Journal of Clinical Microbiology and Biochemical Technology
Unveiling the connections: Chlorpyrifos and its association with breast cancer
Eliana Estrella Akselrad1, María de la Cabeza Fernández2, Paula Moyano3 and María Victoria Naval1*
2Department of Chemistry and Pharmaceutical Sciences, Pharmacy School, Complutense University of Madrid, 28041 Madrid, Spain
3Department of Pharmacology and Toxicology, Veterinary School, Complutense University of Madrid, 28040-Madrid, Spain
María Victoria Naval, Department of Pharmacology, Pharmacognosy and Bothanic, Pharmacy School, Complutense University of Madrid, 28041 Madrid, Spain, Tel: +34913942276; Fax: + 34 91 394 17 69; E-mail: +34913942276; E-mail: [email protected]
Cite this as
Akselrad EE, Cabeza Fernández MDL, Moyano P, Naval MV (2023) Unveiling the connections: Chlorpyrifos and its association with breast cancer. J Clin Microbiol Biochem Technol 9(1): 022-029. DOI: 10.17352/jcmbt.000055Copyright
© 2023 Akselrad EE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Chlorpyrifos, a broad-spectrum insecticide categorized within the organophosphate family, is recognized for its potent inhibition of the enzyme Acetylcholinesterase (AChE), resulting in the manifestation of cholinergic syndrome in humans. Beyond its well-established toxicity in the central nervous system, recent studies have explored additional pathways through which this pesticide may adversely impact human health.
Breast cancer, characterized by the uncontrolled proliferation of epithelial cells in the mammary gland, stands as the most diagnosed cancer in women and is a leading global cause of female cancer-related deaths.
Chlorpyrifos, extensively employed worldwide for pest control in agriculture, domestic settings, and industries, has notably faced recent bans in the European Union, marking a significant regulatory shift. This bibliographical review aims to unravel the intricate mechanisms by which chlorpyrifos may contribute to the development of breast cancer.
Collating findings from human studies, as well as in vitro and in vivo research spanning the past decade, the review sheds light on chlorpyrifos as a potent endocrine disruptor. It influences female sex hormones, exhibits estrogenic effects on breast cancer cells, and induces alterations in breast tissue. Additionally, chlorpyrifos acts as an agonist of Estrogen Receptor α(ERα) and Aryl hydrocarbon Receptor (AhR), contributing to cell proliferation, oxidative stress, and engaging epigenetic and angiogenic mechanisms.
This comprehensive review underscores the compelling association between chlorpyrifos exposure and mammary cancer. It emphasizes the urgent need for further research on pesticide usage to mitigate potential adverse health consequences.
Introduction
Chlorpyrifos is globally used as a broad-spectrum pesticide, both for industrial and household, as well as agricultural purposes. In households, it is employed for controlling cockroaches, fleas, and termites. It is also present in anti-flea and anti-tick collars for pets and is utilized in livestock to combat ticks. In crop cultivation, it is applied directly to the soil or leaves through sprayings [1].
For instance, it is effective against pests such as aphids, beetles, caterpillars, cicadas, and scale insects, among others, and is applied in crops like citrus fruits, nuts, cereals such as corn and wheat, and various fruits and vegetables [2]. Exposure to chlorpyrifos can occur directly through its handling or indirectly through the fumigation of crops, food items, or objects containing residues, or through contaminated drinking water [3].
In recent years, the regulation of chlorpyrifos in the European Union has changed. At the end of 2019, during the meeting of the Standing Committee on Plants, Animals, Food, and Feed (PAFF Committee), Member States voted on two draft Implementation Regulations proposing not to renew approvals for chlorpyrifos. In July 2020, a Regulation was approved, reducing Maximum Residue Limits (MRLs) in different food products to 0.01 mg/kg [4]. Although the use of chlorpyrifos is now banned in the European Union, it cannot be ruled out that it may still be imported or used in other countries. Therefore, recently, on April 7, 2021, the decision was made by the Council of the European Union to include this plant protection compound in Annex A of the Stockholm.
Convention on Persistent Organic Pollutants for elimination, both in production and use [5].
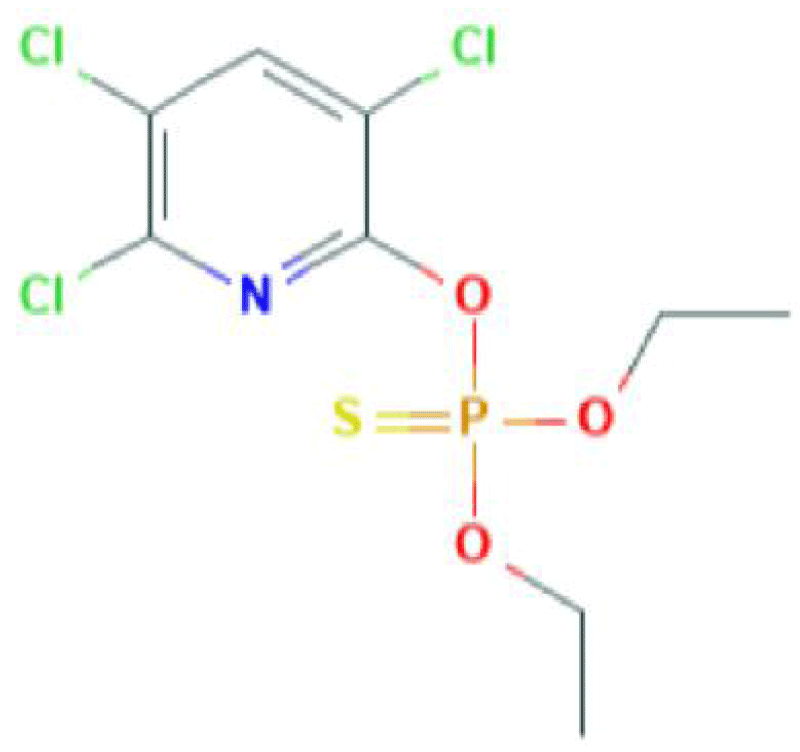
Chlorpyrifos, whose IUPAC name is O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate (Figure 1), is an insecticide belonging to the organophosphorus family. It appears as a crystalline solid, ranging in color from white to colorless, with a strong odor. It is soluble in organic solvents and insoluble in water. Chlorpyrifos is the active ingredient in a large number of commercially available products, with Dursban® and Lorsban® being among the most well-known. The mechanism of action of chlorpyrifos begins with oral, inhalation, or dermal exposure. By the first metabolic pathway, this compound is bioactivated in the liver via cytochrome P450 by a chlorpyrifos-oxon is hydrolyzed by type A esterases, giving rise to the compounds Diethylphosphate (DEP) and 3,5,6-Trichloropyridinol (TCP). On the other hand, by the second metabolic pathway, chlorpyrifos by dearylation, also mediated by cytochrome P450, forms the compounds Diethylthiophosphate (DETP) and TCP. TCP is the dominant non-toxic metabolite of chlorpyrifos in humans and is therefore used in urine as a biomarker after exposure to this insecticide [6]. The risk of toxicity may be modified depending on the cytochrome P450 isoform that metabolizes chlorpyrifos [7].
Chlorpyrifos is recognized for its potent inhibition of the enzyme Acetylcholinesterase (AChE), resulting in the manifestation of cholinergic syndrome in humans. Beyond its well-established toxicity in the central nervous system, recent studies have explored additional pathways through which this pesticide may adversely impact human health. Chlorpyrifos has been detected in local air monitoring at levels that raise concerns for residents living in the agricultural regions where it is used [8].
The Aryl Hydrocarbon Receptor (AHR) is a ligand-activated transcription factor that influences tumor growth. It can affect both the cancerous characteristics of tumor cells and the immune system’s response to them. AHR can make tumors grow faster, but it can also slow them down depending on the kind and stage of the tumor. The aryl hydrocarbon receptor (AhR) interacts with different types of ligands that range from environmental pollutants such as chlorpyrifos to AhR-responsive drugs and beneficial plant compounds [9].
Breast cancer is the uncontrolled proliferation of epithelial cells that line the ducts or lobules of the mammary gland [10]. It is the most diagnosed cancer in women, accounting for 24% of all female cancers, and is the leading cause of female cancer-related deaths worldwide [11].
Endocrine-disrupting chemicals are compounds that can mimic or modulate hormones in the endocrine systems of humans and animals. The natural estrogens include Estrone (E1) and 17β-Estradiol (E2), which are either produced endogenously by animals or used as pharmaceutical products in both human and veterinary medicine. Diethylstilbestrol (DES) is a synthetic nonsteroidal estrogen that has been widely used not only in livestock production to promote growth but also as a treatment for estrogen deficiency [12].
This bibliographical review aims to unravel the intricate mechanisms by which chlorpyrifos may contribute to the development of breast cancer.
Methodology
A bibliographic review was made of articles from the last 10 years, in English and Spanish, from high-impact index journals, in Google Scholar, PubMed, Scielo, the Complutense Library Cisne Catalog, and Eur-Lex.
Fifty-eight articles were selected according to the reading of their abstracts and the language of publication (English and Spanish). Once the abstracts were read, the original articles were searched and finally, 40 articles were used according to the criteria of relevance and length. Among them, we found clinical cases (cohorts and case controls), in vitro and in vivo studies, reviews, and legislation.
The keywords used were: “Clorpirifos”, “Chlorpyrifos”, “Chlorpyrifos AND Breast Cancer”, “Chlorpyrifos AND Cancer”, “Chlorpyrifos AND Carcinogenesis”, “Endocrine Disruptor”, “Organophosphate”, “Aryl hydrocarbon receptor”, “Estrogen”.
Significant clinical research
A. Studies in vitro
Chlorpyrifos (CPF) acts as an endocrine disruptor, inducing proliferation in breast cancer cells. Chlorpyrifos interferes with the natural hormonal response by binding to sex hormones [12] and acting as an estrogen α-receptor agonist [13,14]. In addition, this organophosphate promotes cell proliferation [13,14] or decreases it [13-15] causing cell cycle arrest in the S and G2/M phases [13] and increasing oxidative stress [16,17].
As we have previously advanced, endocrine-disrupting chemicals are compounds that can mimic or modulate hormones in the endocrine systems of humans and animals. Endocrine disruptors can activate or inhibit Estrogen Receptors (ER) or Androgen Receptors (AR) directly. They can also stimulate the Aryl Hydrocarbon Receptor (AhR) pathway, which produces an anti-estrogenic effect without direct action on the ER receptor. The binding of chemical substances to the above-mentioned receptors test their ability as endocrine disruptors [18].
Recent prospective studies have provided strong evidence that high serum levels of estrogens, low levels of sex hormone-binding globulin, and, hence, high circulating levels of free steroid sex hormones increase the risk of developing breast cancer in postmenopausal women. Animal studies show that injections of some pesticides can stimulate the development of breast cancers in male mice normally resistant to breast cancer. In a study, the interaction of chlorpyrifos with three sex hormones was studied in vitro using HS-SPME to measure the freely available concentration of pesticide and the lowering of the concentration due to binding to hormones. The results confirmed a high cohesion between chlorpyrifos and the hormones estrone and 17-βestradiol, whereas significantly less binding was found with the hormone diethylstilbestrol [12]. As expected, 17-β estradiol promotes cell proliferation in the MCF-7 line as it is estrogen-dependent. In contrast, the 1000 nM concentration of this hormone significantly decreased the viability of the MDAMB-231 and MCF-10A cell line, as the latter cell line is also not estrogen-dependent and are normal mammary epithelial cell. Similarly, in another study [15], no effect was associated with these last two cell lines in contact with chlorpyrifos. However, alterations were observed in MCF-7 cells. They found that at the 10µM concentration of the pesticide cell viability was reduced by 37%, although these results were not statistically significant. Therefore, they deduced that estrogen receptors played an important role in these results.
According to several studies, the organophosphates chlorpyrifos and glyphosate, as well as the neonicotinoid imidacloprid, are among the most widely used pesticides that can alter mammary gland development. Chlorpyrifos may act as a weak estrogenic compound affecting ER expression, also displaying anti-androgenic, thyroid, and AhR agonistic activities. In in vitro studies, chlorpyrifos induced cell proliferation in MCF-7 and MDA-MB-231 human breast cell lines by a mechanism involving ER activation, at least in ER-responsive cells [19].
Vascular Endothelial Growth Factor-A (VEGF-A), Cyclooxygenase-2 (COX-2), and Nitric Oxide (NO) are associated with angiogenesis. In a study, the action of CPF, a molecule that selectively binds to the aromatic hydrocarbon receptor, was evaluated in angiogenesis in cellular models of breast cancer, using in vivo models with MCF-7 cells. In MCF-7 cells 50 μM CFP induces invasion in cells. In addition, 0.05 μM and 50 μM CPF increases migration in cell lines [20]. At low doses, CPF increased the expression of VEGF-A and COX-2, accompanied by elevated levels of nitric oxide synthases (NOS) and NO release in MCF-7, and at high doses, it intensified the levels of VEGF-A and COX-2 [21].
Cell proliferation is associated with low concentrations of the pesticide (0.05 µM), mediated by the stimulation of ERα [13], AhR, enzymes such as KIAA1363 and CYP1A [14,22], or the PCNA protein. The latter, studied in rats, shows an increase in cell concentration at doses of 1 mg/kg/day (p < 0.01) [23]. At high doses (10 µM to 100 µM), it induces a decrease in cell viability [14] due to the expression of the AChE-S variant [17] and may even halt the cell cycle [13], characterizing chlorpyrifos as cytotoxic at these concentrations. Oxidative stress is also a significant mechanism. At high concentrations of chlorpyrifos (10 µM to 100 µM), the production of ROS is promoted [14,17]. Moyano, et al. [17] also observed that chlorpyrifos-induced cell proliferation at concentrations of 0.1 µM to 1 µM, this increase is significantly greater in the MDAMB- 231 cell line than in MCF-7. However, it decreased cell viability at concentrations of 10 µM and 100 µM. They studied what could be the mechanism causing cell proliferation triggered by 1 µM chlorpyrifos. The results showed that at this concentration of chlorpyrifos, cell development was partially reversed after treatment with the antioxidant N-acetylcysteine or atropine, or after silencing of β-catenin and AChE in both cancer cell lines. Also, they looked at the level of oxidative stress generated by chlorpyrifos. They demonstrated the increase of ROS at concentrations of 10 μM and 100 µM, so there is a decrease in the expression of antioxidant enzymes NRF2 and HO-1. On the contrary, these enzymes increase when the cell lines are exposed to concentrations of 0.1 μM and 1 µM. Next, chlorpyrifos treatment with N-acetylcysteine was studied by reversing the expression of antioxidant enzymes. Thus, it was demonstrated that ROS induces the expression of NRF2 and HO-1. Furthermore, this study [17] shows that the biocide induces the Wnt/ β-catenin signaling pathway at low concentrations (0.1 μM and 1 µM), producing an increase in the expression of β-catenin, c-Myc, and cyclin D1, while decreasing GSK-3β activity. In contrast, at high concentrations (10 μM and 100 µM) these processes reverse.
Inhibition of GSK-3β was seen to increase the expression of NRF2 and HO-1 enzymes. Therefore, it was deduced that the effect on the NRF2 pathway could be through the action of chlorpyrifos on the Wnt/ β-catenin signaling pathway by downregulation of GSK3β. Similarly, it was suggested that the inhibition of GSK-3β could be by the production of ROS. ROS was reported to inactivate PI3K, inducing GSK-3β activity.
Also, the S and R variants of the AChE enzyme were studied. AChE-R was overexpressed at concentrations of 0.1 µM chlorpyrifos, inducing cell proliferation. In contrast, at concentrations of 10 μM and 100 µM, when cell viability decreased, the AChE-S variant was expressed.
Therefore, this recent study demonstrates that chlorpyrifos at low concentrations promotes cell proliferation through the Wnt/ β-catenin pathway, AChE-R expression, and ROS production.
Oxidative stress produced by chlorpyrifos is associated with alterations in the antioxidant defense system. Ventura, et al. [16] concluded that 50 µM chlorpyrifos significantly increased ERK1/2 phosphorylation in both MCF-7 and MDA-MB-231 cell lines and thereby inhibited cell proliferation. Among the ROS, Hydrogen Peroxide (H2O2) was analyzed in this study. To make sure, the cells were grown in the presence and absence of the MEK1 inhibitor PD98059 for 24 hours. They found that the inhibitor could not suppress the increase in ROS. Ultimately, they concluded that chlorpyrifos caused an increase in H2O2, which led to phosphorylation of the ERK1/2 pathway and thus inhibition of cell proliferation in both cancer cell lines.
Chlorpyrifos led to the increased production of Reactive Oxygen Species (ROS) accompanied by a decrease in the level of reduced glutathione [24].
In summary, chlorpyrifos affects intracellular pathways that regulate various cellular processes, including cell proliferation and survival. At the level of the Wnt/β-catenin pathway, it is a signalling pathway that plays a fundamental role in cellular development and homeostasis. Regarding the inhibition of GSK-3β, this inhibition may be linked to the production of Reactive Oxygen Species (ROS) induced by chlorpyrifos.
B. Studies in vivo
CPF induces the proliferation of breast cancer cells both in vivo and in vitro. The main mechanism of the pesticide, according to Ventura, et al. [23], would be through cellular proliferation and alterations in mammary cells, considering it as an endocrine disruptor. The pesticide increases the number of ducts and alveolar structures in the mammary gland of rats that were chronically exposed to low doses, and it raises the incidence of proliferative benign lesions in the mammary gland in these animals. In addition, chlorpyrifos decreases circulating steroid hormones and gonadotropin levels. No significant signs of intoxication were found in rats chronically exposed to chlorpyrifos at 0.01 and 1 mg/kg/day. It alters the morphology of mammary tissue and the hormonal balance in rats, indicating the susceptibility of the gland to this pesticide. The mammary glands of male and female rats and mice have been shown to be susceptible to exposure to various pesticides, including non-organochlorine pesticides such as vinclozolin, atrazine, glyphosate, and chlorpyrifos, as well as organochlorine pesticides including endosulfan, methoxychlor, and hexachlorobenzene. In experimental models, these compounds have demonstrated a range of effects, from alterations in mammary development to changes in cell proliferation, steroid receptor expression, and signaling, and an increase in malignant cellular transformation, tumor development, and angiogenesis. These findings suggest that agrochemical pesticides have the capacity to induce or promote significant changes in the development, differentiation, and possibly malignant transformation of the mammary gland [8].
In a conducted study, two groups were treated with oral doses of CPF in vegetable oil (0.1 and 2.5 mg/kg/day), and the third group received vegetable oil for 8 weeks. Whole mounts of the mammary glands revealed a significant increase (p < 0.05–0.0001) in ductal thickness, number of branches, alveoli, and terminal end buds, as well as the diameter of terminal end buds. The results indicated that subchronic exposure to CPF induces oxidative stress and negative effects on the reproductive organs of rats, suggesting an increase in cancer incidence [25].
Since epigenetics is essential for gene expression, alterations in epigenetic mechanisms leading to breast cancer have been studied in rats [26]. These changes included DNA methylation, in genes such as BRCA1, and histone acetylation and deacetylation, which are very important in gene expression. Histone acetylation is regulated by the enzyme histone acetyltransferase, while histone deacetylation is regulated by Histone Deacetylase Enzymes (HDACs). The results of this study were negative for alterations in DNA methylation after exposure to chlorpyrifos. However, they analyzed histone acetylation in the mammary gland in rats through HDAC1 mRNA levels. These animals treated with chlorpyrifos (0.01 mg/kg/day) had a 55% increase in expression of the enzyme in mammary tissue (p < 0.001), acting on ERα expression by decreasing it. They also examined that there was a decrease in the tumor latency period and an increase in the number of tumors per rat, but no relationship was found between the decrease in the latency period and the increase in tumor growth. They also analyzed the effect of chronic exposure to low doses of chlorpyrifos on CDKN1B and BRCA1 methylation. Both genes are involved in breast cancer, CDKN1B being a gene that regulates cell cycle progression. However, they found that chlorpyrifos does not significantly alter them.
Therefore, in this study they concluded that chlorpyrifos does affect the initiation of mammary tumorogenesis, accelerating the process, but not the subsequent proliferation; they demonstrated an increase in tumor incidence in animals exposed to the pesticide. In addition, it causes an overexpression of HDAC1 in mammary glands in animals leading to decreased ERα expressivity.
Exposure to AhR agonists stimulates pathways that promote the development of breast cancer and may contribute to tumor progression. Given the widespread use of industrial and agricultural chemicals, continuous evaluation of their effects in laboratory assays and preclinical studies in breast cancer at environmentally relevant doses is considered essential [27].
Despite previous evidence designating it as an endocrine disruptor, it is imperative to underscore that Chlorpyrifos (CPF) remains extensively utilized in diverse nations. A recent investigation meticulously assessed the thyroid-disrupting proclivities of CPF through a regimen of low-dose oral exposure administered to female rats, subdivided into groups (n = 8/group). These groups were subjected to oral gavage treatments with a vehicle (control) and varying concentrations of chlorpyrifos (0.01, 0.1, 1, and 10 mg/kg) over a span of 5 days. Comprehensive blood analyses were conducted to meticulously scrutinize thyroid hormones, hepatic enzymes, bilirubin levels, and estradiol concentrations. The findings of this study culminated in a noteworthy escalation in total Triiodothyronine (T3) levels, even at infinitesimal doses, thereby emphasizing the thyroid-disrupting propensities of CPF at remarkably low concentrations. Of particular note, the highest administered dose elicited considerable morphological alterations in the thyroid gland. This observation underscores that brief exposure to CPF at low concentrations precipitates consequential disruptions in thyroid function in female rats [28].
Another study compared the toxicological effects of chlorpyrifos and other pesticides at real exposure concentrations occurring in children, as detected in urine samples in biomonitoring studies, in two human mammary gland cell lines (MCF-7 and MCF-12A). To assess the toxicological effects, a battery of tests was performed, including assessment of cell vitality and proliferation, apoptosis and necrosis, reactive oxygen species (ROS) and ATP intracellular amount, 17β-estradiol secretion, and gene expression of nuclear receptors involved in mammary gland development [19].
Chlorpyrifos acts as an endocrine disruptor, inducing cellular proliferation in vivo and in vitro models. Chronic exposure to low doses alters the morphology of mammary cells and decreases steroid hormones. Additionally, it increases the incidence of benign lesions. Subchronic exposure to Chlorpyrifos in rats results in significant changes in mammary glands, indicating oxidative stress and negative effects on reproductive organs. This suggests a potential increase in breast cancer incidence. Another confirmed aspect from various studies is that Chlorpyrifos affects epigenetic processes, such as HDAC1 expression, accelerating the initiation of mammary tumors and reducing ERα expression. These findings suggest a significant impact on the development and malignant transformation of mammary glands.
C. Studies in humans
Studies in humans demonstrate that women exposed to the pesticide are more likely (OR = 3.22, 95% CI = 1.38-7.53 [29], HR = 1.4, 95% CI: 1.0-2.0 [4], and RR = 2.26, 95% CI: 1.07-4.75 [30]) to develop breast carcinoma compared to those who are not exposed. Specifically, it would increase the risk of cancer in premenopausal women (HR = 1.9; 95% CI: 1.0-3.8 [31]. Tumor incidence would also increase in rats after exposure to the organophosphate [26]. The increase of the TCP metabolite in urine (p < 0.01) after spraying with chlorpyrifos would demonstrate in a human study that the insecticide enters the human body, remaining in it for several days [32].
Following the literature search, four human studies of interest were found. In 2016, Wang, et al. [32] published a case-control study with the aim to determine the concentration of chlorpyrifos metabolites in urine in the adult Chinese population and to evaluate the effects after spraying of this insecticide. A total of 120 urine samples were collected from 20 farmers, including both sexes, three days before spraying their crops and three days after spraying. At the same time, 45 urinary samples were collected from 15 adults, also male and female, living 25 km from the crop fields for three days. Urine creatinine was used to adjust the chlorpyrifos concentration and thus, the results were obtained through the geometric mean creatinine concentration. It was concluded that farmers significantly (p < 0.01) increased urinary TCP metabolite levels three days after spraying with chlorpyrifos compared to adults living in urban areas. However, no significant urinary TCP results were found between controls and cases before spraying the fields with the pesticide. In addition, in this study, the biomarker 8-OHdG of increased DNA oxidative stress in urine was found in farmers after spraying chlorpyrifos on crops.
In 2019, a study [33] investigated the relationship between pesticide exposure and the occurrence of breast cancer through a case-control study in the cities of Fresno, Tulare, and Kern in the state of California, USA. Cases (n = 155) were recruited through the Central California Cancer Registry and controls (n = 150) were taken from a database of another previous study living in the same geographic area. The results they obtained were that breast cancer was three times more likely among women exposed to chlorpyrifos compared to those who were not (OR = 3.22; 95% CI = 1.38-7.53).
From a prospective cohort study in the states of Iowa and North Carolina in the United States, the Agricultural Health Study [29] gave way to two new studies. Among them is the one conducted by Engel, et al. [4] in 2017, where he observed among exposed women (n = 30,594), slightly significant associations between breast cancer risk and chlorpyrifos insecticide use (HR = 1.4; CI95%:1.0-2.0). In the other study [34], significant associations between an increased risk of estrogen and progesterone receptor-negative breast cancer were found (RR = 2.26; CI95%: 1.07-4.75). But, the results among postmenopausal women were nonsignificant (RR = 1.53; CI95%: 0.96- 2.44) for breast cancer risk and chlorpyrifos exposure.
A longitudinal prospective study examined the association between prenatal exposure to chlorpyrifos and deficits in inhibitory control in children. Measurements of prenatal exposure to chlorpyrifos and assessments of inhibitory control were conducted in a cohort of children. The results indicated a significant association between prenatal exposure to chlorpyrifos and deficits in inhibitory control, suggesting potential neurotoxic effects [35].
In summary, various studies in humans demonstrate that women exposed to the pesticide chlorpyrifos have a higher likelihood of developing breast carcinoma, with risk rates (OR) ranging between 2.26 and 3.22. This risk is particularly notable in cases of premenopausal women. Tumor incidence also increases in rats after exposure to the organophosphate. Women exposed to chlorpyrifos had three times higher odds of developing breast cancer compared to those not exposed. There is sufficient evidence to determine a clear relationship with neurotoxic issues related to the use of pesticides Table 1.
Discussion
After pooling all the selected articles, our review has revealed that the mechanisms of action by which chlorpyrifos causes the development of breast cancer may be due to its joint action through several pathways.
Most studies, both in vitro and in vivo, mention that chlorpyrifos acts as a potent endocrine disruptor, altering circulating levels [23] and bioavailability of sex hormones, such as E1 or E2 [12] and acting as an agonist of ERα [13] Ventura [23] shows that there is an increase of mammary ducts in rats when they are exposed to 0.1 mg/kg/day of the pesticide (p < 0.01) and at this same concentration also increases the appearance of lobular adenosis (p < 0.05), while at a higher concentration of 1 mg/kg/day, the rats suffer duct hyperplasia (p < 0.05).
At low concentrations of the pesticide (0.05 µM) cell proliferation is associated with stimulation of ERα [25], AhR, enzymes such as KIAA1363 and CYP1A [26], or PCNA protein. This last protein studied in rats demonstrates cellular increase at concentrations of 1mg/kg/day (p < 0.01) [23]. In addition, it has recently been studied that this cellular increase could be due to the Wnt/ β-catenin signaling pathway that induces the expression of β-catenin, c-Myc, and cyclin D1, and decreases the activity of GSK-3β. The expression of the AChE-R variant would also participate in this proliferative process [17]. However, at high doses (10 µM to 100 µM) it produces a decrease in cell viability [12,15] by the expression of the AChE-S variant [17] and even stopping the cell cycle [13] considering chlorpyrifos as cytotoxic at these concentrations.
Oxidative stress is also an important mechanism. At high concentrations of chlorpyrifos (10 µM to 100 µM) ROS production is promoted [14,17] H2O2 promotes ERK1/2 phosphorylation thereby inhibiting cell proliferation [16]. In addition, the oxidative stress biomarker 8-OHdG was found in the urine after spraying with chlorpyrifos [32],
Other processes such as the epithelial-mesenchymal transition of cells cause a morphological change [20] the increase of VEGF-A, COX2, and nitric oxide factors cause angiogenesis [22] a decrease in the tumor latency period and an increase in tumor promotion [26].
Regarding epigenetic mechanisms, animals treated with chlorpyrifos presented an increase of HDAC1 in mammary tissue (p < 0.001), decreasing the expressivity of ERα [26].
Metabolite levels increase in the urine of farmers after spraying with chlorpyrifos, and this would show that the insecticide enters and remains in the human body for a few days [32]. In addition, studies in humans show that women exposed to the pesticide are more likely to develop mammary carcinoma compared to those who are not exposed to the pesticide [34].
In summary, chlorpyrifos acts as an endocrine disruptor, inducing cellular proliferation in vivo and in vitro models. Chronic exposure to low doses alters the morphology of mammary cells and decreases steroid hormones. Additionally, it increases the incidence of benign lesions. Various studies in humans demonstrate that women exposed to the pesticide chlorpyrifos have a higher likelihood of developing breast carcinoma. These findings suggest a significant impact on the development and malignant transformation of mammary glands.
Conclusion
Chlorpyrifos is a potent endocrine disruptor. This compound induces cell proliferation and oxidative stress, leads to cell cycle arrest and morphological changes in cells, and promotes biomarkers associated with epigenetic and angiogenic mechanisms.
Thanks to the findings from these studies and the collaboration with regulatory agencies, the European Union has taken a significant step by banning this recognized carcinogenic active substance. The next crucial advancement would be to implement international measures for its complete eradication. This underscores the importance of continuously monitoring all types of insecticides to identify those that are least harmful to health.
- Lasagna M, Ventura C, Hielpos MS, Mardirosian MN, Martín G, Miret N, Randi A, Núñez M, Cocca C. Endocrine disruptor chlorpyrifos promotes migration, invasion, and stemness phenotype in 3D cultures of breast cancer cells and induces a wide range of pathways involved in cancer progression. Environ Res. 2022 Mar;204(Pt A):111989. doi: 10.1016/j.envres.2021.111989. Epub 2021 Sep 7. PMID: 34506784.
- Solomon KR, Williams WM, Mackay D, Purdy J, Giddings JM, Giesy JP. Properties and uses of chlorpyrifos in the United States. Rev Environ Contam Toxicol. 2014;231:13-34. doi: 10.1007/978-3-319-03865-0_2. PMID: 24723132.
- Engel LS, Hill DA, Hoppin JA, Lubin JH, Lynch CF, Pierce J, Samanic C, Sandler DP, Blair A, Alavanja MC. Pesticide use and breast cancer risk among farmers' wives in the agricultural health study. Am J Epidemiol. 2005 Jan 15;161(2):121-35. doi: 10.1093/aje/kwi022. PMID: 15632262.
- Commission Regulation (EU) 2020/1085 of 23 July 2020 amending Annexes II and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue limits of chlorpyrifos and chlorpyrifos-methyl.
- Council Decision (EU) 2021/592 of 7 April 2021 on the submission, on behalf of the European Union, of a proposal for the inclusion of chlorpyrifos in Annex A to the Stockholm Convention on persistent organic pollutants.
- Wang L, Liu Z, Zhang J, Wu Y, Sun H. Chlorpyrifos exposure in farmers and urban adults: Metabolic characteristic, exposure estimation, and potential effect of oxidative damage. Environ Res. 2016 Aug;149:164-170. doi: 10.1016/j.envres.2016.05.011. Epub 2016 May 18. PMID: 27208467.
- Croom EL, Wallace AD, Hodgson E. Human variation in CYP-specific chlorpyrifos metabolism. Toxicology. 2010; 276(3):184–91. http://dx.doi.org/10.1016/j.tox.2010.08.005
- Kass L, Gomez AL, Altamirano GA. Relationship between agrochemical compounds and mammary gland development and breast cancer. Mol Cell Endocrinol. 2020 May 15;508:110789. doi: 10.1016/j.mce.2020.110789. Epub 2020 Mar 9. PMID: 32165172.
- Safe S, Han H, Goldsby J, Mohankumar K, Chapkin RS. Aryl Hydrocarbon Receptor (AhR) Ligands as Selective AhR Modulators: Genomic Studies. Curr Opin Toxicol. 2018 Oct-Dec;11-12:10-20. doi: 10.1016/j.cotox.2018.11.005. Epub 2018 Nov 22. PMID: 31453421; PMCID: PMC6709982.
- Ma X, Hu X, Zhu Y, Jin H, Hu G, Ding L, Ning S. Sesamol inhibits proliferation, migration and invasion of triple negative breast cancer via inactivating Wnt/β-catenin signaling. Biochem Pharmacol. 2022 Dec;206:115299. doi: 10.1016/j.bcp.2022.115299. Epub 2022 Oct 13. PMID: 36244446.
- Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, Wang Z, Li W, Geldsetzer P, Bärnighausen T, Bloom DE, Wang C. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023 Apr 1;9(4):465-472. doi: 10.1001/jamaoncol.2022.7826. PMID: 36821107; PMCID: PMC9951101.
- Farhadi K, Tahmasebi R, Biparva P, Maleki R. In vitro study of the binding between chlorpyrfos and sex hormones using headspace solid-phase microextraction combined with high-performance liquid chromatography: A new aspect of pesticides and breast cancer risk. Hum Exp Toxicol. 2015 Aug;34(8):819-27. doi: 10.1177/0960327114559990. Epub 2015 Feb 11. PMID: 25677505.
- Ventura C, Núñez M, Miret N, Martinel Lamas D, Randi A, Venturino A, Rivera E, Cocca C. Differential mechanisms of action are involved in chlorpyrifos effects in estrogen-dependent or -independent breast cancer cells exposed to low or high concentrations of the pesticide. Toxicol Lett. 2012 Sep 3;213(2):184-93. doi: 10.1016/j.toxlet.2012.06.017. Epub 2012 Jul 4. PMID: 22771950.
- Moyano P, García J, García JM, Pelayo A, Muñoz-Calero P, Frejo MT, Anadon MJ, Lobo M, Del Pino J. Chlorpyrifos-induced cell proliferation in human breast cancer cell lines differentially mediated by estrogen and aryl hydrocarbon receptors and KIAA1363 enzyme after 24 h and 14 days exposure. Chemosphere. 2020 Jul;251:126426. doi: 10.1016/j.chemosphere.2020.126426. Epub 2020 Mar 6. PMID: 32171938.
- Rich JD, Gabriel SM, Schultz-Norton JR. In vitro effects of herbicides and insecticides on human breast cells. ISRN Toxicol. 2012 Oct 14;2012:232461. doi: 10.5402/2012/232461. PMID: 23762632; PMCID: PMC3671687.
- Ventura C, Venturino A, Miret N, Randi A, Rivera E, Núñez M, Cocca C. Chlorpyrifos inhibits cell proliferation through ERK1/2 phosphorylation in breast cancer cell lines. Chemosphere. 2015 Feb;120:343-50. doi: 10.1016/j.chemosphere.2014.07.088. Epub 2014 Aug 31. PMID: 25180937.
- Moyano P, García JM, García J, Pelayo A, Muñoz-Calero P, Frejo MT, Anadon MJ, Naval MV, Flores A, Mirat VA, Del Pino J. Chlorpyrifos induces cell proliferation in MCF-7 and MDA-MB-231 cells, through cholinergic and Wnt/β-catenin signaling disruption, AChE-R upregulation and oxidative stress generation after single and repeated treatment. Food Chem Toxicol. 2021 Jun;152:112241. doi: 10.1016/j.fct.2021.112241. Epub 2021 Apr 27. PMID: 33930485.
- Jabłońska-Trypuć A, Wydro U, Wołejko E, Makuła M, Krętowski R, Naumowicz M, Sokołowska G, Serra-Majem L, Cechowska-Pasko M, Łozowicka B, Kaczyński P, Wiater J. Selected Fungicides as Potential EDC Estrogenic Micropollutants in the Environment. Molecules. 2023 Nov 5;28(21):7437. doi: 10.3390/molecules28217437. PMID: 37959855; PMCID: PMC10648374.
- Coppola L, Tait S, Fabbrizi E, Perugini M, La Rocca C. Comparison of the Toxicological Effects of Pesticides in Non-Tumorigenic MCF-12A and Tumorigenic MCF-7 Human Breast Cells. Int J Environ Res Public Health. 2022 Apr 7;19(8):4453. doi: 10.3390/ijerph19084453. PMID: 35457321; PMCID: PMC9030493.
- Lasagna M, Hielpos MS, Ventura C, Mardirosian MN, Martín G, Miret N, Randi A, Núñez M, Cocca C. Chlorpyrifos subthreshold exposure induces epithelial-mesenchymal transition in breast cancer cells. Ecotoxicol Environ Saf. 2020 Dec 1;205:111312. doi: 10.1016/j.ecoenv.2020.111312. Epub 2020 Sep 18. PMID: 32956863.
- Zárate LV, Pontillo CA, Español A, Miret N V., Chiappini F, Cocca C. Angiogenesis signaling in breast cancer models is induced by hexachlorobenzene and chlorpyrifos, pesticide ligands of the aryl hydrocarbon receptor. Toxicol Appl Pharmacol. 2020; 401(January):115093. https://doi.org/10.1016/j.taap.2020.115093
- Wagner C, Hois V, Taschler U, Schupp M, Lass A. KIAA1363-A Multifunctional Enzyme in Xenobiotic Detoxification and Lipid Ester Hydrolysis. Metabolites. 2022 Jun 2;12(6):516. doi: 10.3390/metabo12060516. PMID: 35736449; PMCID: PMC9229287.
- Ventura C, Nieto MR, Bourguignon N, Lux-Lantos V, Rodriguez H, Cao G, Randi A, Cocca C, Núñez M. Pesticide chlorpyrifos acts as an endocrine disruptor in adult rats causing changes in mammary gland and hormonal balance. J Steroid Biochem Mol Biol. 2016 Feb;156:1-9. doi: 10.1016/j.jsbmb.2015.10.010. Epub 2015 Oct 27. PMID: 26518068.
- Hsu SS, Lin YS, Chen HC, Liang WZ. Involvement of oxidative stress-related apoptosis in chlorpyrifos-induced cytotoxicity and the ameliorating potential of the antioxidant vitamin E in human glioblastoma cells. Environ Toxicol. 2023 Sep;38(9):2143-2154. doi: 10.1002/tox.23850. Epub 2023 Jun 7. PMID: 37283489.
- Nishi K, Hundal SS. Chlorpyrifos induced toxicity in reproductive organs of female Wistar rats. Food and Chemical Toxicology. 2013; 62:732–8. http://dx.doi.org/10.1016/j.fct.2013.10.006
- Ventura C, Zappia CD, Lasagna M, Pavicic W, Richard S, Bolzan AD, Monczor F, Núñez M, Cocca C. Effects of the pesticide chlorpyrifos on breast cancer disease. Implication of epigenetic mechanisms. J Steroid Biochem Mol Biol. 2019 Feb;186:96-104. doi: 10.1016/j.jsbmb.2018.09.021. Epub 2018 Oct 2. PMID: 30290214.
- Miret NV, Pontillo CA, Buján S, Chiappini FA, Randi AS. Mechanisms of breast cancer progression induced by environment-polluting aryl hydrocarbon receptor agonists. Biochem Pharmacol. 2023 Oct;216:115773. doi: 10.1016/j.bcp.2023.115773. Epub 2023 Sep 1. PMID: 37659737.
- Otênio JK, Souza KD, Alberton O, Alberton LR, Moreno KGT, Gasparotto Junior A, Palozi RAC, Lourenço ELB, Jacomassi E. Thyroid-disrupting effects of chlorpyrifos in female Wistar rats. Drug Chem Toxicol. 2022 Jan;45(1):387-392. doi: 10.1080/01480545.2019.1701487. Epub 2019 Dec 12. PMID: 31826669.
- Yang KJ, Lee J, Park HL. Organophosphate Pesticide Exposure and Breast Cancer Risk: A Rapid Review of Human, Animal, and Cell-Based Studies. Int J Environ Res Public Health. 2020 Jul 13;17(14):5030. doi: 10.3390/ijerph17145030. PMID: 32668751; PMCID: PMC7399930.
- Kudavidanage EP, Dissanayake DMI, Keerthirathna WLR, Nishshanke NLW, Peiris LDC. Commercial Formulation of Chlorpyrifos Alters Neurological Behaviors and Fertility. Biology (Basel). 2020 Mar 7;9(3):49. doi: 10.3390/biology9030049. PMID: 32156097; PMCID: PMC7150932.
- Engel LS, Werder E, Satagopan J, Blair A, Hoppin JA, Koutros S, Lerro CC, Sandler DP, Alavanja MC, Beane Freeman LE. Insecticide Use and Breast Cancer Risk among Farmers' Wives in the Agricultural Health Study. Environ Health Perspect. 2017 Sep 6;125(9):097002. doi: 10.1289/EHP1295. PMID: 28934092; PMCID: PMC5915194.
- Wang L, Liu Z, Zhang J, Wu Y, Sun H. Chlorpyrifos exposure in farmers and urban adults: Metabolic characteristic, exposure estimation, and potential effect of oxidative damage. Environ Res. 2016 Aug;149:164-170. doi: 10.1016/j.envres.2016.05.011. Epub 2016 May 18. PMID: 27208467.
- Tayour C, Ritz B, Langholz B, Mills PK, Wu A, Wilson JP, Shahabi K, Cockburn M. A case-control study of breast cancer risk and ambient exposure to pesticides. Environ Epidemiol. 2019 Oct 14;3(5):e070. doi: 10.1097/EE9.0000000000000070. PMID: 32166211; PMCID: PMC7028467.
- Lerro CC, Koutros S, Andreotti G, Friesen MC, Alavanja MC, Blair A, Hoppin JA, Sandler DP, Lubin JH, Ma X, Zhang Y, Beane Freeman LE. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup Environ Med. 2015 Oct;72(10):736-44. doi: 10.1136/oemed-2014-102798. Epub 2015 Jul 6. PMID: 26150671; PMCID: PMC4909328.
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011 Aug;119(8):1196-201. doi: 10.1289/ehp.1003160. Epub 2011 Apr 21. PMID: 21507777; PMCID: PMC3237355.
- Islam JY, Hoppin J, Mora AM, Soto-Martinez ME, Gamboa LC, Castañeda JEP, Reich B, Lindh C, van Wendel de Joode B. Respiratory and allergic outcomes among 5-year-old children exposed to pesticides. Thorax. 2023 Jan;78(1):41-49. doi: 10.1136/thoraxjnl-2021-218068. Epub 2022 Feb 24. PMID: 35210357; PMCID: PMC9533533.
- Jean-Pierre M, Michalovicz LT, Kelly KA, O’Callaghan JP, Nathanson L, Klimas N. A pilot reverse virtual screening study suggests toxic exposures caused long-term epigenetic changes in Gulf War Illness. Comput Struct Biotechnol J. 2022; 20:6206–13. https://doi.org/10.1016/j.csbj.2022.11.006
- Lyu R, Lei Y, Zhang C, Li G, Han R, Zou L. An ultra-sensitive photoelectrochemical sensor for chlorpyrifos detection based on a novel BiOI/TiO2 n-n heterojunction. Anal Chim Acta. 2023 Sep 22;1275:341579. doi: 10.1016/j.aca.2023.341579. Epub 2023 Jul 7. PMID: 37524465.
- AlKahtane AA, Ghanem E, Bungau SG, Alarifi S, Ali D, AlBasher G, Alkahtani S, Aleya L, Abdel-Daim MM. Carnosic acid alleviates chlorpyrifos-induced oxidative stress and inflammation in mice cerebral and ocular tissues. Environ Sci Pollut Res Int. 2020 Apr;27(11):11663-11670. doi: 10.1007/s11356-020-07736-1. Epub 2020 Jan 22. PMID: 31965510.
- Mishra AK, Gopesh A, Singh KP. Acute toxic effects of chlorpyrifos on pseudobranchial neurosecretory system, brain regions and locomotory behavior of an air-breathing catfish, Heteropneustes fossilis (Bloch 1794). Drug Chem Toxicol. 2022 Mar;45(2):670-679. doi: 10.1080/01480545.2020.1762631. Epub 2020 May 15. PMID: 32408778.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
