Open Journal of Bioinformatics and Biostatistics
In Silico Identification and Characterization of Potential Red Seaweed Allergens
Department of Food Science and Nutrition, College of Home Economics, University of the Philippines, Diliman, Quezon City Philippines
Author and article information
Cite this as
Gaspan DS, Tolentino MPS (2023) In Silico Identification and Characterization of Potential Red Seaweed Allergens. Open J Bioinform Biostat. 2023; 7(1): 001-017. Available from: 10.17352/ojbb.000013
Copyright License
© 2023 Gaspan DS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Red seaweeds such as Kappaphycus alvarezii and Porphyra yezoensis have many applications, especially in the food industry, which suggests the need for knowing their potential allergenicity. In silico approaches can be used to determine if a protein is an existing allergen or has the ability to cross-react with one. In this study, 318 sequences for Kappaphycus alvarezii and 641 protein sequences for Porphyra yezoensis were screened for potential allergens using AlgPred 2.0 and AllergenOnline, followed by the FAO allergenicity test using Allermatch. Data from this were used to predict the B-cell epitopes using the IEDP prediction tool and T-cell epitopes using MHC2Pred and were modeled using SWISS-MODEL and PyMOL to highlight specific epitopes. These models were assessed for quality using Global Quality Model Estimate (GQME) scores, ERRAT scores, and VERIFY 3D. Results showed fourteen (14) potential red seaweed allergens, four (4) of which were found in Kappaphycus alvarezii and ten (10) in Porphyra yezoensis. Several proteins of red seaweeds shared structural similarities with species normally associated with food allergies, such as common hazel, Atlantic salmon, and shark catfish, as well as other types of allergens such as those in house-dust mites, that could potentially induce cross-reactivity. Additionally anticipated were specific B-cell and T-cell epitopes and their specific peptide sequences that were incorporated in the 3D models, which were created for further comparison with other molecular structures of recognized allergens. Almost all of the 3D models had a GQME score of above 0.7 and had a high ERRAT score for overall quality but some failed to pass the VERIFY 3D test. This study could serve as a preliminary yet robust approach to identifying allergenic proteins in red seaweed and narrowing down potential existing cross-reactive allergens from various species that could aid in future in vitro and in vivo allergenicity studies.
IgE: Immunoglobulin E; Th2: T helper type 2; BLASTP: Basic Local Alignment Search Tool for Proteins); ARP: Allergen Representative Peptide; MEME: Multiple EM for Motif Elicitation; MAST: Motif Alignment And Search Tool; IEDB: Immune Epitope Database: TCR: T-Cell Receptor; APC: Antigen-Presenting Cells; MHC: Major Histocompatibility Complex; UniProt: Universal Protein Resource; HLA: Human Leukocyte Antigens; GMQE: Global Model Quality Estimate; SVM: Support Vector Machine; HSP70: Heat Shock Protein 70; TIM: Triosephosphate Isomerase; NRL: Natural Rubber Latex; HDM: House Dust Mites; HWP: Hydrolyzed Wheat Protein; GWAS: Genome-Wide Association Study
Introduction
Seaweeds are marine macroalgae that can be found in oceans, lakes, and rivers [1]. They are non-toxic and can be consumed directly or processed as food due to their high nutritional value, including proteins, essential amino acids, vitamins, unsaturated fatty acids, and low calories [2]. The demand for seaweed as functional food has been growing globally, with increasing usage in Western countries [3]. Seaweeds are processed into various products such as burgers, laver sheets, chips, jams, and jellies. They are particularly valuable in the production of hydrocolloids, with carrageenan being the most popular seaweed extract used as a thickener and gelling agent in food [4].
Red seaweeds, particularly Kappaphycus alvarezii and Eucheuma denticulatum, are widely used for carrageenan production [5]. In the Philippines, red seaweeds are abundant and cultivated in regions like Tawi-Tawi, Palawan, and Bohol, traditionally consumed as part of the Filipino diet [6-8]. Kappaphycus alvarezii, known locally as ‘guso’ or ‘tambalang,’ constitutes 80% of the country’s seaweed exports and is a major raw material for κ-carrageenan worldwide [9]. Seaweed farming has become a vital livelihood in coastal communities [10,11]. Red seaweeds have diverse applications in bio-packaging, biofuels, bio-medical, bio-remediation, biostimulants, cosmetics, nutraceuticals, and pharmaceuticals [12-19]. They also show potential in medicine, including anticancer, anti-diabetic, anti-inflammatory, and cardioprotective effects [20]. As the demand for seaweed in the food industry increases, particularly in the Philippines and Indonesia, the development of standards for seaweed-derived products is crucial [11]. Compliance with EU law is necessary for exported seaweed as an alternative protein source, considering the potential sensitization or allergenic response in the population [21]. However, there is currently a lack of available data on processing standards and limited assessment of the allergenic potential of seaweed products in the Philippines.
Allergies are disorders where the immune system causes hypersensitivity reactions as a response to an allergen by boosting the synthesis of immunoglobulin E (IgE), which happens especially among genetically susceptible individuals [22,23]. A protein’s allergenicity is determined by its potential to elicit both humoral and cellular T helper type 2 (Th2) immune responses, resulting in the production of allergen-specific IgE and Th2 cytokines [23]. Given that seaweeds are widely used as food and a promising alternative protein source, the allergenicity of seaweed should then be investigated further. There is currently minimal research on the allergenicity of proteins from seaweeds [24-25]. Further studies are then needed to explore the potential allergenic properties of seaweed proteins that consider cost-effectiveness, speed, and the ability to analyze a large number of proteins or substances in a relatively short amount of time, and one way to do this is through the use of in silico methods.
in silico approaches determine protein allergenicity and cross-reactivity [26]. Data generated from in silico methods can be complemented by in vitro and in vivo testing, such as serum screening, as recommended by the Codex Alimentarius Commission [26,27]. Matching amino acid sequences of a novel protein to known allergenic proteins in databases is a common in silico approach for assessing protein allergenicity [28]. While proteins consist of extensive amino acid chains, only a few regions serve as antibody binding sites that define allergenicity. Computational protocols and algorithms are useful for evaluating IgE cross-reactivity, focusing on specific methods rather than overall allergenicity [29]. This study used an in silico approach to assess the potential allergenicity risks of red seaweed products. Specifically, the study identified putative allergens from Kappaphycus alvarezii and Porphyra yezoensis using bioinformatic tools, characterized the protein structures of the identified allergens, and identified IgE epitopes homologous to known allergens. These findings provide valuable insights for future in vitro and in vivo research on red seaweed allergens.
Review of related literature
Red seaweed
Red seaweed, a phylogenetically old organism, belongs to the Primoplantae clade and exhibits diverse morphological and reproductive features [30]. They are photosynthetic, containing chlorophyll a and d, carotenoids, and phycobiliproteins (phycoerythrin, phycocyanin, and allophycocyanin) [31]. Red seaweeds have a unique polysaccharide composition and rely on floridean starch from the cytoplasm as a reserve, lacking starch in chloroplasts [32]. Among red seaweeds, Kappaphycus alvarezii is a nutrient-dense food with high-quality protein and carrageenan content, making it a potential alternative protein source. It finds applications in the food, pharmaceutical, and cosmetic industries [33]. Environmental factors such as season, temperature, and light affect the protein content of Kappaphycus alvarezii, and salinity plays a crucial role in its cultivation success [30-37]. Red seaweeds, in general, are low in calories but rich in protein, dietary fiber, vitamins, and minerals [38]. Kappaphycus alvarezii is gaining popularity as a functional food due to its nutritional potential, bioactive peptide content, umami flavor, and suitability for alternative protein products [39-41]. Porphyra yezoensis, another red seaweed, is known for its protein and carbohydrate content, vitamins (riboflavin and niacin), and mineral richness. It is widely consumed in Asia, particularly in Japan and Korea [42-46].
Uses of seaweed in the food industry
Seaweeds provide phycocolloids such as agar, carrageenan, and alginate, which are widely used in the food industry as gelling, stabilizing, and thickening agents. Agar, derived from red seaweeds like Gelidiaceae, Gelidiellaceae, and Gracilariaceae, forms gels in a specific temperature range and is utilized as a stabilizer and thickening agent in various food products [47-49]. Carrageenan, primarily sourced from Kappaphycus alvarezii and Eucheuma denticulata, acts as a gelling, thickening, and stabilizing agent in foods like ice cream, cheese, syrups, and meat products [50]. Alginate, derived from brown seaweeds like Ecklonia, Macrocystis, Undaria, Laminaria, Durvillea, Turbinaria, and Sargassum, increases viscosity, generates non-melting gels, and serves as an emulsifier in the food industry [51]. Nori, a type of dried or baked red seaweed, is widely used in the food processing industry. Kappaphycus alvarezii and Porphyra sp. are commonly used for making nori, with Porphyra sp. being the primary source [52,53]. Commercial nori has a moisture content of 16.09%, ash content of 5.12%, fat content of 0.10%, protein content of 6.15%, and carbohydrate content of 72.54% [54].
Seaweed allergy
The consumption of seafood products has increased with international trade, but it can trigger serious adverse reactions in susceptible individuals [55]. Seafood allergy is common in adults and young children, and allergies to seaweed-derived products have also been reported. Non-edible green algae allergens can worsen respiratory symptoms in children with allergies, and occupational pulmonary hypersensitivity has been observed in workers exposed to brown seaweed dust [57-59]. Seaweed allergies manifest as skin rash, itching, blister formation, swollen eyes, nasal and throat irritation, skin sores, oral and intestinal discomfort, headache, fatigue, and fever [59]. The prevalence of seaweed allergies is unclear, but the risk may be overlooked.
Allergen contamination can occur during the cultivation of seaweed, especially when combined with other marine species like shrimp and crustaceans [60]. Nori products can contain significant amounts of amphipods, which may vary between batches [61]. Clinical reports have shown allergic reactions to seaweed, confirmed through positive skin prick tests [62,63]. Seaweed allergies are often IgE-mediated, potentially caused by past seaweed ingestion. Despite some knowledge of amino acid composition, the protein structure and biological characteristics of seaweed are understudied. Allergens in seaweed include phycobiliproteins and phycolectins [30]. Cross-reactivity has been observed among seaweed species, and patients sensitive to seaweed should avoid products containing seaweed and its compounds unless tolerance has been established [62,63].
In-silico tools for allergen determination
in silico methods can determine allergenicity and cross-reactivity potential of novel proteins, but not distinguish between sensitization and elicitation phases of allergy [64]. These methods compare amino acid sequences to known allergens in databases to assess protein allergenicity [26]. A sequence identity of at least 35% over 80 amino acids with recognized allergens designates a protein as a potential allergen [28]. Linear sequence comparison tools like FASTA and BLASTP are commonly used for similarity analysis [65-67]. Homologous proteins share structural and epitopic similarities, making them more likely to share allergenic properties [67]. AllergenOnline provides sequence identity matches and a searchable database for allergen comparison [68-70]. AlgPred 2.0 predicts allergenic proteins and antigenic sites within proteins using various methods [71]. Allermatch predicts allergenicity based on similarity to known allergenic proteins using a sliding window technique [73]. B-cell epitopes can be identified using synthetic peptides, recombinant proteins, or immunoblot techniques [74]. T-cell epitope prediction focuses on identifying immunogenic peptides that stimulate CD4 and CD8 T-cells, with MHC-peptide binding being a key determinant [76-79].
Methodology
Protein sequence retrieval
A total number of 318 protein sequences of Kappaphycus alvarezii and 641 protein sequences of Porphyra yezoensis were available from the Universal Protein Resource (UniProt) database for these species and were retrieved for analysis.
Prediction of allergenic protein by using 5 prediction approaches
The retrieved protein sequences of Kappaphycus alvarezii and Porphyra yezoensis were processed with the AlgPred 2.0 server and AllergenOnline. These were used to scan for allergenic proteins on the basis of IgE mapping, Support Vector Machine prediction on amino acid composition, hybrid approach, Multiple Em for Motif Elicitation, and Basic Local Alignment Search Tool on allergen representative peptides. Consequently, positive results obtained from any one of these five prediction approaches were designated as a potentially allergenic protein for further analysis.
Food and agriculture organization of the United Nations/World Health Organization allergenicity test
The Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) allergenicity test was performed in Allermatch™ by scanning the query protein sequences obtained from the AlgPred 2.0 and AllergenOnline against all the available protein sequences in the UniProt and WHO and International Union of Immunological Societies Allergen Nomenclature Subcommittee databases. A full FASTA alignment was done for the query sequences with both databases, which helps in the positioning of regions of potential allergenicity in the whole primary structure of the protein. The FASTA output was parsed, and information from the allergen database was added and presented.
B-cell Epitope Prediction
The Bepipred Linear Epitope 2.0 and Parker Hydrophilicity Prediction methods were used to detect and confirm possible allergenic protein sequences obtained from the AllerMatch as the data input for antibody epitope prediction. Bepipred Linear Epitope Prediction 2.0 uses a random forest algorithm trained on epitopes annotated from antibody-antigen protein structures to predict the location of linear B-cell epitopes. The antigenicity and hydrophilicity of the predicted B-cell epitopes of the potential allergens were determined using two algorithms: (1) Bepipred Linear Epitope 2.0, and (2) Parker Hydrophilicity Prediction technique. Comparing the projected results from Bepipred and Parker Hydrophilicity test yielded the final consensus B-cell epitope result.
T-cell Epitope Prediction
T-cell epitopes were predicted using MHC2Pred to predict the binding of peptides to major histocompatibility cell (MHC) Class II complexes. The potential allergenic protein sequences obtained from AllerMatch were used as the data input for T‑cell epitope prediction. Three alleles were selected based on a study by Schubert [80], specifically, HLA-DQB1*03, HLA-DQB1*0301, and HLA-DQB1*0302 alleles, which were related to fungal allergy. These alleles were selected and used to predict HLA‑DQ‑based T‑cell epitopes from proteins in seaweeds. A default threshold setting of 0.5 was chosen for the analysis. The highest peptide scores and their corresponding epitope sequences were highlighted and presented as potential T-cell epitopes.
Protein tertiary structure prediction and validation
The potential allergenic protein sequences obtained from AllerMatch were further processed with the SWISS-MODEL structure prediction server for the construction of the three‑dimensional (3D) models of potential seaweed allergens, highlighting their potential B-cell epitopes using PyMOL software. The Global Model Quality Estimate (GMQE) scores were obtained from SWISS-MODEL to validate the stereochemical quality of the 3D model generated. A score of 0.7 and above was considered a good model based on a study by Biasini [81]. ERRAT scores for overall quality were also determined and the 3D-1D profile of the models was tested using VERIFY 3D to further determine if the generated models were of good quality using the prompt “PASS” or “FAIL” that was shown after the test.
Results and discussion
Sequence-based analysis of red seaweed allergens
A total of fourteen (14) potential allergens were predicted as red seaweed allergens using AlgPred 2.0 and AllergenOnline. Four (4) of these were found in Kappaphycus alvarezii as shown in Table 1, which included the following proteins: Heat shock protein 70, Thioredoxin, Photosystem II CP47 reaction center protein, and Thiol-specific antioxidant protein. The Heat Shock protein 70 (HSP70) had shared 80.20% similarity with an allergen of Common hazel (Corylus avellana) namely Cor a 10.010, which can be found in tree pollens, and could be inhaled, and cause an allergenic response. Thioredoxin also shared 75% identity with Mala s 13.0101 in Malassezia sympodialis. Mala s 13.0101 is a protein found in the yeast species Malassezia sympodialis, which is commonly found on human skin. Photosystem II CP47 reaction center protein shared 38.40% similarity with Sal s 7 present in Atlantic salmon (Salmo salar) and 35.04% with Onc m alpha2I found in Rainbow trout (Oncorhynchus mykiss). Lastly, the Thiol-specific antioxidant protein shared 56% similarity with Bomb m 1 found in Domestic silkworms (Bombyx mori). It also exhibited 38.8% similarity with Tri a 32.0101 found in Common wheat (Triticum aestivum). Out of the four predicted allergens present in Kappaphycus alvarezii, only HSP70 was determined to have an exact 8 contiguous amino acid identical to the known allergen Cor a 10.0101.
Heat Shock Protein 70 (HSP70) has a subtle relationship with allergic diseases. Previous studies have found that HSP70 was an important mediator to mediate allergic reactions, and was capable of binding IgE antibodies in allergic patients [82]. The levels of HSP70 were significantly elevated in patients with allergic rhinitis [83]. Interestingly, Hsp70 was widely present in plant pollen as a pan-allergen, which could be responsible for a part of the allergenic cross-reactivity between proteins from different pollens and plant food such as common hazel [84]. Common hazel (Corylus avellana) was used as the best material to study pollen and food allergens in one species. According to a study by Vanto, et al. [85], hazelnut HSP70 was found to be a strong immunogenic protein, eliciting IgE-mediated responses in a significant proportion of hazelnut-allergic patients. Another study by Araya, et al. [86], confirmed the allergenic potential of hazelnut HSP70 and suggested that it could be a relevant target for the diagnosis and treatment of hazelnut allergy. Mala s 13.0101 has been documented as a potential allergen due to its ability to induce an immune response in some individuals. According to a study by Kim, et al. [87], Mala s 13.0101 was found to be a strong allergen, eliciting positive skin prick test reactions in patients with atopic dermatitis, and found that Mala s 13.0101 was capable of eliciting IgE-mediated responses in patients with atopic dermatitis, suggesting that it may play a role in the development of this skin condition. On the other hand, Thioredoxin shared 37% identity with Cop c 2 present in Common ink cap (Coprinus comatus), which could induce delayed-type reactions in atopic individuals, particularly in those with atopic dermatitis (AD), and since spores of Coprinus species are ubiquitous, these basidiomycetes were considered as possible aeroallergens when investigating causes of eczematous skin lesions in AD [88]. Photosystem II CP47 reaction center protein also shared 38.40% similarity with Sal s 7 present in Salmo salar (Atlantic salmon), and 35.04% with Onc m alpha2I found in Rainbow trout (Oncorhynchus mykiss), which were all fish allergens and could be correlated with parvalbumins. They are members of the calcium-binding EF-hand protein family characterized by a conserved protein structure, and they represent highly cross-reactive allergens for patients with specific IgE to conserved epitopes [89]. Thiol-specific antioxidant protein was found to be 56% similar to Bomb m 1 found in Domestic silkworms (Bombyx mori), and 38.8% similar to Tri a 32.0101 present in Common wheat (Triticum aestivum). A few proteins such as Bomb m 1 related to Bombyx mori (Bm) respiratory allergies have been identified and characterized but none of them are from the adult stage of the silkworm moth. Most of the allergens described were related to food allergies after pupa ingestion, particularly in Asian countries where it is a traditional food, and after exposure to larvae and pupa through skin or respiratory mucosa in silk industry workers [90,91]. Triticum aestivum is also an important allergen source responsible for various clinical manifestations of allergy like food allergy, pollen allergy, and respiratory allergy to flour-Baker’s asthma. A study by Pahr [92] revealed that Tri a 32.0101 was recognized by serum IgE from 35.7% of their tested patients, and was the most frequently recognized allergen in the patient population.
On the other hand, ten (10) putative allergens were found in Porphyra yezoensis as indicated in Table 2, which includes the following: Chitinase, Putative heat shock protein, Chaperone protein dnaK, Glyceraldehyde-3-phosphate dehydrogenase, Fructose-bisphosphate aldolase, Triosephosphate isomerase, Superoxide dismutase, Heat shock protein 70, Calmodulin, and Tubulin beta chain.
Chitinase was found to be 35.20% similar to Pun g 14 found in Pomegranate (Punica granatum). In 2011, 9 cases of allergic reactions to pomegranate have already been reported, but the responsible allergens have so far not been characterized in detail [92]. There were only three allergens originating from the pomegranate that was registered by WHO/IUIS, only one of which was chitinase III (Pun g 14), which has its structure determined [93]. However, Pun g 14 seems to be a minor allergen in pomegranate [94].
The Putative Heat Shock Protein (PHTP) was found to be 78.80% similar to an allergen named Asp f 12, which could be found in Neosartorya fumigata, one of the major Aspergillus species responsible for fungal respiratory disorders worldwide [95]. PHTP also shared 35.70% similarity to Pan h 3 found in Shark catfish (Pangasianodon hypophthalmus) native to the rivers of Southeast Asia [96]. Well-known allergens from different fish species frequently share between 50% and 75% of sequence identities resulting in cross-reactivity to different species for many fish-sensitized individuals [96]. Cross-reactivity in fish allergy was more common compared to other food allergies, such as wheat and egg. This is why people with fish allergies were advised to avoid consuming seaweed and seaweed products [97]. Chaperone Protein dnaK was also found to be 77.80% similar to an allergen Cor a 10.0101 found in Common hazel (Corylus avellana), which was also similar to allergens found in Kappaphycus alvarezii, as seen in Table 1. Food groups such as shellfish, fish, tree nuts, and peanuts have very high rates of cross-reactivity, which might explain its frequent recurrence when comparing allergens [98]. Glyceraldehyde-3-phosphate dehydrogenase was found to have a high percentage of similarity to Tri a 34.0101 found in Common wheat (Triticum aestivum) with 87.5% and was also similar to Pan h 3 in Shark catfish (Pangasianodon hypophthalmus) with 86.20%. Wheat is one of the six foods (the other five being hen’s egg, cow’s milk, peanuts, soy, and fish) responsible for more than 90% of all allergies in children [99]. Even low titers of IgE can cause an allergy, and in other cases, very high levels do not give clinical symptoms, which makes studies on the cross-reactivity of wheat allergens unpredictable [100]. Fructose-bisphosphate aldolase was found to be 67.50% similar to fish allergens namely Pan h 3 and Sal s 3.010, as well as 62.5% similar to Thu a 3 found in Shark catfish (Pangasianodon hypophthalmus), Atlantic salmon (Salmo salar), and Yellowfin tuna (Thunnus albacares) respectively. Fish enolase and aldolase have been identified as important new fish allergens. Inter-species cross-reactivity, even though limited, was found for enolases and aldolases by IgE-inhibition ELISA. In fish allergy diagnosis, IgE to enolase and aldolase were especially relevant when IgE to parvalbumin was absent. Cross-reactivity among aldolases was found to be variable in allergic patients [89]. Most fish-allergic patients react to multiple fish-related species, even when they belong to taxonomically distinct fish families. Cross-sensitization of over 80% has been shown by serological studies and skin testing [101].
Triosephosphate isomerase also had the highest similarity with Pro c 8.0101 found in Louisiana crawfish (Procambarus clarkii), which has a percentage of 70%. Triosephosphate isomerase (TIM) is a key enzyme in glycolysis and has been identified as an allergen in saltwater products. In a study by Yang [102], TIM with a molecular mass of 28 kDa was purified from the freshwater crayfish (Procambarus clarkii) muscle and was found to be cross-reactive with other enzymes such as filamin C. On the other hand, Superoxide dismutase was found to be 45.7% similar to Hev b 10.0101 in the Pará rubber tree (Hevea brasiliensis). IgE-mediated type 1 allergy to natural rubber latex (NRL) is caused by proteins present in diverse latex products. Latex is the milky sap produced by the laticiferous cells of the tropical rubber tree Hevea brasiliensis of the family Euphorbiaceae. Sensitization occurs via the skin, mucosal or wound contact, or inhalation of airborne allergens released from powdered latex gloves [103]. Superoxide dismutase was also found to be 45% similar to Pis v 4.0101 in Pistachio nut (Pistacia vera). Some people with a pistachio nut allergy developed hypersensitivity to other tree nuts as well. A study by Noorbakhsh, et al. [104] indicated that exposure of people to pistachio significantly affected the prevalence of its allergic reactions, and such exposure may affect the co-sensitivities with other nuts and other allergens. The plant taxonomic classification of pistachio and other tree nuts does appear to predict allergenic cross-reactivity, which could imply its cross-reactivity to other novel allergens. Furthermore, the Heat shock protein 70 in Porphyra yezoensis also shared similar results with HSP70 in Kappaphycus alvarezii, which exhibited 88.80% similarity with Pen c 19 found in Penicillium citrinum, and 78.80% similarity with Cor a 10.0101 present in Corylus avellana.
Calmodulin was found to be 43.80% similar to an allergen named Jun o 2 found in Prickly juniper (Juniperus oxycedrus), which is an evergreen tree that has plenty of applications where its leaf, berries, stem, and oil extracted from its wood were used for medicine [105]. Its oil was commonly used as a fragrance in perfumes, and skin creams, and was sometimes used in the meat industry to impart a smoky flavor to meats, fish, and sauces [106]. The last potential allergen present in Porphyra yezoensis was the Tubulin beta chain, which was found to be 51.2% similar to Lep d 2 found in storage mites (Lepidoglyphus destructor). As part of the allergenicity assessment, bioinformatic approaches were used to compare the amino-acid sequence of candidate proteins with sequences in a database of known allergens to predict potential cross-reactivity between novel food proteins and proteins to which people have become sensitized. Two criteria commonly used for these queries were searches over 80 amino-acid stretches for > 35% identity and searches for 8 amino-acid contiguous matches [107]. It was found using AllergenOnline that HSP70 (Table 1) was the only potential allergen found in Kappaphycus alvarezii that shared > 35% identity and contained an exact 8 contiguous amino acid identical to Cor a 10.0101 in Corylus avellana. Thus, it correlated well with the highly significant homology, which suggested Kappaphycus alvarezii to be cross‑reactive with Corylus avelana. On the other hand, almost all putative allergens from Porphyra yezoensis (Table 2) shared > 35% identity and contained an exact 8 contiguous amino acids identical to their respective known similar allergens, except for Chitinase and Putative Heat Shock Protein, which would suggest the high possibility of cross-reactivity.
Percentage-based analysis
Understanding the potential allergenicity of red seaweed is crucial in assessing the safety and potential health risks associated with their consumption. However, in the case of red seaweeds, it is important to determine and examine other potential cross-reactive allergens aside from food-induced allergies. Results from AllerMatch indicated various species, which the potential red seaweed allergens in Kappaphycus alvarezii and Porphyra yezoensis were associated (Figure 1) but the most common species that always come up were house dust mites (Dermatophagoides pteronyssinus) with 64.29%, followed by American house dust mites (Dermatophagoides farinae) with 57.14% of potential red seaweed allergens, and the fungus Malassezia sympodialis, as well as mold mite (Tyrophagus putrescentiae) with 42.86%.
House dust mites (HDM) thrive in the humid tropical Asian environment, and there is a high prevalence of HDM allergy and IgE sensitization in our Asian population. Seafood allergies and mite allergies may be linked and be cross-reactive due to the presence of Tropomyosins. Tropomyosins from HDMs have a high sequence homology to shellfish tropomyosins, and cross-reactivity between HDM and shrimp tropomyosins has been demonstrated. Exposure to inhaled tropomyosins from house dust mites has been postulated to be the primary sensitizer for seafood allergy particularly shellfish, in a reaction analogous to the oral allergy (inhalant-food) syndrome. This notion was supported by indirect data from the effects of HDM immunotherapy on shellfish allergy, and strong correlations between shellfish and HDM sensitization [108]. Furthermore, the induction of seafood allergy, with positive skin prick tests in previously non-allergic patients receiving HDM immunotherapy, has been reported as well, which further strengthened their correlation to one another [109]. Studies of HDM and shellfish sensitization occurring in populations with little or no exposure to either HDMs or shellfish provided evidence to suggest primary sensitization with subsequent cross-reactivity. A study of unexposed Jewish people who observed strict Kosher dietary rules that prohibit the consumption of shellfish showed that sensitization to seafood, particularly shrimp, was related to cross-reacting tropomyosin allergens in HDMs [110]. They also showed that the IgE cross-reactivity translated into clinical allergy to shrimp in some of the subjects. Reciprocally, a population-based study of young adults from Iceland, where exposure to HDMs was extremely rare, showed that HDM sensitization was associated with sensitization to seafood [111].
B-cell epitope analysis
B-cell epitope analysis played a crucial role in understanding the allergenicity of proteins and substances. It helped in identifying specific regions within these molecules that are recognized by antibodies produced by B cells of the immune system. It provided insights into the structural and functional properties of allergenic molecules and helped in understanding the molecular interactions between allergens and the immune system, shedding light on the mechanisms underlying allergic responses [112]. The finalized consensus result of the B-cell epitope chosen for each potential allergen in Kappaphycus alvarezii (Table 3) showed that almost all the B‑cell epitopes that were predicted by the Bepipred were found to be fitting suitably into the hydrophilic regions, which were subjected to the Parker Hydrophilicity Prediction. The amino acid residues present in B‑cell epitopes of Chaperone protein DnaK, Thioredoxin, Photosystem II CP47 reaction center protein, and Thiol-specific antioxidant protein were all hydrophilic. Thereby, these protein regions with greater distribution of hydrophilic residues were exposed to the external surface, which would most probably be capable of inducing B‑cell responses.
A study by Oezguen, et al. [113] showed that five amino acids were more likely to be in epitopes: Ala, Ser, Asn, Gly, and most particularly, Lysine. At least 2 of these common amino acids (Table 3) were present in every predicted B-cell epitope of Kappaphycus alvarezii. This indicated that these B-cell epitopes of Kappaphycus alvarezii may possess a higher likelihood of eliciting an immune response, and potentially exhibiting allergenic properties. The presence of these common amino acids in the predicted B-cell epitopes suggested that they could play a crucial role in the interaction between allergens and the immune system. Further investigations, including experimental validation, are necessary to confirm the allergenic potential of Kappaphycus alvarezii and understand the specific mechanisms underlying its allergenicity.
Potential allergens from Porphyra yezoensis were also subjected to B-cell epitope analysis, and the finalized consensus result of the B-cell epitope (Table 4) chosen for each potential allergen showed the specific peptide sequence that could bind to specific antibodies when an allergic response is triggered. This table also showed the position of the specific peptide in the whole protein sequence of the potential Porphyra yezoensis allergens.
Almost all of the B‑cell epitopes in Porphyra yezoensis, that were predicted by the Bepipred, were found to fit suitably into the hydrophilic regions found using Parker Hydrophilicity Prediction, thus, these protein regions with greater distribution of hydrophilic residues were being exposed to the external surface, which would most probably be capable of inducing B‑cell responses except for Triosephosphate isomerase, which has 9 non-polar amino acids present in the epitope. Similar to the study of Oezguen, et al. [113], at least 2 of the common amino acids found in B-cell epitopes (Ala, Ser, Asn, Gly, Lys), were present in every predicted B-cell epitopes of Porphyra yezoensis, especially Lysine, and Alanine. This implied that the consistent presence of common amino acids, such as Ala, Ser, Asn, Gly, Lysine, and Alanine in the predicted B-cell epitopes further supported the likelihood of these epitopes triggering B-cell responses. The fitting of the predicted B-cell epitopes into hydrophilic regions suggested their exposure on the external surface, enhancing their accessibility to interact with B cells, and initiating immune reactions. However, it was worth noting that the protein Triosephosphate isomerase, with its presence of non-polar amino acids in the epitope, may exhibit a different mechanism or potentially lower allergenicity compared to the other identified epitopes.
T-cell epitope analysis
T-cell epitope analysis plays a critical role in allergen research by identifying specific regions within allergenic proteins that are recognized by T-cell receptors, thus providing insights into the immunological response associated with allergies. This analysis helps in understanding the mechanisms of T-cell activation, the release of inflammatory mediators, and the development of immune tolerance. The top predicted peptide scores of T-cell epitopes of potential Kappaphycus alvarezii allergens (Table 5) that bind on HLA-DQ alleles and their corresponding epitope sequence was presented in Table 6.
From the T‑cell epitope prediction results, the highest peptide scores for each of the putative allergens were reported in these two alleles, which were HLA-DQB1*03 and HLA-DQB1*0301 with 1.256 and 1.318 as the highest scores, with the T-cell epitope sequences QAIENDNYD and LSGMLFLAS respectively, which suggested that the predicted T‑cell epitopes were highly associated with HLA-DQB1*03 and HLA-DQB1*0301 while mediating T‑cell immune responses, especially for HSP70 and Photosystem II CP47 reaction center proteins in Kappaphycus alvarezii.
A study by Boehncke [114] showed that grass pollen allergy was associated with an increased frequency of HLA-DQB1*0301 when compared to the control population. Furthermore, a study by Movahedi, et al. [115] showed that HLA-DQB1*0301 was associated with high levels of IgE production in asthmatic patients. Blanco, et al. [116] also searched for underlying genetic traits in latex and latex-fruit allergic patients by assessing genetic markers in the HLA Class II, IL 4, and FcεRI-βca gene, which they found that latex-fruit allergy was associated with loci HLA-DQB1 alleles These studies highlighted some preliminary research into the genetics of food-related allergy. Furthermore, a study by Madore, et al. [117] investigated the role of HLA-DQ genotypes in peanut allergy. The researchers found that specific HLA-DQ genotypes, including HLA-DQB102:01 and HLA-DQB102:02, were significantly associated with an increased risk of peanut allergy. This suggested that genetic factors, specifically certain HLA-DQ genotypes, play a crucial role in the development of allergies. Moreover, a study by Dimitrov, et al. [118] examined the genetic basis of egg allergy and identified an association between HLA-DQB1*01:02 and increased susceptibility to egg allergy. The presence of this specific HLA-DQB1 allele was found to be associated with a higher likelihood of developing IgE-mediated allergic reactions to eggs. These studies provided further evidence for the involvement of specific HLA-DQB1 alleles in the genetic predisposition to food allergies.
The top predicted peptide scores of T-cell epitopes of potential Porphyra yezoensis allergens (Table 7) that bind on HLA-DQ alleles and their corresponding epitope sequences (Table 8) were presented, and the highest peptide scores for each of the putative allergens were reported in these two alleles: HLA-DQB1*03 and HLA-DQB1*0301 with 1.209 and 1.678 as the highest scores respectively, which suggested that the predicted T-cell epitopes were highly associated with HLA-DQB1*03 and HLA-DQB1*0301 while mediating T-cell immune responses, especially for Chitinase and Superoxide dismutase proteins in Porphyra yezoensis.
According to Saetang [119], T-cell epitopes on seafood allergy, particularly shellfish, were observed to overlap with predicted B-cell epitopes, indicating that the sequences in the present study may be important for all types of both B-cell and T-cell response. Furthermore, these epitopes showed specificity to the highly distributed HLA DQ alleles, which could make these epitopes the cause of sensitization in most people. In a Genome-Wide Association Study (GWAS) that was performed in Japan, the researchers examined the potential relationship between wheat allergy, specifically on the hydrolyzed wheat protein (HWP) and HLA alleles. The results showed a positive association between HWP allergy and HLA alleles class II confirming the substantial involvement of the HLA system in food allergies like wheat, which was strongly associated with red seaweed allergy (Tables 1,2) [120]. It was worth mentioning that a considerable proportion of individuals who carry specific HLA class II risk alleles do not present food allergies, which were probably related to genome epigenetic modifications or protective functions, still unconnected to allergy and genetic loci. Furthermore, a variety of food allergens that have not previously been fully researched, such as seaweed, fungi, fish, shellfish, soy, and wheat, provided a strong incentive for additional research into their putative linkage with the HLA polymorphic allele [121].
Structure-based analysis
A three-dimensional (3D) model-based analysis is of significant importance for in silico determination of allergens because this allows researchers to gain valuable insights into the structural characteristics and properties of allergenic proteins, aiding in the prediction and assessment of their allergenic potential. By employing computational methods and algorithms, 3D modeling enabled the visualization and examination of the allergen’s molecular structure, identifying critical regions such as epitopes and potential binding sites. This analysis provided information on the spatial arrangement of amino acids, their interactions, and the overall conformation of the allergenic protein. Understanding these structural features was crucial for predicting protein stability, protein-protein interactions, and the potential for IgE binding [122]. A good quality 3D model is of utmost importance for in silico determination of allergens since it enables the identification and characterization of important regions within the allergenic protein, such as epitopes, which are crucial for binding to antibodies or T-cell receptors [123]. The Collated Global Model Quality Estimate (GMQE) values, ERRAT scores, and VERIFY 3D results of the predicted 3D models of the potential allergens in the 2 red seaweed species (Table 9) were presented and evaluated using the GMQE range between 0 and 1. A value above 0.7 was considered reliable and of good quality [81]. On the other hand, ERRAT measured the overall quality factor for non-bonded atomic interactions, and the higher scores mean higher quality [124]. Normally, the accepted range was more than 50 for a high-quality model, which was the case for the ERRAT scores in Table 9, ranging from 83% to 99%. Lastly, VERIFY 3D determined the compatibility of an atomic model (3D) with its own amino acid sequence (1D) by assigning a structural class based on its location and environment (alpha, beta, loop, polar, nonpolar, etc) and comparing the results to good structures. VERIFY3D was useful in the evaluation of undetermined protein models, based on low-resolution electron-density maps, on NMR spectra with inadequate distance constraints, or on computational procedures [125].
GMQE (Global Model Quality Estimation) was expressed as a number between 0 and 1, which higher numbers indicating higher reliability, reflecting the expected accuracy of a model built with that alignment and template, and normalized by the coverage of the target sequence [126,127]. All of the generated models were above 0.7, which indicated good quality predicted 3D models for the potential red seaweed allergens, except for Chitinase, Putative heat shock protein, and Heat Shock Protein 70 found in Porphyra yezoensis.
ERRAT scores were also shown in the table which indicated the overall quality factor for nonbonded atomic interactions, with higher scores indicating higher quality, and the generally accepted range was > 50 for a high-quality model [128]. All of the models generated obtained a high score for their overall model quality. However, only five 3D models; Chitinase, Fructose-bisphosphate aldolase, Superoxide dismutase, Calmodulin, and Tubulin beta chain, have passed the VERIFY 3D test, where at least 80% of the amino acids should have scored > = 0.1 in the 3D/1D profile [129]. 3D Models that failed to pass the VERIFY3D test (as seen in Table 9) should be reconstructed using other modeling software.
Highlighting B-cell epitope regions in 3D models provided valuable insights into the molecular basis of antigen-antibody interactions and immune responses. The predicted 3D models of Kappaphycus alvarezii (Figure 2) showed the highlighted regions with their respective predicted B-cell epitopes, which were highlighted in red. The 3D models could extensively enhance our knowledge of allergen structures for they will facilitate further analysis of the common properties of IgE binding sites of allergenic proteins [113].
On the other hand, Figure 3 showed the predicted 3D models of potential allergens present in Porphyra yezoensis, which were Chitinase, Putative heat shock protein, Chaperone protein dnaK, Glyceraldehyde-3-phosphate dehydrogenase, Fructose-bisphosphate aldolase, Triosephosphate isomerase, Superoxide dismutase, Heat shock protein 70 (HSP70), Calmodulin, and Tubulin beta chain. The highlighted regions showed their respective predicted B-cell epitopes, which were highlighted in red.
These models and epitope predictions could help develop further in vitro and in vivo testing, which could eventually lead to the ease of development of novel immunotherapy methods for allergic patients. T-cell epitope peptide therapy harnesses the established immunological dogma that dominant T-cell epitope peptides could induce anergy of specific T cells if delivered in a way that fails to activate the T cell [129]. Mapping known continuous IgE epitopes on the surface of the 3D models showed that only selected residues were surface-exposed. This is especially the case for long peptides that are larger than 10 amino acids. The 3D models could be useful in refining the sequences of these peptides to better identify the real site of IgE binding. This could facilitate the design of apo-allergenic proteins [113,129]. The 3D models and known experimental structures, in combination with the findings of the amino acid distribution on the epitopes, could be used to develop new methods and increase the predictive power of existing ones for the prediction of allergenicity and cross-reactivity [130].
Summary and conclusion
A total of four (4) potential allergens were found in Kappaphycus alvarezii, and ten (10) potential allergens were found in Porphyra yezoensis using an in silico approach. It was found that species that were related to food allergies like common hazel, common wheat, Atlantic salmon, and shark catfish posed allergens that have a similar structure with proteins in red seaweed, which could potentially induce cross-reactivity. Thus, patients allergic to these foods are advised to be wary of red seaweed. Furthermore, house-dust mite allergens were also prevalent in showing similarity with potential red seaweed allergens. This implies that the presence of common structural motifs and similarities between allergens found in Kappaphycus alvarezii, Porphyra yezoensis, and known food and environmental allergens raises concerns about the potential for cross-reactivity and allergic reactions for patients who are already allergic to common hazel, common wheat, Atlantic salmon, shark catfish, or house-dust mites and they may be at an increased risk of developing allergic reactions to red seaweed proteins due to their shared structural features. The presence of plenty analogous proteins to putative red seaweed allergens highlighted the importance of considering cross-reactivity when assessing the allergenic potential of novel food that utilizes seaweed as its main ingredient and emphasized the need for comprehensive allergen profiling and risk assessment to ensure the safety of individuals with known allergies. Specific B-cell and T-cell epitopes from Kappaphycus alvarezii and Porphyra yezoenis were predicted and 3D models were generated for further comparison with other molecular structures of known allergens. The presence of the highlighted B-cell epitopes in all of the 3D models indicated the presence of immunogenic regions within the allergen. This suggested that these regions have the potential to induce an immune response in individuals who were sensitized to the potential allergens. It also suggested that individuals who were sensitized to these epitopes may experience allergic reactions upon consuming red seaweed, which highlighted the need for clear labeling of allergenic ingredients in food products that contain seaweed. On the other hand, the presence of T-cell epitopes in the 3D models suggested that the identified regions of the allergen have the capacity to elicit T-cell-mediated immune responses, leading to the release of pro-inflammatory cytokines, and the recruitment of immune cells to the site of allergen exposure. These T-cell epitopes in the 3D models could provide insights into the potential severity and persistence of allergic reactions. These results could help in understanding the molecular basis of T-cell-mediated allergic responses, and their potential impact on allergy progression and persistence. Using the predicted allergens and the specific B-cell and T-cell epitopes involved in allergic reactions could guide the development of hypoallergenic variants of red seaweed proteins or potential allergen-specific immunotherapies, and could be used as baseline data for further in vitro and in vivo testing.
Recommendations
One of the challenges of this study was determining which in silico tool to use considering the wide range of algorithms and variables that these bioinformatic tools utilize. This study was also limited to sequence-based analysis using full FASTA alignment, determination of 8 exact contiguous amino acids, and the determination of more than 35% identity with a known allergen. Thus, utilization of other in silico tools that focus on physicochemical property-based approaches such as AllerTOP and AllergenFP, was recommended to provide a complementary perspective to sequence-based approaches in the determination of allergens. Physicochemical property-based tools could identify specific structural and physicochemical determinants associated with allergenicity, such as hydrophobicity, charge distribution, flexibility, and stability of proteins. Understanding these determinants could provide valuable information about the structural features that contribute to the allergenic potential of a protein, aiding in the identification and characterization of allergens. Furthermore, environmental factors such as exposure of red seaweed to common seafood allergens like fish and shrimp were not included in the scope of this study thus it is recommended for future researchers to explore its correlation and effect to the initial protein sequence. It was also recommended to use other software to generate more accurate and better quality 3D models like I-Tasser, and use other types of structure-based analysis in predicting allergens like UCSF Chimera that employs superimposition of 3D structure to a known allergen since these tools generate more accurate 3D models, and provide more data on how the model was generated. These also provide interactive visualization and analysis of molecular structures and related data, including density maps, supramolecular assemblies, sequence alignments, docking results, trajectories, and conformational ensembles. Lastly, it was recommended by the researcher to do further in vitro allergenicity assessment like Specific IgE ELISA for both Kappaphycus alvarezii and Porphyra yezoensis with patients that are allergic to common hazelnut, Atlantic salmon, shark catfish, and house dust mites to confirm the current findings of the study, and to establish the clinical relevance of the identified potential allergens in seaweed. Experimental validation through in vitro assays is crucial to confirm the presence and allergenicity of the identified potential red seaweed allergens.
Authors’ contribution
DSG collected and analyzed the data, performed B Cell and T Cell epitope mapping, and homology modelling, and wrote the initial draft of the research manuscript; MPT validated the results, helped in presenting the data, contributed to writing, and finalized the manuscript.
- Cheney D. Toxic and harmful seaweeds. Seaweed in Health and Disease Prevention. 2016; 407–421. https://doi.org/10.1016/b978-0-12-802772-1.00013-0
- Peñalver R, Lorenzo JM, Ros G, Amarowicz R, Pateiro M, Nieto G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar Drugs. 2020 Jun 5;18(6):301. doi: 10.3390/md18060301. PMID: 32517092; PMCID: PMC7345263.
- Cornish L. Those curious and delicious seaweeds: A fascinating voyage from biology to gastronomy. Phycologia. 2019; 58: 578–579.
- Sartal CG, Alonso MCB, Barrera BP. Application of Seaweeds in the Food Industry. In Handbook of Marine Macroalgae, S.-K. Kim (Ed.). 2011. https://doi.org/10.1002/9781119977087.ch34
- O'Sullivan L, Murphy B, McLoughlin P, Duggan P, Lawlor PG, Hughes H, Gardiner GE. Prebiotics from marine macroalgae for human and animal health applications. Mar Drugs. 2010 Jul 1;8(7):2038-64. doi: 10.3390/md8072038. PMID: 20714423; PMCID: PMC2920542.
- Sulayao N, Tagarino R, Kick C. Seaweed Farming In The Philippines: Its Prospects In Northeast Sorsogon. 1991. https://www.doc-developpement-durable.org/file/Culture/culture-algues/algoculture/SEAWEED%20FARMING%20IN%20THE%20PHILIPPINES.pdf.
- Mateo, J. 2021. Understanding biosecurity: knowledge, attitudes, and practices of seaweed farmers in the Philippines. Journal of Applied Phycology. 33. 10.1007/s10811-020-02352-5.
- Montaño M. Seaweeds in Philippine Food: Traditional Uses and Recent Developments. Fisheries science. 2002; 68: 1457-1459. 10.2331/fishsci.68.sup2_1457.
- Buschmann AH, Camus C, Infante J, Neori A, Israel Á, Hernández-González MC, Critchley AT. Seaweed production: Overview of the global state of exploitation, farming, and emerging research activity. European Journal of Phycology. 2017; 52(4): 391–406. https://doi.org/10.1080/09670262.2017.1365175
- Ask EI, Azanza RV. Advances in cultivation technology of commercial eucheumatoid species: a review with suggestions for future research. Aquaculture. 2002; 206: 257–277.
- FAO. The global status of seaweed production, trade, and utilization. 2018. https://issuu.com/globefish/docs/the_global_status_of_seaweed_production__trade_and/1?ff&showOtherPublicationsAsSuggestions=true
- Battacharyya D, Babgohari M.Z, Rathor P, Prithiviraj B. Seaweed extracts as biostimulants. Sci. Hort. 2015; 196: 39–48.
- Bedoux G, Hardouin K, Burlot AS, Bourgougnon N. Bioactive components from seaweeds: Cosmetic application and future applications. In: (B. Nathalie, ed) Advances in botanical research. Elsevier, London. 2014; 345–378.
- Chaoroensiddhi S, Conlon M.A. Franco CMM, Zhang W. The development of seaweed0-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017; 70: 20–33.
- Filippo-Herrera DA, Munoz-Ochoa M, Hernandez-Herrera RM. Biostimulant activity of individual and blended seaweed extracts on the germination and growth of the mung bean. J. Appl. Phycol. 2017; 31: 1–13.
- Gaurav NS, Sivasankari GS, Kiran A. Ninawe, Selvin J. Utilization of bioresources for sustainable biofuels: a review. Renew. Sust. Energ. Rev. 2017; 73: 205–2014.
- Lomartire S, Marques JC, Gonçalves AMM. An Overview of the Alternative Use of Seaweeds to Produce Safe and Sustainable Bio-Packaging. Appl. Sci. 2022; 12: 3123. https://doi.org/10.3390/ app12063123
- Wang HD, Li XC, Lee DJ, Chang JS. Potential biomedical applications of marine algae. Bioresour Technol. 2017 Nov;244(Pt 2):1407-1415. doi: 10.1016/j.biortech.2017.05.198. Epub 2017 Jun 3. PMID: 28697977.
- Zeraatkar AK, Ahmadzadeh H, Talebi AF, Moheimani NR, McHenry MP. Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manage. 2016 Oct 1;181:817-831. doi: 10.1016/j.jenvman.2016.06.059. Epub 2016 Jul 5. PMID: 27397844.
- Collins KG, Fitzgerald GF, Stanton C, Ross RP. Looking Beyond the Terrestrial: The Potential of Seaweed Derived Bioactives to Treat Non-Communicable Diseases. Mar Drugs. 2016 Mar 18;14(3):60. doi: 10.3390/md14030060. PMID: 26999166; PMCID: PMC4820313.
- van den Burg SWK, Stuiver M, Veenstra FA, Bikker P, Lopez Contreras AM, Palstra AP, van Raamsdonk LWD. A Triple P review of the feasibility of sustainable offshore seaweed production in the North Sea. Wageningen, the Netherlands: Wageningen UR. 2013.
- Sicherer SH. Determinants of systemic manifestations of food allergy. J Allergy Clin Immunol. 2000 Nov;106(5 Suppl):S251-7. doi: 10.1067/mai.2000.110158. PMID: 11080740.
- Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, Fiocchi A, Chiang W, Beyer K, Wood R, Hourihane J, Jones SM, Lack G, Sampson HA. ICON: food allergy. J Allergy Clin Immunol. 2012 Apr;129(4):906-20. doi: 10.1016/j.jaci.2012.02.001. Epub 2012 Feb 23. PMID: 22365653.
- Farrokhi S, Nabipour I, Assadi M. Seaweeds: some pharmaco-immunological effects. Iran J Immunol. 2012 Jun;9(2):145-8. PMID: 22735802.
- Fleurence J, Morançais M, Dumay J, Decottignies P, Turpin V, Munier M , Jaouen P. What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends in Food Science & Technology. 2012; 27(1): 57–61. https://doi.org/10.1016/j.tifs.2012.03.004
- Goodman RE, Vieths S, Sampson HA, Hill D, Ebisawa M, Taylor SL, van Ree R. Allergenicity assessment of genetically modified crops--what makes sense? Nat Biotechnol. 2008 Jan;26(1):73-81. doi: 10.1038/nbt1343. Erratum in: Nat Biotechnol. 2008 Feb;26(2):241. PMID: 18183024.
- Codex Alimentarius Commission guidelines. Foods derived from modern biotechnology (2003 reprinted in the second edition in 2009). World Health Organization and Agricultural Organization of the United Nations. 2009.
- Mirsky HP, Cressman RF Jr, Ladics GS. Comparative assessment of multiple criteria for the in silico prediction of cross-reactivity of proteins to known allergens. Regul Toxicol Pharmacol. 2013 Nov;67(2):232-9. doi: 10.1016/j.yrtph.2013.08.001. Epub 2013 Aug 9. PMID: 23933007.
- Schein CH, Ivanciuc O, Braun W. Bioinformatics approaches to classifying allergens and predicting cross-reactivity. Immunol Allergy Clin North Am. 2007 Feb;27(1):1-27. doi: 10.1016/j.iac.2006.11.005. PMID: 17276876; PMCID: PMC1941676.
- Cian RE, Drago SR, de Medina FS, Martínez-Augustin O. Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Mar Drugs. 2015 Aug 20;13(8):5358-83. doi: 10.3390/md13085358. PMID: 26308006; PMCID: PMC4557026.
- Denis C, Ledorze C, Jaouen P, Fleurence J. Comparison of different procedures for the extraction and partial purification of R-phycoerythrin from the red macroalga Grateloupia turuturu. Bot. Mar. 2009; 52: 278–281. doi: 10.1515/BOT.2009.034
- Yu SK, Blennow A, Bojko M, Madsen F, Olsen CE, Engelsen SB. Physico-chemical characterization of floridean starch of red algae. Starch-Starke. 2002; 54: 66–74. doi: 10.1002/1521-379X(200202)54:2<66::AID-STAR66>3.0.CO;2-B.
- Rodrigueza MRC, Montaño MNE. Bioremediation potential of three carrageenophytes cultivated in tanks with seawater from fish farms. Journal of Applied Phycology. 2007; 19: 755–762.
- Yunque DA, Tibubos KR, Hurtado AQ, Critchley AT. Optimization of culture conditions for tissue culture production of young plants of carrageenophyte Kappaphycus. Journal of Applied Phycology. 2011; 23: 433-438.
- Yong YS, Yong WTL, Thien VY, Ng SE, Anton A, Yasiir S. Acclimatization of micropropagated Kappaphycus alvarezii (Doty) Doty ex Silva (Rhodophyta, Solieriaceae) in outdoor nursery system. Journal of Applied Phycology, 2014; 27: 413-419.
- Harwinda FK, Satyantini WH, Masithah ED. The effects of salinity and temperature shock on Kappaphycus alvarezii seaweed spores release. IOP Conf. Series: Earth and Environmental Science. 2018; 137: 012019.
- Bindu MS, Levine IA. The commercial red seaweed Kappaphycus alvarezii—an overview on farming and environment. J Appl Phycol. 2011; 23: 789–796. https://doi.org/10.1007/s10811-010-9570-2
- Gamero-Vega G, Palacios-Palacios M, Quitral V. Nutritional composition and bioactive compounds of red seaweed: A mini-review. Journal of Food and Nutrition Research. 2020; 8(8): 431–440. https://doi.org/10.12691/jfnr-8-8-7
- Admassu H, Gasmalla MAA, Yang R, Zhao W. Identification of Bioactive Peptides with α-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra spp). J Agric Food Chem. 2018 May 16;66(19):4872-4882. doi: 10.1021/acs.jafc.8b00960. Epub 2018 Apr 30. PMID: 29667406.
- Buschman AH, Correa JA, Westermeier R, Hernandez-Gonzalez M, Norambuena R. Red algal farming in Chile: a review. Aquaculture. 2001; 194: 203-220.
- GFI. Is red seaweed the next big thing in plant-based protein? The Good Food Institute. 2022. [accessed 2022 Jun 1]. https://gfi.org/blog/red-seaweed-research-with-beth-zotter/
- Ragan MA, Bird CJ, Rice EL, Gutell RR, Murphy CA, Singh RK. A molecular phylogeny of the marine red algae (Rhodophyta) based on the nuclear small-subunit rRNA gene. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7276-80. doi: 10.1073/pnas.91.15.7276. PMID: 8041780; PMCID: PMC44382.
- Cao J, Wang J, Wang S, Xu X. Porphyra Species: A Mini-Review of Its Pharmacological and Nutritional Properties. J Med Food. 2016 Feb;19(2):111-9. doi: 10.1089/jmf.2015.3426. Epub 2015 Dec 10. PMID: 26653974.
- Watanabe F, Takenaka S, Katsura H, Miyamoto E, Abe K, Tamura Y, Nakatsuka T, Nakano Y. Characterization of a vitamin B12 compound in the edible purple laver, Porphyra yezoensis. Biosci Biotechnol Biochem. 2000 Dec;64(12):2712-5. doi: 10.1271/bbb.64.2712. PMID: 11210144.
- L Venkatraman K, A Syed A, Indumathi P, Mehta A. VITPOR AI, A Coagulation Factor XIIa Inhibitor from Porphyra yezoensis: in vivo Mode of Action and Assessment of Platelet Function Analysis. Protein Pept Lett. 2020;27(3):243-250. doi: 10.2174/0929866526666191026111056. PMID: 31738131.
- Tian Y, Jiang Y, Guo Y, Zhao Y, Li N, Yao L. Research progress on nutritional quality and edible value of Porphyra. J Food Saf Qual. 2021; 12: 4929–36. 10.19812/j.cnki.jfsq11-5956/ts.2021.12.033
- Porse H, Rudolph B. The seaweed hydrocolloid industry:2016 updates, requirements, and outlook. J. Appl. Phycol. 2017; 29: 2187–2200.
- Panchanan B Ashim B, Mandal AB. Effect of Kappaphycus alvarezii in Development of Functional Based Reformulated Chicken Sausages Utilizing Spent Hen Meat. Journal of Meat Science. 2020; 15: 34-39. 10.5958/2581-6616.2020.00005.5.
- Gonzalez C, Garcıa Alvarez O. Mıguez Rodr´ıguez L. Algas marinas de Galicia: Biolox ıa, gastronom´ıa, industria. Edicions Xerais de Galicia, S.A., Spain. 1998.
- Pereira L, Critchley AT, Amado AM, Ribeiro-Claro PJA. A comparative analysis of phycocolloids produced by underutilized versus industrially utilized carrageenophytes (Gigartinales, Rhodophyta). J. Appl. Phycol. 2009; 21: 599–605.
- Kaliaperumal N. Products from Seaweeds, SDMRI Research Publication N◦3, India, 33. 2003.
- Giury MD. Nori Cultivation. http://www.seaweed.ie/aquaculture.ph. (April 8, 2023). 2006.
- Riyanto T, Rohidin G, Yusuf B. Nori Imitation Sheet With Edible Film Concept Based on Myofibrillar Protein Tilapia Fish. Indonesian Fisheries Products Processing Journal. 2014; 17:262-279.
- Hwang ES, Ki KN, Chung HY. Proximate composition, amino Acid, mineral, and heavy metal content of dried laver. Prev Nutr Food Sci. 2013 Jun;18(2):139-44. doi: 10.3746/pnf.2013.18.2.139. PMID: 24471123; PMCID: PMC3892503.
- Ruethers T, Taki AC, Johnston EB, Nugraha R, Le TTK, Kalic T, McLean TR, Kamath SD, Lopata AL. Seafood allergy: A comprehensive review of fish and shellfish allergens. Mol Immunol. 2018 Aug;100:28-57. doi: 10.1016/j.molimm.2018.04.008. Epub 2018 May 30. PMID: 29858102.
- Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, Schleimer RP, Nadeau KC. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw Open. 2019 Jan 4;2(1):e185630. doi: 10.1001/jamanetworkopen.2018.5630. PMID: 30646188; PMCID: PMC6324316.
- Mims JW, Veling MC. Inhalant allergies in children. Otolaryngol Clin North Am. 2011 Jun;44(3):797-814, xi. doi: 10.1016/j.otc.2011.03.013. Epub 2011 Apr 29. PMID: 21621062; PMCID: PMC7172761.
- Scott D, Fell DO. Seaweed irritation rash treatment, symptoms & first aid. eMedicineHealth. 2021. https://www.emedicinehealth.com/wilderness_seaweed_irritation/article_em.htm
- Food & Allergy Consulting & Testing Center. 2022. Seaweed: Do we know enough? FACTS SA. https://www.factssa.com/news/seaweed-do-we-know-enough/
- Motoyama K, Hamada Y, Nagashima Y, Shiomi K. Allergenicity and allergens of amphipods found in nori (dried laver). Food Addit Contam. 2007 Sep;24(9):917-22. doi: 10.1080/02652030701305454. PMID: 17691004.
- Thomas I, Siew LQC, Watts TJ, Haque R. Seaweed allergy. J Allergy Clin Immunol Pract. 2019 Feb;7(2):714-715. doi: 10.1016/j.jaip.2018.11.009. Epub 2018 Nov 22. PMID: 30471363.
- Kular H, Dean J, Cook V. A case of carrageenan allergy in a pediatric patient. Annals of Allergy, Asthma & Immunology. 2018. 121(5). Available from https://doi.org/10.1016/j.anai.2018.09.395
- Goodman RE. Biosafety: Evaluation and regulation of genetically modified(GM) crops in the United States. J Huazhong Agric Univ. 2014;33(6):85–113.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403-10. doi: 10.1016/S0022-2836(05)80360-2. PMID: 2231712.
- Ferrari IV, Patrizio P. Study of basic local alignment search tool (BLAST) and multiple sequence alignment (clustal- X) of monoclonal mice/human antibodies. 2021. https://doi.org/10.1101/2021.07.09.451785
- Pearson WR. Flexible sequence similarity searching with the FASTA3 program package. Methods Mol Biol. 2000;132:185-219. doi: 10.1385/1-59259-192-2:185. PMID: 10547837.
- Siruguri V, Bharatraj DK, Vankudavath RN, Mendu VV, Gupta V, Goodman RE. Evaluation of Bar, Barnase, and Barstar recombinant proteins expressed in genetically engineered Brassica juncea (Indian mustard) for potential risks of food allergy using bioinformatics and literature searches. Food Chem Toxicol. 2015 Sep;83:93-102. doi: 10.1016/j.fct.2015.06.003. Epub 2015 Jun 14. Erratum in: Food Chem Toxicol. 2015 Dec;86:386-7. PMID: 26079618.
- Moran DL, Tetteh AO, Goodman RE, Underwood MY. Safety assessment of the calcium-binding protein, apoaequorin, expressed by Escherichia coli. Regul Toxicol Pharmacol. 2014 Jul;69(2):243-9. doi: 10.1016/j.yrtph.2014.04.004. Epub 2014 Apr 24. PMID: 24768935.
- Ladics GS. Current codex guidelines for assessment of potential protein allergenicity. Food Chem Toxicol. 2008 Oct;46 Suppl 10:S20-3. doi: 10.1016/j.fct.2008.07.021. Epub 2008 Jul 30. PMID: 18708115.
- Sharma N, Patiyal S, Dhall A, Pande A, Arora C, Raghava GPS. AlgPred 2.0: an improved method for predicting allergenic proteins and mapping of IgE epitopes. Brief Bioinform. 2021 Jul 20;22(4):bbaa294. doi: 10.1093/bib/bbaa294. PMID: 33201237.
- Fiers MW, Kleter GA, Nijland H, Peijnenburg AA, Nap JP, van Ham RC. Allermatch, a webtool for the prediction of potential allergenicity according to current FAO/WHO Codex alimentarius guidelines. BMC Bioinformatics. 2004 Sep 16;5:133. doi: 10.1186/1471-2105-5-133. PMID: 15373946; PMCID: PMC522748.
- Pomés A. Relevant B cell epitopes in allergic disease. Int Arch Allergy Immunol. 2010;152(1):1-11. doi: 10.1159/000260078. Epub 2009 Nov 26. PMID: 19940500; PMCID: PMC2956005.
- Vita R, Mahajan S, Overton JA, Dhanda SK, Martini S, Cantrell JR, Wheeler DK, Sette A, Peters B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019 Jan 8;47(D1):D339-D343. doi: 10.1093/nar/gky1006. PMID: 30357391; PMCID: PMC6324067.
- Sun B, Zhang Y. Overview of orchestration of CD4+ T cell subsets in immune responses. Adv Exp Med Biol. 2014;841:1-13. doi: 10.1007/978-94-017-9487-9_1. PMID: 25261202.
- Ahmed RK, Maeurer MJ. T-cell epitope mapping. Methods Mol Biol. 2009;524:427-38. doi: 10.1007/978-1-59745-450-6_31. PMID: 19377963.
- Ahmad TA, Eweida AE, El-Sayed LH. T-cell epitope mapping for the design of powerful vaccines. Vaccine Reports. 2016; 6:13-22. doi: 10.1016/j.vacrep.2016.07.002.
- Lafuente EM, Reche PA. Prediction of MHC-peptide binding: a systematic and comprehensive overview. Curr Pharm Des. 2009;15(28):3209-20. doi: 10.2174/138161209789105162. PMID: 19860671.
- Schubert MS, Hutcheson PS, Graff RJ, Santiago L, Slavin RG. HLA-DQB1 *03 in allergic fungal sinusitis and other chronic hypertrophic rhinosinusitis disorders. J Allergy Clin Immunol. 2004 Dec;114(6):1376-83. doi: 10.1016/j.jaci.2004.08.029. Erratum in: J Allergy Clin Immunol. 2005 Feb;115(2):329. PMID: 15577839.
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014 Jul;42(Web Server issue):W252-8. doi: 10.1093/nar/gku340. Epub 2014 Apr 29. PMID: 24782522; PMCID: PMC4086089.
- Ortona E, Margutti P, Delunardo F, Vaccari S, Riganò R, Profumo E, Buttari B, Teggi A, Siracusano A. Molecular and immunological characterization of the C-terminal region of a new Echinococcus granulosus Heat Shock Protein 70. Parasite Immunol. 2003 Mar;25(3):119-26. doi: 10.1046/j.1365-3024.2003.00617.x. PMID: 12911519.
- Min HJ, Kim KS, Yoon JH, Kim CH, Cho HJ. T-helper 2 cytokine-induced heat shock protein 70 secretion and its potential association with allergic rhinitis. Int Forum Allergy Rhinol. 2017 May;7(5):530-535. doi: 10.1002/alr.21905. Epub 2017 Jan 5. PMID: 28054753.
- Gruehn S, Suphioglu C, O'Hehir RE, Volkmann D. Molecular cloning and characterization of hazel pollen protein (70 kD) as a luminal binding protein (BiP): a novel cross-reactive plant allergen. Int Arch Allergy Immunol. 2003 Jun;131(2):91-100. doi: 10.1159/000070924. PMID: 12811017.
- Vanto T, Turjanmaa K, Reunala T, Kroker G. Hazelnut (Corylus avellana) heat shock protein 70 is a strong immunogenic protein. Clinical and Experimental Allergy. 2000; 30(5):706-711. doi:10.1046/j.1365-2222.2000.00788.x
- Araya M, Morero R, Rodriguez A, Sánchez J, Villalba M. Identification of Hazelnut Heat Shock Proteins and Assessment of Their Allergenic Potential. Journal of Agricultural and Food Chemistry. 2010; 58(3):1476-1482. doi:10.1021/jf903166c
- Kim HJ, Jeong SY, Lee JH, Hong SC, Lee SJ. Clinical relevance of Malassezia sympodialis allergen 13.0101 in patients with atopic dermatitis. Journal of Dermatological Science. 2017; 87(1):38-42. doi:10.1016/j.jdermsci.2017.03.007
- Fischer B, Yawalkar N, Brander KA, Pichler WJ, Helbling A. Coprinus comatus (shaggy cap) is a potential source of aeroallergen that may provoke atopic dermatitis. J Allergy Clin Immunol. 1999 Oct;104(4 Pt 1):836-41. doi: 10.1016/s0091-6749(99)70295-2. PMID: 10518829.
- Kuehn A, Hilger C, Lehners-Weber C, Codreanu-Morel F, Morisset M, Metz-Favre C, Pauli G, de Blay F, Revets D, Muller CP, Vogel L, Vieths S, Hentges F. Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: component resolved diagnosis using parvalbumin and the new allergens. Clin Exp Allergy. 2013 Jul;43(7):811-22. doi: 10.1111/cea.12117. PMID: 23786287.
- Jeong KY, Son M, Lee JY, Park KH, Lee JH, Park JW. Allergenic Characterization of 27-kDa Glycoprotein, a Novel Heat Stable Allergen, from the Pupa of Silkworm, Bombyx mori. J Korean Med Sci. 2016 Jan;31(1):18-24. doi: 10.3346/jkms.2016.31.1.18. Epub 2015 Dec 24. PMID: 26770033; PMCID: PMC4712575.
- Zuo J, Lei M, Yang R, Liu Z. Bom m 9 from Bombyx mori is a novel protein related to asthma. Microbiol Immunol. 2015 Jul;59(7):410-8. doi: 10.1111/1348-0421.12271. PMID: 26094648.
- Pahr S, Constantin C, Mari A, Scheiblhofer S, Thalhamer J, Ebner C, Vrtala S, Mittermann I, Valenta R. Molecular characterization of wheat allergens specifically recognized by patients suffering from wheat-induced respiratory allergy. Clin Exp Allergy. 2012 Apr;42(4):597-609. doi: 10.1111/j.1365-2222.2012.03961.x. PMID: 22417217.
- Petersen A, Kleinheinz A, Jappe U. Anaphylactic reactions to pomegranate: identification and characterization of eliciting IgE-reactive components. Clin Transl Allergy. 2011 Aug 12;1(Suppl 1): 88. doi: 10.1186/2045-7022-1-S1-P88. PMCID: PMC3354222.
- Zoccatelli G, Dalla Pellegrina C, Consolini M, Fusi M, Sforza S, Aquino G, Dossena A, Chignola R, Peruffo A, Olivieri M, Rizzi C. Isolation and identification of two lipid transfer proteins in pomegranate (Punica granatum). J Agric Food Chem. 2007 Dec 26;55(26):11057-62. doi: 10.1021/jf072644x. Epub 2007 Nov 27. PMID: 18038997.
- Masuda T, Zhao G, Mikami B. Crystal structure of class III chitinase from pomegranate provides the insight into its metal storage capacity. Biosci Biotechnol Biochem. 2015;79(1):45-50. doi: 10.1080/09168451.2014.962475. Epub 2014 Sep 25. PMID: 25252615.
- Banerjee B, Greenberger PA, Fink JN, Kurup VP. Immunological characterization of Asp f 2, a major allergen from Aspergillus fumigatus associated with allergic bronchopulmonary aspergillosis. Infect Immun. 1998 Nov;66(11):5175-82. doi: 10.1128/IAI.66.11.5175-5182.1998. PMID: 9784519; PMCID: PMC108645.
- Klueber J, Schrama D, Rodrigues P, Dickel H, Kuehn A. Fish allergy management: from component-resolved diagnosis to unmet diagnostic needs. Curr Treat Options Allergy. 2019; 6:322–37. 10.1007/s40521-019-00235-w
- Seszak-Greinecker G, Balic N, Wolfgang H, Ines S. Exclusive Sensitization to Striped Catfish (Pangasianodon hypophthalmus) and Closely Related Fish Species. Journal of Allergy and Clinical Immunology. 2012; 129: AB170. 10.1016/j.jaci.2011.12.401.
- Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010 Feb;125(2 Suppl 2):S116-25. doi: 10.1016/j.jaci.2009.08.028. Epub 2009 Dec 29. PMID: 20042231.
- Kamath SD, Bublin M, Kitamura K, Matsui T, Ito K, Lopata AL. Cross-reactive epitopes and their role in food allergy. J Allergy Clin Immunol. 2023 May;151(5):1178-1190. doi: 10.1016/j.jaci.2022.12.827. Epub 2023 Mar 15. PMID: 36932025.
- Gotua M, Lomidze N, Dolidze N, Gotua T. IgE-mediated food hypersensitivity disorders. Georgian Med News. 2008 Apr;(157):39-44. PMID: 18487689.
- Ogino R, Chinuki Y, Yokooji T, Takizawa D, Matsuo H, Morita E. Identification of peroxidase-1 and beta-glucosidase as cross-reactive wheat allergens in grass pollen-related wheat allergy. Allergol Int. 2021 Apr;70(2):215-222. doi: 10.1016/j.alit.2020.09.005. Epub 2020 Oct 21. PMID: 33616048.
- Van Do T, Elsayed S, Florvaag E, Hordvik I, Endresen C. Allergy to fish parvalbumins: studies on the cross-reactivity of allergens from 9 commonly consumed fish. J Allergy Clin Immunol. 2005 Dec;116(6):1314-20. doi: 10.1016/j.jaci.2005.07.033. Epub 2005 Oct 3. PMID: 16337465.
- Yang Y, Zhang YX, Liu M, Maleki SJ, Zhang ML, Liu QM, Cao MJ, Su WJ, Liu GM. Triosephosphate Isomerase and Filamin C Share Common Epitopes as Novel Allergens of Procambarus clarkii. J Agric Food Chem. 2017 Feb 1;65(4):950-963. doi: 10.1021/acs.jafc.6b04587. Epub 2017 Jan 23. PMID: 28072528.
- Noorbakhsh R, Mortazavi SA, Sankian M, Shahidi F, Tehrani M, Azad FJ, Behmanesh F, Varasteh A. Pistachio allergy-prevalence and in vitro cross-reactivity with other nuts. Allergol Int. 2011 Dec;60(4):425-32. doi: 10.2332/allergolint.10-OA-0222. Epub 2011 May 25. PMID: 21593580.
- Al-Snafi Ali. Pharmacological and therapeutic effects of Juniperus oxycedrus-a review. 2018. 10.5281/zenodo.1214996.
- Nehme R, Andrés S, Pereira RB, Ben Jemaa M, Bouhallab S, Ceciliani F, López S, Rahali FZ, Ksouri R, Pereira DM, Abdennebi-Najar L. Essential Oils in Livestock: From Health to Food Quality. Antioxidants (Basel). 2021 Feb 23;10(2):330. doi: 10.3390/antiox10020330. PMID: 33672283; PMCID: PMC7926721.
- Herman RA, Song P, Thirumalaiswamysekhar A. Value of eight-amino-acid matches in predicting the allergenicity status of proteins: an empirical bioinformatic investigation. Clin Mol Allergy. 2009 Oct 29;7:9. doi: 10.1186/1476-7961-7-9. PMID: 19874602; PMCID: PMC2773230.
- Wong L, Huang CH, Lee BW. Shellfish and House Dust Mite Allergies: Is the Link Tropomyosin? Allergy Asthma Immunol Res. 2016 Mar;8(2):101-6. doi: 10.4168/aair.2016.8.2.101. Epub 2015 Jul 14. PMID: 26739402; PMCID: PMC4713872.
- van Ree R, Antonicelli L, Akkerdaas JH, Garritani MS, Aalberse RC, Bonifazi F. Possible induction of food allergy during mite immunotherapy. Allergy. 1996 Feb;51(2):108-13. PMID: 8738516.
- Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003 Jul;33(7):956-61. doi: 10.1046/j.1365-2222.2003.01722.x. PMID: 12859453.
- Adalsteinsdottir B, Sigurdardottir ST, Gislason T, Kristensen B, Gislason D. What characterizes house dust mite sensitive individuals in a house dust mite free community in Reykjavik, Iceland? Allergol Int. 2007 Mar;56(1):51-6. doi: 10.2332/allergolint.O-06-447. Epub 2007 Jan 29. PMID: 17259810.
- Nugraha R, Kamath SD, Johnston E, Karnaneedi S, Ruethers T, Lopata AL. Conservation Analysis of B-Cell Allergen Epitopes to Predict Clinical Cross-Reactivity Between Shellfish and Inhalant Invertebrate Allergens. Front Immunol. 2019 Nov 19;10:2676. doi: 10.3389/fimmu.2019.02676. PMID: 31803189; PMCID: PMC6877653.
- Oezguen N, Zhou B, Negi SS, Ivanciuc O, Schein CH, Labesse G, Braun W. Comprehensive 3D-modeling of allergenic proteins and amino acid composition of potential conformational IgE epitopes. Mol Immunol. 2008 Aug;45(14):3740-7. doi: 10.1016/j.molimm.2008.05.026. Epub 2008 Jul 14. PMID: 18621419; PMCID: PMC2593650.
- Boehncke WH, Loeliger C, Kuehnl P, Kalbacher H, Böhm BO, Gall H. Identification of HLA-DR and -DQ alleles conferring susceptibility to pollen allergy and pollen associated food allergy. Clin Exp Allergy. 1998 Apr;28(4):434-41. doi: 10.1046/j.1365-2222.1998.00246.x. PMID: 9641569.
- Movahedi M, Moin M, Gharagozlou M, Aghamohammadi A, Dianat S, Moradi B, Nicknam MH, Nikbin B, Amirzargar A. Association of HLA class II alleles with childhood asthma and Total IgE levels. Iran J Allergy Asthma Immunol. 2008 Dec;7(4):215-20. PMID: 19052351.
- Blanco C, Sánchez-García F, Torres-Galván MJ, Dumpierrez AG, Almeida L, Figueroa J, Ortega N, Castillo R, Gallego MD, Carrillo T. Genetic basis of the latex-fruit syndrome: association with HLA class II alleles in a Spanish population. J Allergy Clin Immunol. 2004 Nov;114(5):1070-6. doi: 10.1016/j.jaci.2004.06.022. PMID: 15536412.
- Madore AM, Vaillancourt VT, Asai Y, Alizadehfar R, Ben-Shoshan M, Michel DL, Kozyrskyj AL, Becker A, Chan-Yeung M, Clarke AE, Hull P, Daley D, Sandford AJ, Laprise C. HLA-DQB1*02 and DQB1*06:03P are associated with peanut allergy. Eur J Hum Genet. 2013 Oct;21(10):1181-4. doi: 10.1038/ejhg.2013.13. Epub 2013 Feb 27. PMID: 23443026; PMCID: PMC3778350.
- Dimitrov I, Doytchinova I. Associations between Milk and Egg Allergens and the HLA-DRB1/DQ Polymorphism: A Bioinformatics Approach. Int Arch Allergy Immunol. 2016;169(1):33-9. doi: 10.1159/000444172. Epub 2016 Mar 9. PMID: 26953725.
- Saetang J, Tipmanee V, Benjakul S. in silico Prediction of Cross-Reactive Epitopes of Tropomyosin from Shrimp and Other Arthropods Involved in Allergy. Molecules. 2022 Apr 21;27(9):2667. doi: 10.3390/molecules27092667. PMID: 35566021; PMCID: PMC9104922.
- Noguchi E, Akiyama M, Yagami A, Hirota T, Okada Y, Kato Z, Kishikawa R, Fukutomi Y, Hide M, Morita E, Aihara M, Hiragun M, Chinuki Y, Okabe T, Ito A, Adachi A, Fukunaga A, Kubota Y, Aoki T, Aoki Y, Nishioka K, Adachi T, Kanazawa N, Miyazawa H, Sakai H, Kozuka T, Kitamura H, Hashizume H, Kanegane C, Masuda K, Sugiyama K, Tokuda R, Furuta J, Higashimoto I, Kato A, Seishima M, Tajiri A, Tomura A, Taniguchi H, Kojima H, Tanaka H, Sakai A, Morii W, Nakamura M, Kamatani Y, Takahashi A, Kubo M, Tamari M, Saito H, Matsunaga K. HLA-DQ and RBFOX1 as susceptibility genes for an outbreak of hydrolyzed wheat allergy. J Allergy Clin Immunol. 2019 Nov;144(5):1354-1363. doi: 10.1016/j.jaci.2019.06.034. Epub 2019 Jul 10. PMID: 31301374.
- Kostara M, Chondrou V, Sgourou A, Douros K, Tsabouri S. HLA Polymorphisms and Food Allergy Predisposition. J Pediatr Genet. 2020 Jun;9(2):77-86. doi: 10.1055/s-0040-1708521. Epub 2020 Apr 1. PMID: 32341809; PMCID: PMC7183399.
- Power TD, Ivanciuc O, Schein CH, Braun W. Assessment of 3D models for allergen research. Proteins. 2013 Apr;81(4):545-54. doi: 10.1002/prot.24239. Epub 2013 Jan 15. PMID: 23239464; PMCID: PMC3593753.
- Roche DB, Buenavista MT, Tetchner SJ, McGuffin LJ. The IntFOLD server: an integrated web resource for protein fold recognition, 3D model quality assessment, intrinsic disorder prediction, domain prediction and ligand binding site prediction. Nucleic Acids Res. 2011 Jul;39(Web Server issue):W171-6. doi: 10.1093/nar/gkr184. Epub 2011 Mar 31. PMID: 21459847; PMCID: PMC3125722.
- Omar S, Mohd Tap F, Shameli K, Rasit Ali R, Che Jusoh N, Ahmad Khairudin NB. Sequence Analysis and comparative modelling of nucleocapsid protein from Pseudomonas stutzeri. IOP Conference Series: Materials Science and Engineering. 2018; 458:012025. https://doi.org/10.1088/1757-899x/458/1/012025
- Eisenberg D, Lüthy R, Bowie JU. VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997;277:396-404. doi: 10.1016/s0076-6879(97)77022-8. PMID: 9379925.
- Bonvin Lab. (nd). Homology modeling of the mouse MDM2 protein. Bonvin Lab. https://www.bonvinlab.org/education/molmod_online/modelling/#:~:text=GMQE%20(Global%20Model%20Quality%20Estimation,Higher%20numbers%20indicate%20higher%20reliability.
- Mora Lagares L, Minovski N, Caballero Alfonso AY, Benfenati E, Wellens S, Culot M, Gosselet F, Novič M. Homology Modeling of the Human P-glycoprotein (ABCB1) and Insights into Ligand Binding through Molecular Docking Studies. Int J Mol Sci. 2020 Jun 5;21(11):4058. doi: 10.3390/ijms21114058. PMID: 32517082; PMCID: PMC7312539.
- Messaoudi A, Belguith H, Ben Hamida J. Homology modeling and virtual screening approaches to identify potent inhibitors of VEB-1 β-lactamase. Theor Biol Med Model. 2013 Apr 2;10:22. doi: 10.1186/1742-4682-10-22. PMID: 23547944; PMCID: PMC3668210.
- Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010 Aug;11(8):674-80. doi: 10.1038/ni.1899. Epub 2010 Jul 20. PMID: 20644573; PMCID: PMC3249647.
- Aalberse RC, Stadler BM. in silico predictability of allergenicity: from amino acid sequence via 3-D structure to allergenicity. Mol Nutr Food Res. 2006 Jul;50(7):625-7. doi: 10.1002/mnfr.200500270. PMID: 16764015.
- Bonds RS, Midoro-Horiuti T, Goldblum R. A structural basis for food allergy: the role of cross-reactivity. Curr Opin Allergy Clin Immunol. 2008 Feb;8(1):82-6. doi: 10.1097/ACI.0b013e3282f4177e. PMID: 18188023.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
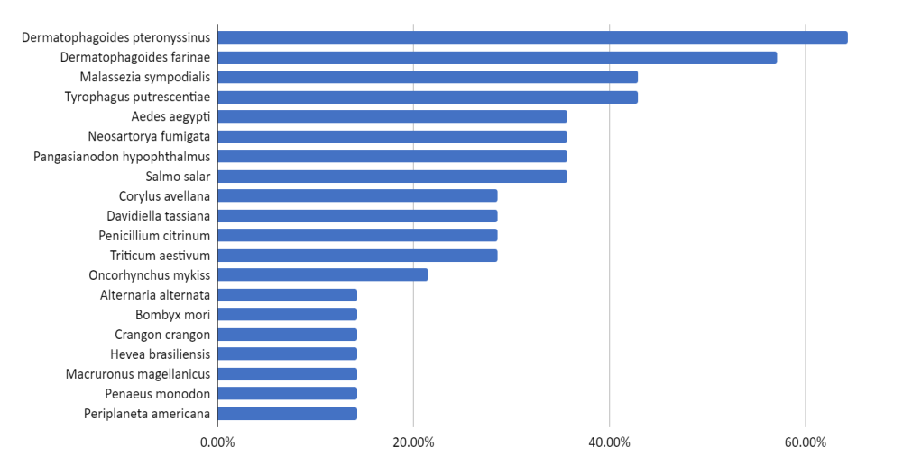
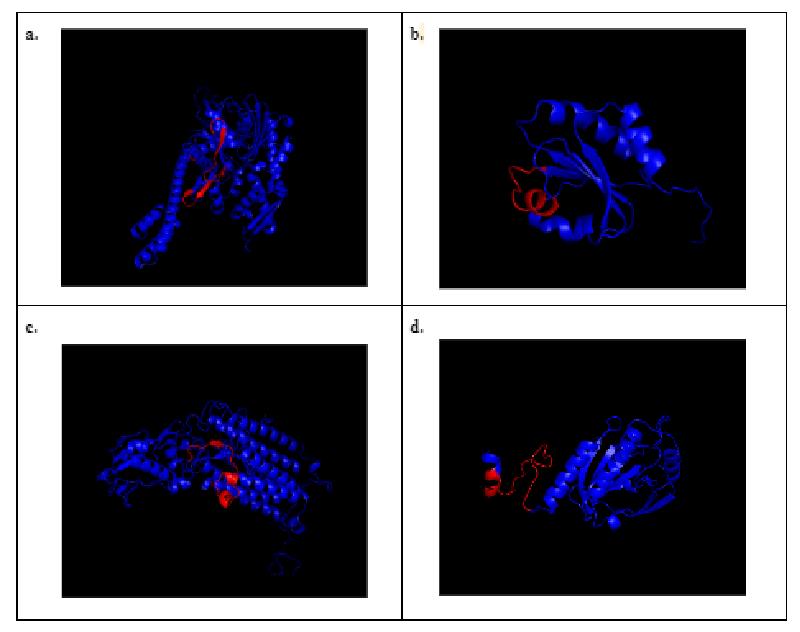
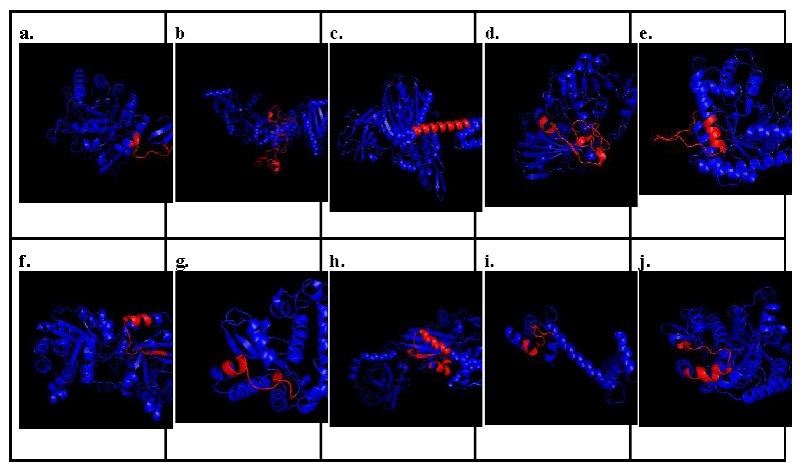


 Save to Mendeley
Save to Mendeley
