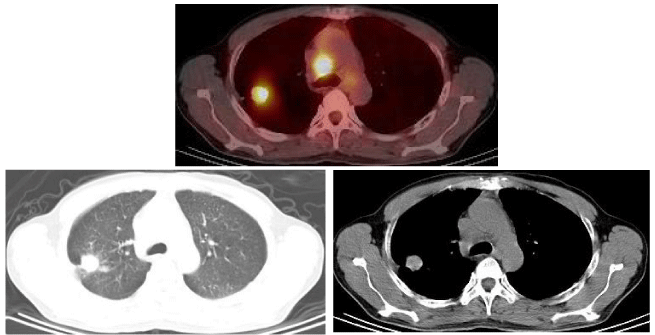
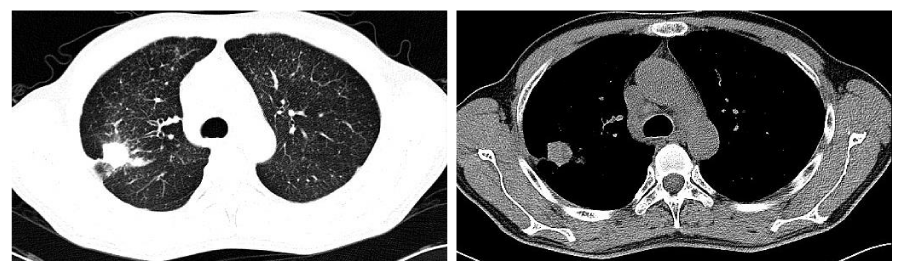
The carcinoembryonic antigen (CEA) serum level tested on January 31, 2013, was 276.37ug/l (the normal value of limitation is 5.0ug/l). Chest Computed tomography (CT) on February 5, 2013, showed a large number of miliary tubercles scattered in two lungs, which like metastatic lesions; one well-defined lesion lied in the posterior segment of the right upper lobe, whose edge is not smooth and diameter is about 2cm, among which pleural indentation sign and spicule sign should be found; a few of enlarged lymph nodes located in the mediastinum, among which the maximum diameter is 2.3cm. A CT-guided percutaneous needle biopsy was performed on February 18, 2013, and the pathological findings showed adenocarcinoma. Imaging by PET–CT on February 28, 2013, showed intense fluorodeoxyglucose activity in the right upper-lobe nodule, SUV max was7.3, diameter was about 2.92cmx2.72cm (Figure 1). Extensive fluorodeoxyglucose activity was also evident within the following lymph nodes: double hilars, mediastinal, right neck, right subclavian, right upper trachea, retrocaval, and carinal. Of which the SUV max was 12.17 and the maximum diameter is 3.36cmx2.92cm .At the same time, it showed miliary nodules in double lung seem to be metastatic lesions with great possibility.
At the time of his initial medical oncology consultation, the patient declined chemotherapy and thus was tested for the EGFR mutation in pathological section using the ARMS method. However, he was found to be EGFR mutation-negative (wildtype EGFR). Chemotherapy was offered again, the patient refused once more. Meanwhile,the patient and his families strongly required to try EGFR-TKI treatment. The doctor’s persuasion did not work anymore.
First-line therapy with erlotinib was initiated and the patient received his first dose of erlotinib (150 mg daily) on March 31, 2013. Before the treatment, we kept the data about chest CT images and the CEA serum level. The former showed that, compared with the initial CT images acquired on February 5, 2013, the lesion in the right upper lobe had increased in size,which now measured 2.4cmx2.2cm. The latter showed the result was>160.0ng/ml (the normal value limitation is 10.0ng/ml).On day 9 of erlotinib therapy, he developed a grade 2 rash (extensive but not severe, with modest itch of skin and xerosis cutis) on his head, face and neck. With close follow-up instead of medication, the rash relieved to grade 1 when seen in follow-up on July 21, 2013. During subsequent visits, the rash on the patient’s face and neck further relieved.
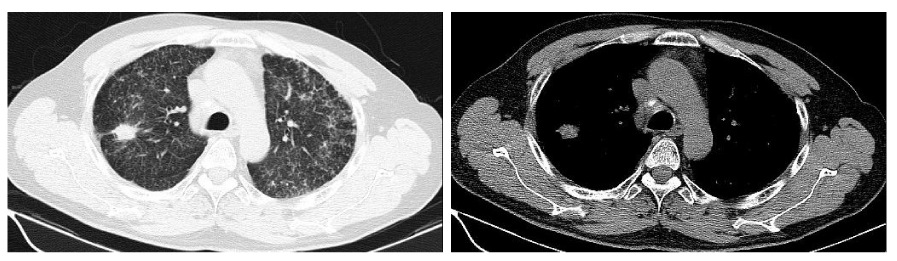
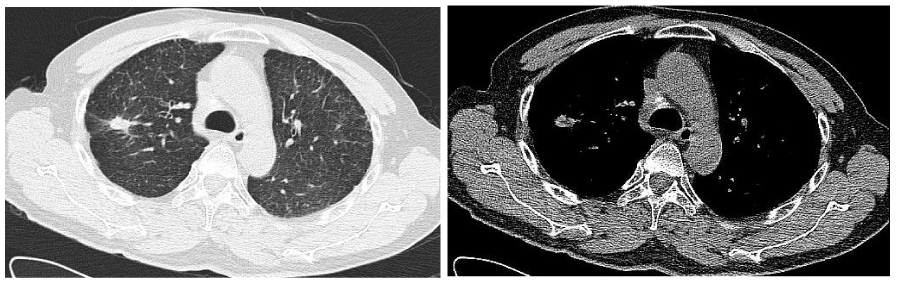
Follow-up the chest CT images of the patient on May 8, 2014, (Figure 2), showed that the lesion in the right upper lobe and the lymph nodes in double hilars and mediastinum had decreased in size which now measured 1.3cm and 1.6cm respectively; the miliary nodules in double lung changed little. Subsequent chest CTs images were tested every two months, which showed that all lesions in lungs are stable compared with the last CT image, while the lesion in the right upper lobe had achieved a partial response (PR) according to the Response Evaluation Criteria in Solid Tumors (RECIST), which measured 1.5cm×1.0 cm, compared with the initial CT images acquired March 24, 2013 (Figure 3).
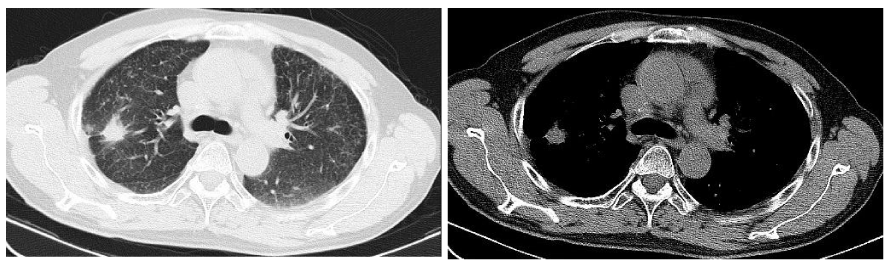
All lesions in lungs are stable until September 4, 2015, on which the lesion in the right upper lobe had increased in size which now measured 1.9cm×1.7 cm (Figure 4). At the same time, the IL-6 serum level was 7.72ng/l (the normal value is 7.0ng/l). Combination of imaging and IL-6, we speculated that the resistance of EGFR-TKI must be appearance. Therefore, we add metformin (1000mg, twice a day)to reverse the drug resistance. Follow-up CT imaging of the patient’s chest on October 30, 2015, showed that the lesion in the right upper lobe had decreased in size which now measured 1.3cm; the other lesions in double lung changed little. Subsequent CTs imaging of the chest also tested every two months, showed that all lesions in lungs are stable. The last CT imaging of the patient’s chest on February 26, 2016 showed the lesion in the right upper lobe measured 1.3cm.
The CEA levels was tested within one month after treatment. The result was >160ng/ml, 146.88ng/ml, respectively on seventh day and thirtieth day after treatment. The Follow-up result is as table1 figure 5.
At the time of March 2016, the patient died in a car accident, even though he was still getting benefit from erlotinib.
Response to first-line erlotinib in an egfr mutation-negative patient
Discussion
Paez et al. (2004) firstly pointed out that some gene mutations may bring benefit to the efficacy of EGFR-TKI [1]. The later studies confirmed the hypothesis and found that the mutations at the exons 18 to 21(especially deletion in exon 19 and missense mutation in exon 21) of the EGFR tyrosine kinase coding region was the strongest predictor of response to TKIs [2-4]. Several papers showed that EGFR mutation test should be a prerequisite for first-line treatment of advanced NSCLC with gefitinib,while a reduction in survival was observed in patients with wild-type EGFR who were given gefitinib.
However, about 70% of lung cancer patients had local invasion or distant metastasis at the time of diagnosis, and surgery was not necessary. So the pathological diagnosis and EGFR gene mutation detection mainly depend on small specimens obtained by percutaneous lung biopsy, transbronchial biopsy, pleural biopsy and so on. But for small samples, obtaining how much proportion of cancerous cells depends on lesion’s characteristics, technique of material operator, experience of diagnosis and other factors. Travis et al. [5] believed that the representativeness of the small sample was poor, which can not reflect the overall situation of lung cancer. It even lead to erroneous diagnosis sometimes. They indicated that the main reason underlying this error is the existence of the inherent histologic heterogeneity in a subset of NSCLC. This feature makes lung cancer tissue from small specimens be classified by mistake due to different local structure, squamous cell carcinoma accounted for 10%, adenocarcinoma 14%, large cell carcinoma 50% [6]. Recently, a clinical study of 239 cases of NSCLC patients, compared the clinical small specimens with surgical specimens. The results showed that substantial proportion of the small specimens can not go on EGFR testing or lead the error testing result due to the low proportion of tumor cells [7]. In addition, different detection methods may have great influence on the positive rate of EGFR mutations, such as the positive rate is lowest in gene sequencing, which can only detect the specimen with mutation content greater than 30%, even ARMS (amplification refractory mutation system) which had been widely recognized also need the sample containing 1% EGFR mutations among cancer cells to detect [8-10]. All of the above factors may lead false negative results in the detection of EGFR gene mutation, which may cause those patients with advanced NSCLC losing the opportunity to receive EGFR-TKI treatment.
Therefore, when the EGFR gene mutation comes out negative, patients surely do not benefit from EGFR-TKI? In our case report, the NSCLC patient with EGFR negative was treated with erlotinib as the first line therapy, benefiting 29 months PFS and 36 months OS, which must be longer if there was no accident happend to him. And the side effect is modest. The result about efficacy of EGFR-TKI in our case is much better than Chemotherapy, and the side effect is more modest than Chemotherapy. In a clinical study of erlotinib, the patient with no EGFR gene mutations or high expression also had good efficacy [11]. Therefore, it is an urgent problem to find a simple, convenient indicator to predict the EGFR mutation and efficacy of EGFR-TKI.
In our case, the initial CEA serum level before treatment was >160ng/ml. The vaule was >160 ng/ml, 146.88 ng/ml, repectively on seventh day and thirtieth day after treatment. CEA directly produced by tumor cells is a clinical common tumor marker. It is closely related to tumor recurrence and metastasis in lung cancer, which also plays certain value of early diagnosis, curative effect evaluation and disease development. Are there any relationship among CEA level, EGFR mutation and efficacy of EGFR-TKI?
At present, some studies suggested that serum CEA level was closely related to EGFR mutation of lung adenocarcinoma [12-14]. The trial conducted by Pan et al. showed among lung adenocarcinoma patients the serum CEA level in EGFR gene mutation group were significantly higher than the wild. Conversely, the higher serum CEA level, the more EGFR gene mutation rate [15]. The same conclusion occured in recurrent lung adenocarcinoma patients after surgery [16]. It happens that there is a similar trial completed by Yang et al, which provided a positive correlation between serum CEA expression level and EGFR mutation status in NSCLC patients, namely the EGFR mutation-positive rate increases as the serum CEA expression level rises within a certain range (≥20 ng/mL, especially 20~49 ng/mL) [17]. Shoji et al [13]. Found the EGFR mutation rate is up to 87.5% in recurrent lung adenocarcinoma when the serum CEA≥20ng /ml.
At the same time, there was strongly relationship between serum CEA response with efficacy of EGFR-TKI.It has been found that in advanced NSCLC, 1 months after EGFR-TKI treatment, patients in descending type group had better efficacy than the other two groups [18]. Chiu et al. studied CEA, Ca 125, and Ca 19.9 in 89 Asiatic NSCLC patients unknown for EGFR status, showing that objective tumor response to gefitinib was closely correlated with individual tumor marker responses at 4 weeks [19]. In particular, CEA response after 4 weeks, defined as decline of 50% or more in CEA level, was also predictive for PFS and OS. In summary, elevated serum CEA is a positive predictor for EGFR mutation and the efficacy of EGFR-TKI; the CEA response can be early predictor of EGFR-TKI outcome in EGFR wild-type.
How did CEA and EGFR generate contact? The downstream molecules of EGFR, such as Akt and STAT, played a key role in the anti-apoptotic pathway [20, 21]. The CEA protein had an anti-apoptotic effect, and the question is whether it is also a downstream product of EGFR. Okamoto T [15], speculated the activation of the EGFR transduction pathway, due to gene mutations, might promote the expression of the CEA protein, which is released into the blood. So, if CEA is a signal marker of EGFR gene mutation,its elevated serum concentration is a feature in judging the efficacy of EGFR-TKI, which needs further basic research and clinical trial experience. Although this mechanism needs further research, but the correlation between the serum concentration of CEA and EGFR gene mutation has been basically established, which provide a simple, noninvasive method to forecast EGFR mutation and efficacy of EGFR-TKI.
In our case, the side effect specific to this class of agents is the development of a rash primarily on the head, face, neck, which occurs after 9 days of treatment. It is similar to the studies, which showed the rash occurs during 1-3 weeks after treatment, reaches maximum intensity after 3-5 weeks, and its incidence rate is 50%-75% [9,22,23]. Multiple trials across different tumour types treated by EGFR inhibitors have confirmed a relationship between the incidence and severity of rash with both response and survival. In Study BR.21, it confirmed these relationships between presence of rash with overall survival increased with rash severity grade: grade 1 versus no rash (HR= 0.41, P < 0.001) and grade ≧2 versus no rash (H= 0.29, P <0.001) [24]. Similar results were observed for PFS and Disease control. In Study PA.3, grade ≧rash (but not grade 1) had a strongly correlation with overall survival improvement: grade≧2 versus no rash (HR=0.47, P <0.001). In PFS and disease control, it had the similar results [24]. Subgroup analyses from the TRUST study showed PFS and OS were both significantly longer in patients who developed erlotinib-related rash compared to those with no rash [25]. In summary, it appears that patients who develop worse rashes from this class of drugs show more benefit.
In our case, the SUVmax of the lesions in the posterior segment of the right upper lobe and in the septum is 7.3, 12.17 respectively, which is much higher than the normal value .As we known, 18F-FDG PET as a noninvasive diagnostic modality has been widely used for the staging of initial tumors, recurrent tumors and evaluation of the treatment response in patients with NSCLC [26]. FDG uptake, as assessed using the SUVmax of a tumor, is proportional to the glucose metabolic rate of viable tumor cells; therefore, this might help predict the biological aggressiveness of a tumor. Some studies have reported that the degree of tumor FDG uptake is a significant prognostic factor in NSCLC [27-29]. Thus, we presumed that FDG uptake on PET might be correlated with EGFR mutation.It has been confirmed in the study conducted by Kai-Hsiung Ko et al. showed patients of pulmonary adenocarcinoma were more likely to have EGFR mutations when with SUVmax ≥6 (p=0.002) and CEA level ≥5 (p=0.013). Similar results were observed for The CT characteristics of larger tumors (≥3 cm) (p=0.023) and tumors with a nonspiculated margin (p=0.026) [30]. Similarly, Huang et al. reported that a higher FDG uptake (SUVmax >9.5) was more likely to be associated with EGFR mutation in an Asian population [31]. Conversely, Mak et al. showed that a high FDG uptake (SUVmax >5) was correlated with wildtype EGFR status, and Na et al. concluded that patients with a low SUVmax were more likely to have EGFR mutations [32,33]. The possible reasons for the different results observed between these studies are as follows: first, the latter two studies reported a lower mutation rate (21 % vs. 24 %); second, in the study performed by Na et al., the histological type of the study cases was not only adenocarcinoma, but also included squamous cell carcinoma (44%), which has been shown to have a different FDG uptakeand distinct tumor biology [34]. Although the SUVmax cutoff between the former studies is different, but as we known, SUVmax is a semiquantitative index that varies between different centers or PET scanners, depending on the fasting duration, level of plasma glucose, time to imaging, reconstruction algorithms and region-ofinterest parameters.
Conclusion
The identification of the mutation status of EGFR is important for the optimization of treatment in patients with NSCLC, but because of the tissue heterogeneity in NSCLC, EGFR gene testing via small sample may lead false negative results sometimes. Combined this case with previous literatures, we believe that those patients with EGFR-negative, like no smoking, female, adenocarcinoma, Asian, high serum CEA level, especialy ≥20 ng/Ml,high FDG uptake, tumors with a nonspiculated margin,should be taken into account of the false negative. Therefore, if those patients who can not accept or endure chemotherapy,they can try EGFR-TKI treatment with closely follow-up the chest CT images, rash and CEA response. But previous studies only focused on the relathionship among one parameter alone with EGFR mutation and efficacy of EGFR-TKI.As we known, one parameter is not sufficiently powerful and confident for discrimination of false negative EGFR and predicting the efficacy of EGFR-TKI. Therefore A larger, more comprehensive, multi-institutional study is needed to combine these predictors to evaluate the sensitivity and specificity of predictors for predicting EGFR mutation.
Make no assumptions. Combination of the clinical predictors of outcome previously reported in the literature should replace one parameter alone even EGFR mutation status to distinguish the person who may benefit from EGFR-TKI therapy.
- Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, et al. (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 304:1497-500. Link: https://goo.gl/LpzUYP
- Mok TS, Wu YL, FACS, Thongprasert S, Yang CH, et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 361: 947-57. Link: https://goo.gl/LiQTrp
- Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, et al (2005) Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 353: 133-44. Link: https://goo.gl/KtdfRK
- Sequist LV, Bell DW, Lynch TJ, Haber DA (2007) Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 25: 587-95. Link: https://goo.gl/TgnXMi
- Travis WD, Rekhtman N, Riley GJ, Geisinger KR, Asamura H, et al. (2010) Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology: a paradigm shift. J Thorac Oncol. 5: 411-4. Link: https://goo.gl/uriXMz
- Cataluna JJ, Perpiñá M, Greses JV, Calvo V,Padilla JD, et al. (1996) Cell type accuracy of bronchial biopsy specimens in primary lung cancer. Chest.109: 1199-203. Link: https://goo.gl/V1EEC2
- Smouse JH, Cibas ES, Jänne PA, Joshi VA, Zou KH, et al. (2009) EGFR mutations are detected comparably in cytologic and surgical pathology specimens of nonsmall cell lung cancer. Cancer. 117: 67-72. Link: https://goo.gl/ewja9C
- Chen S 2010 Detection of epidermal growth factor receptor mutations in non-small cell lung cancer tumor specimens from various ways by denaturing high-performance liquid chromatography. Zhongguo Fei Ai Za Zhi. 13: 850-5. Link: https://goo.gl/f4LJp3
- Liu Y, Liu B, Li XY, Li JJ, Qin HF, et al. (2011) A comparison of ARMS and direct sequencing for EGFR mutation analysis and tyrosine kinase inhibitors treatment prediction in body fluid samples of non-small-cell lung cancer patients. J Exp Clin Cancer Res. 30: 111. Link: https://goo.gl/LKYL5N
- Liu J, Zhao R, Zhang J. Zhang J (2015) ARMS for EGFR mutation analysis of cytologic and corresponding lung adenocarcinoma histologic specimens. J Cancer Res Clin Oncol. 141: 221-7. Link: https://goo.gl/bo17h2
- Shepherd FA, Pereira JR, Ciuleanu T, Tan EH, Hirsh V, et al. (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 353: 123-32. Link: https://goo.gl/xk3iR4
- Jung M, Kim SH, Lee YJ, Hong S, Kang YA, et al. (2011) Prognostic and predictive value of CEA and CYFRA 21-1 levels in advanced non-small cell lung cancer patients treated with gefitinib or erlotinib. Exp Ther Med. 2: 685-693. Link: https://goo.gl/HTszAn
- Shoji F, Yoshino I, Yano T, Kometani T, Ohba T, et al. (2007) Serum carcinoembryonic antigen level is associated with epidermal growth factor receptor mutations in recurrent lung adenocarcinomas. Cancer. 110: 2793-8. Link: https://goo.gl/mL5pMi
- Kappers I, Vollebergh MA, Tinteren HV, Korse CM, Nieuwenhuis LL, et al. (2010) Soluble epidermal growth factor receptor (sEGFR) and carcinoembryonic antigen (CEA) concentration in patients with non-small cell lung cancer: correlation with survival after erlotinib and gefitinib treatment. Ecancermedicalscience. 4: 178. Link: https://goo.gl/8U3cnJ
- Pan JB, Hou YH, Zhang GJ (2013) Correlation between EGFR mutations and serum tumor markers in lung adenocarcinoma patients. Asian Pac J Cancer Prev. 14: 695-700. Link: https://goo.gl/6RbhYH
- Okamoto T, Nakamura T, Lkeda J, Maruyama R, Shoji F, et al. (2005) Serum carcinoembryonic antigen as a predictive marker for sensitivity to gefitinib in advanced non-small cell lung cancer. Eur J Cancer. 41: 1286-90. Link: https://goo.gl/9r9A2u
- Yang ZM (2014) Analysis of CEA expression and EGFR mutation status in non-small cell lung cancers. Asian Pac J Cancer Prev. 15: 3451-5.
- Jin B (2014) Value of carcinoembryonic antigen levels in predicting the efficacy of EGFR-TKI in advanced non-small cell lung cancer harboring EGFR mutations. Zhonghua Yi Xue Za Zhi. 94: 2327-31. Link: https://goo.gl/BCyYDv
- Chiu CH, Shihbc YN, Tsaiac CM, Lioua JL, Chena YM, et al. (2007) Serum tumor markers as predictors for survival in advanced non-small cell lung cancer patients treated with gefitinib. Lung Cancer. 57: 213-21. Link: https://goo.gl/pLnnoN v
- Sordella R, Bell DW, Haber DA, Settleman J (2004) Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 305: 1163-7. Link: https://goo.gl/YQH8Zf
- Cappuzzo F, Magrini E, Ceresoli GL, Bartolini S, Rossi E, et al. (2004) Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 96: 1133-41. Link: https://goo.gl/x7i5V5
- Lacouture ME, Melosky BL (2007) Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 12: 1-5. Link: https://goo.gl/yXobdR
- Perez-Soler R, Saltz L (2005) Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 23: 5235-46. Link: https://goo.gl/kju7f5
- Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, et al. (2007) Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 13: 3913-21. Link: https://goo.gl/4Nga7N
- Heigener DF, Wub YL, Zandwijkc NV, Malid P, Horwood K, et al. (2011) Second-line erlotinib in patients with advanced non-small-cell lung cancer: subgroup analyses from the TRUST study. Lung Cancer. 74: 274-9. Link: https://goo.gl/CSuPvb
- Higashi K, Ueda Y, Arisaka Y, Sakuma T, Namb Y, et al. (2002) 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 43: 39-45. Link: https://goo.gl/1nzL1H
- Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, et al. (2005) (18F) fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 23: 1136-43. Link: https://goo.gl/Mtdbxg
- Hanin FX, Lonneux M, Cornet J, Noirhomme P, Coulon C, et al. (2008) Prognostic value of FDG uptake in early stage non-small cell lung cancer. Eur J Cardiothorac Surg. 33: 819-23. Link: https://goo.gl/ZRtxpC
- Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon JE, et al. (2008) Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol. 26: 1459-64. Link: https://goo.gl/2RBJV4
- Ko KH, Huang TW, Gao HW, Shen DHY, Chang WC, et al. (2014) Value of 18F-FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol Imaging. 41: 1889-97. Link: https://goo.gl/XbQUj9
- Huang CT, Yen RF, Cheng MF, Hsu YC, Wei PF, et al. (2010) Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol. 27: 9-15. Link: https://goo.gl/5KVBkV
- Na II, Byunb BH, Kimb KM, Cheonb GJ, Choe DH, et al. (2010) 18F-FDG uptake and EGFR mutations in patients with non-small cell lung cancer: a single-institution retrospective analysis. Lung Cancer. 67: 76-80. Link: https://goo.gl/q1FBrB
- Mak RH, Digumarthy SR, Muzikansky A, Engelman JA, Shepard JAO, et al. (2011) Role of 18F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist. 16: 319-26. Link: https://goo.gl/7oTzi1
- Vesselle H, Alexander Salskov BA, Turcotte E, Linda Wiens BS, Schmidt R, et al. (2008) Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 3: 971-8. Link: https://goo.gl/7o3CF6

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
