Open Journal of Bioinformatics and Biostatistics
PCR Primer Design for in-silico Rapid Detection of Ocular Infection Caused by Candida Species in Humans
Ayesha Kanwal1, Muhammad Rizwan Javed1*, Shinawar Waseem Ali2*, Kishver Tusleem3 and Muhammad Tahir ul Qamar4
2Institute of Agricultural Sciences, University of the Punjab, Quaid-i-Azam Campus, Lahore-54590, Pakistan
3Fatima Jinnah Medical University, Lahore-54000, Pakistan
4College of Informatics, Huazhong Agricultural University, Wuhan-430070, P. R. China
Shinawar Waseem Ali, Institute of Agricultural Sciences, University of the Punjab, Quaid-i-Azam Campus, Lahore-54590, Pakistan, E-Mail: [email protected]
Cite this as
Kanwal A, Javed MR, Ali SW, Tusleem K, ul Qamar MT (2017) PCR Primer Design for in-silico Rapid Detection of Ocular Infection Caused by Candida Species in Humans. Open J Bioinform Biostat 1(1): 004-009. DOI: 10.17352/ojbb.000002Background: Computational analyses have shown great potentials for providing tools for the rapid detection and identification of fungi for medical, scientific and commercial purposes. Various bioinformatics tools have been developed for finding the specific regions within the ribosomal RNA (rRNA) gene complex. Candidais a genus of yeast that includes about 150 different species and is the most common cause of human ocular infections. In the present study, rapid detection method of Candida, based on specific regions (18S, 5.8S and 28S) of ribosomal RNA (rRNA) genes of eight (8) species e.g. C. albicans, C. krusei, C. parapsilosis, C. glabrata, C. guilliermondii, C. kefyr, C. lusitaniae and C. tropicalis has been developed. Rapid diagnosis and early identification of causative agent through computational based methods with high accuracy will result in effective treatment.
Objective: Development of rapid detection method and assay for Candidaspecies based on bioinformatics tools.
Methodology: Ribosomal RNA (18S, 5.8S and 28S) sequences of eight Candidaspecies were retrieved from GenBank/EMBL databases. A set of unique primers were designed based on the conserved region in the given yeast species. To verify the in-silico specificity of the designed primers, the NCBI-BLAST program was employed to search the primers in short, near exact sequences. The primers were further analyzed by the AmplifX tool to determine their specificity and sensitivity against Candidaspecies.
Conclusions: The study resulted in the development of rapid and reproducible detection strategy of Candidaspecies on the basis of computational PCR that will be very helpful for the doctors/practitioners to prescribe targeted medicine against Candidaand related causative agents.
Introduction
Yeasts are the microorganisms commonly found in nature [1], among them Candidais famous genera containing a wide range of species and sub species. Although among Candidaspecies, few are harmless endosymbionts for hosts such as humans. However, many species that are otherwise harmless but if present in improper place can cause disorders. Out of about 200 species of Candida; C. albicans, C. tropicalis, C. glabrata, C. krusei, C. parapsilosis, C. dubliniensis, C. kefyr and C. lusitaniae are known to cause most human ocular infections [2]. A warm, moist climate and a rural agricultural environment may influence the sensitivity of healthy eyes to fungi and fungal infections [3].
To detect fungal species that can cause infections, specific computational polymerase chain reaction was developed that was effective and enabled scientists to know the root cause of fungal eye infections. Conserved regions of 18S ribosomal RNA genes were used to design specific primers to amplify the targeted regions of desired fungi, ultimately to diagnose Candidaand infections developed by Candida. Because effective treatment of any disease can be done only when we know the root cause of disease and we are able to identify and detect the disease causing agents. In this sense computational polymerase chain reaction is more effective way for detection other than conventional microbiological techniques. Because in computational polymerase chain reaction, time saving is main advantage and accuracy of results is more than other techniques [4,5]. Genome of many Candidaspecies is being sequenced, so polymerase chain reaction can specify them by using specific probes with 100% efficacy, sensitivity and specificity. Genome includes ribosomal RNA in this section for development of polymerase chain reaction methods to detect human fungal pathogens by focusing on 18S ribosomal RNA genes, 5.8S and the 5’ end of 28S RNA gene in most of the studies conducted [6-8].
Due to many problems in traditional diagnosis methods for detection of fungal systematics and fungal infections, now it has become very necessary to develop rapid detection methods that should be specific and sensitive [9]. The manual assortment of optimum PCR oligonucleotide primer sets can be quite dull and thus offers itself very naturally for computational analysis. The basic cause which can affect function of the oligonucleotides and their melting temperatures as well as possible homology among primers are well defined and straightforward tasks that are easily encoded in computer software. Software provides a minimum number of candidate set of primers, so that the primers can be easily selected with the help of softwares. Scientists are taking benefits of accurate computed calculations and using all the versions of primer’s placements, length, co-relation with other primers to find out efficient one that meet all the conditions given by the user. Among a wide range of primer pairs examined by computational methods, software can select only those that are appropriate for the experiment. So, by this method over all excellent quality primers can be selected [10,11]. Hundreds of programs have been designed to select and make primer’s sets having variations in specifications. Primers are also available commercially and primer designing software are also available that provides enhanced efficacy in results [12].
Materials and Methods
Retrieval of nucleotide sequences and their alignment:
The rRNA (18S, 5.8S and 28S) nucleotide sequences of eight (8) ocular infection causing Candidaspecies; Candidaalbicans, Candidakefyr, Candidatropicalis, Candidaparapsilosis, Candidakrusei, Candidalusitaniae, Candidaglabrata, and Candidaguilliermondii were retrieved from NCBI (www.ncbi.nlm.nih.gov) and there accession No. are listed in the Table 1. The selected sequences were aligned by using ClustalW (www.genome.jp/tools/clustalw/) to determine the conserved regions. The templates of conserved regions (18S, 5.8S & 28S) were predicted with their corresponding Candidaspecies along with sequence and product size ranges from 110-111 and 190-194 bp [13].
Designing of universal primers against conserved regions
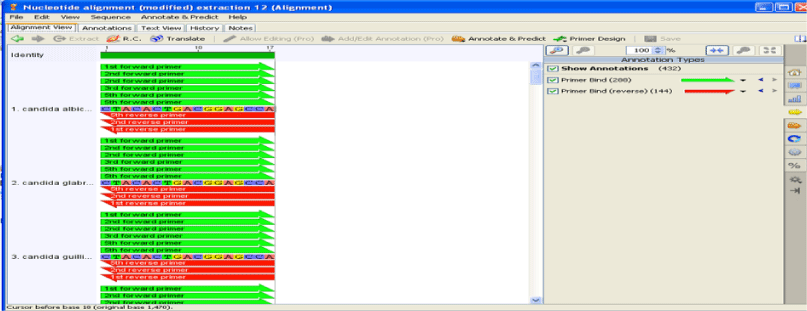
For the sake of designing primers, conserved regions (18S, 5.8S & 28S) were used into the Geneious (version 10.0.9) tool (www.geneious.com/). Two primer sets were designed with the size of 19bp (ACGGGGAAACTCACCAGGTCCA), (TCCCAGCACGACGGAGTTT) and 22bp (GTGATGCCCTTAGACGTTCTGG), (GGGCAGGGACGTAATCAACGCA) respectively.
Primers were then improved and updated primers were then re-analyzed with the help of AmplifX (1.7.0 version) tool (www.amplifx.software.informer.com/1.7/). The modified primers were checked by using parameters such as oligocalc [14] and to make sure that primer have good quality, (Tm (melting temperature), Length of primer, GC content, 3’ end stability, hairpins and Poly X tail parameters were determined.
Analysis and selection of restriction site for Candidaspecies
With the help of NEBcutter (V 2.0) tool (www.nc2.neb.com/NEBcutter2/) the eight Candidaspecies sequences were subjected to restriction digestion using the restriction endonucleases type-II, listed in the REBASE database (www.rebase.neb.com/) that select the enzymes to cut the sequences differently at not more than 5 cleavage sites [15].
Results and Discussion
In newborns, candidal retinitis is the most common intra ocular fungal infection [16,17]. Endogenous candidal chorioretinitis causes pain and decrease in vision due to associated anterior uveitis [18]. The full length sequences of eight Candidaspecies namely C. albicans, C. krusei, C. parapsilosis, C. glabrata, C. guilliermondii, C. kefyr, C. lusitaniae and C. tropicalis were retrieved from NCBI and all these 8 Candidaspecies were then subjected to alignment by using online tool clustalW. The 18S, 5.8S and 28S rRNA nucleotides were chosen as the target regions for this study [19]. Figures 1,2 shows the positions of the primers sequences obtained from “GENEIOUS” software.
Template
The 18S, 5.8S and 28S regions of Candida species were generated with eight templates given in Tables 2,3 respectively.
Template of 18S, 5.8S and 28S rRNA gene
The sequence and size of 18S, 5.8S, and 28S region of template is given below of size ranges from 110 to 111 bp.
Template of 18S, 5.8S and 28S rRNA gene
The sequence and size of 18S, 5.8S and 28S regions of template is given below of size ranges from 190 to 194 bp.
The product sizes of each pair of primers were determined by the help of Candidaprimer annealing map, as listed in (Table 4).
Primer improvement
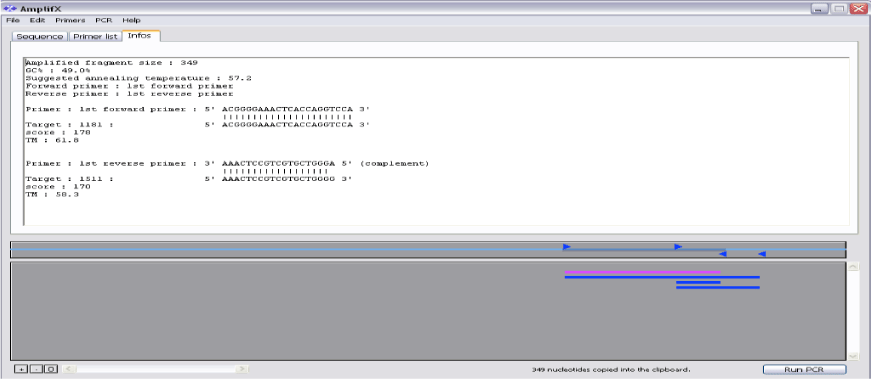
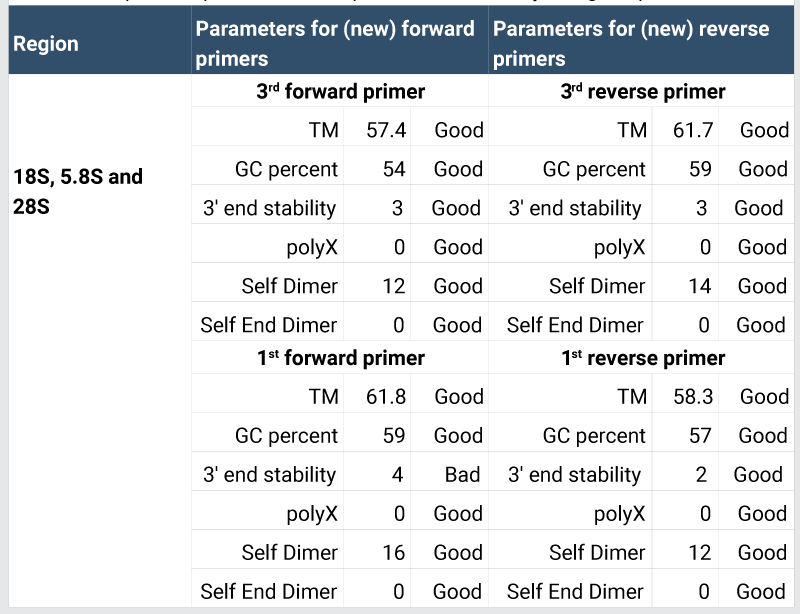
Prior to further process, primers were checked by AmplifX tool as shown in Figure 3. The in-silico primers were designed by using Geneious tool, which is freely available on Just Bio website (www.justbio.com). The results of redesigned primer’s parameters with the help of AmplifX tool are shown in Table 2. These parameters confirmed that new primers were of good quality. Primer amplification efficiencies are given in Table 5 [20,21].
AmplifX was used to seek in a collection of primers, it was used to amplify a fragment into a target sequence. The information was automatically computed by AmplifX (like Tm, Quality, length) associated with each primer.
The selected species were differentiated by using the restriction enzyme digestion of the PCR products. Candidaspeciation would be an important aid to effective patient treatment, facilitating the application of species-specific antifungal therapy, thereby avoiding problems of drug resistance.
For finding the genotype of a particular Candidaspecies and identification of gene, software NEB Cutter was used. This cutter was used for the linear DNA analysis and the restriction enzymes were used to cleave the DNA without need for expensive gene sequencing [22]. In similar manner restriction enzymes were used to digest genomic DNA.
After finalization, the results of each Candidaspecies, the number of restriction sites, nucleotide position of each cut, list of enzymes and specificity of common and unique enzymes were separated manually as shown in Tables 6,7. The enzyme TspRI was found as a common restriction enzyme present in all eight species.
While five enzymes were unique; HinFI, MseI, CviQI, TaqI and BsrDI that would subsequently allow identification of C. glabrata, C. guilliermondii, C. kefyr, C. krusei, C. lusitanae species. These five unique restriction enzymes provide greatest level of species discrimination.
Identification Strategy
Conclusion
We found that rapid identification of Candida species has become more important because of an increase in ocular infections. An advantage of genotypic identification of Candidaspecies is its rapidity and therefore it will be very helpful for the doctors to detect the specific species and help them to prescribe relevant medicine. Furthermore, traditional methods which were used for the identification of Candida species including morphological and biochemical analysis, and serotyping are based on phenotypic expression, which make them unreliable. Traditional tests are also time consuming. However, computational techniques make identification of Candida species very rapid. In limited medical facilities, the prediction of Candida sp. involved in ocular infection will be a valuable addition of information in the field of medicine.
We are thankful to the members of PCR Laboratory, Pakistan Institute of Nuclear Medicine (PINUM) Cancer Hospital, Faisalabad, Pakistan and Bioprocess Engineering Lab, Department of Bioinformatics and Biotechnology, Government College University Faisalabad, Pakistan for their valuable contributions.
- Manolakaki D, Velmahos G, Kourkoumpetis T, Chang Y, Alam HB, et al. (2010) Candidainfection and colonization among trauma patients. Virulence 1: 367-375. Link: https://goo.gl/f230j3
- Jenkinson HF, Douglas LJ (2002) Interactions between Candidaspecies and bacteria in mixed infections. Interaction between Candidaspecies and Bacteria in mixed infections. Polymicrobial Diseases Link: https://goo.gl/V7LTPV
- Klotz SA, Penn CC, Negvesky GJ, Butrus SI (2000) Fungal and parasitic infections of the eye. Clin micro biol rev 13: 662-685. Link: https://goo.gl/bAqkkj
- Rickerts V, Mousset S, Lambrecht E, Tintelnot K, Schwerdtfeger R, et al. (2007) Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin infect dis 44: 1078-1083. Link: https://goo.gl/7nPPUW
- Russell, GELDER V Laboratory Diagnosis of Ocular Infectious Disease. 4: 1. Link: https://goo.gl/qbicbN
- Iwen PC, Hinrichs SH, Rupp ME (2002) Utilization of the internal transcribed spacer regions as molecular targets to detect and identify human fungal pathogens. Med Mycol 40: 87-109. Link: https://goo.gl/7Cw0ZU
- Gaudio PA, Gopinathan U, Sangwan V, Hughes TE (2002) Polymerase chain reaction based detection of fungi in infected corneas. Br J ophthalmol 86: 755-760. Link: https://goo.gl/xEL707
- Khot PD, Ko DL, Fredricks DN (2009) Sequencing and analysis of fungal rRNA Operons for development of broad-range fungal PCR assays. App Envir Microb 75: 1559-1565. Link: https://goo.gl/9MFHMo
- White PL, Shetty A, Barnes RA (2003) Detection of seven Candidaspecies using the Light-Cycler system. J med microbiol 52: 229-238. Link: https://goo.gl/xWlRB7
- Rychlik W, Rhoads RE (1989) A computer program for choosing optimal oligonudeotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic acids res 17: 8543-8551. Link: https://goo.gl/DriVML
- Lowe T, Sharefkin J, Yang SQ, Dieffenbach CW (1990) A computer program for selection of oligonucleotide primers for polymerase chain reactions. Nucleic Acids Re 18: 1757-1761. Link: https://goo.gl/QrnD8X
- Dieffenbach CW, Lowe TMJ, Dveksler GS (1993) General Concepts for PCR Primer Designdata compiled. In 2011 Publication by Cold Spring Harbor Laboratory Press. Supp 3: 30-37. Link: https://goo.gl/h21MS2
- Madico G, Quinn TC, Boman J, Gaydos CA (2000) Touchdown enzyme time release-PCR for detection and identification of Chlamydia trachomatis, C. pneumoniae, and C. psittaci using the 16S and 16S-23S spacer rRNA genes. J Clin Microbiol 38: 1085-1093. Link: https://goo.gl/LK9NIw
- Kibbe WA (2007) OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res 35: W43–W46. Link: https://goo.gl/bwuwOd
- Romi W, Keisam S, Ahmed G, Jeyaram K (2014) Reliable differentiation of Meyerozymaguilliermondii from Meyerozymacaribbica by internal transcribed spacer restriction fingerprinting. BMC Microbiol 14: 52. Link: https://goo.gl/e5jqU0
- Palmer EA (1980) Endogenous Candidaendophthalmitis in infants. Am J Ophthalmol 89: 388-395. Link: https://goo.gl/4gmI6T
- Baley JE, Annable WL, Kliegman RM (1981) Candidaendophthalmitis in the premature infant. Journal of pediatr 98: 458-461. Link: https://goo.gl/Qo1D03
- Ahuja Y, Couch SM, Razonable RR, Bakri SJ (2008) Infectious retinitis. Ret Phys. Link: https://goo.gl/KBXNlC
- Williams DW, Wilson MJ, Lewis MA, Potts AJ (1995) Identification of Candidaspecies by PCR and restriction fragment length polymorphism analysis of intergenic spacer regions of ribosomal DNA. J clin microbiol 33: 2476-2479. Link: https://goo.gl/2oOtPk
- Thomas MC, Thomas DK, Selinger LB, Inglis GD (2011) spyder, a new method for in silico design and assessment of 16S rRNA gene primers for molecular microbial ecology. FEMS Microbiol Letter 320: 152–159. Link: https://goo.gl/tJSeYf
- Culley TM, Stamper TI, Stokes RL, Brzyski JR, Hardiman NA, et al. (2013) An efficient technique for primer development and application that integrates fluorescent labeling and multiplex PCR. App Plant Sci 1: 1300027. Link: https://goo.gl/dmMaOo
- Rasmussen HB (2012) Restriction fragment length polymorphism analysis of PCR-amplified fragments (PCR-RFLP) and gel electrophoresis–valuable tool for genotyping and genetic fingerprinting. In Tech. Link: https://goo.gl/yMwAFj
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
