Journal of Clinical Microbiology and Biochemical Technology
Antibiotic sensitivity of catheter-associated bacterial pathogens in pediatrics patients
1Hitec Institute of Medical Sciences, Taxila Cantt Rawalpindi, Pakistan
2University of Health Sciences Lahore, Medicine Lahore, Punjab, Pakistan
3Department of Bioinformatics and Biotechnology, Government College University, 38000 Faisalabad, Pakistan
Author and article information
Cite this as
Nasir MA, Sher F, Saroosh I, Shakir A, Abdullah M, et al. (2023) Antibiotic sensitivity of catheter-associated bacterial pathogens in pediatrics patients. J Clin Microbiol Biochem Technol. 2023; 9(1): 014-021. Available from: 10.17352/jcmbt.000054
Copyright License
© 2023 Nasir MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Antimicrobial-resistant (AMR) pathogens causing Urinary Tract infection is a serious public health concern in our clinical setting. A total of 200 catheter tips were collected from the different wards (medical, surgical, urology) at the Children’s Hospital Faisalabad. Samples were streaked on nutrient agar plates and the positivity of the samples was noted after 24 hours. Positive samples were processed further for the identification of K. pneumoniae, P. aeruginosa, S. aureus, and E. coli using culture identification, microscopy, and biochemical profiling on the basis of culture characterization, microscopy, biochemical profiling, and antibiotic susceptibility testing. 76 (38%) of the samples showed growth on nutrient agar. In processed samples, the high prevalence was marked for P. aeruginosa (24/200; 12%) followed by E. coli (22/200; 11%) and S. aureus (19/200; 9.5%) while 11 K. pneumoniae isolates (5.5%) were identified in this study. In antibiotic susceptibility profiling of P. aeruginosa, the highest susceptibility was found for colistin (100%) and imipenem (70.83%) followed by gentamicin (54.17%) while the highest resistance was found for tobramycin (54.17%) followed by meropenem, ceftazidime, and cefotaxime (50%).
Conclusion: Advance studies are needed to investigate the real investigations of bacterial contamination; resistance to treatment options and resistance to antibiotics are needed.
In children, urinary tract infection (UTI) is the most prevalent bacterial infection, within the first seven years of life affecting 8% & 2% of girls and boys respectively [1]. Abnormalities of urinary tract abnormalities like congenital can cause a high risk of UTI in some children [2]. In 30% of children with CAKUT (congenital anomalies of kidney and urinary tract) are at danger for the development of UTI in children. Unidirectional flow of urine changes due to vesico-ureteral reflux (VUR) [3], while pyelo-ureteral junction obstruction (PUJO) leads to stasis, in which both increase the risk of multiplying pathogenic microorganisms [4]. At the age of 1 month and 11 years, more than 8% of children will experience at least one UTI, and during the first six to 12 months after an initial UTI, more than 30% of kids and newborns experience repetitive infections [5]. The most common etiology of UTIs is due to more than 95% of bacteria. Escherichia coli (E. coli) is the most frequent causative organism of UTIs and it is responsible for more than 80% of UTIs [6]. In males Proteus mirabilis is more frequent than in females while in newborn infants Streptococcus agalactiae is more common, Streptococcus viridians, Haemophilus influenza, Streptococcus pneumonia, Staphylococcus epidermidis, Staphylococcus aureus, and Streptococcus agalactia may be responsible in children with anomalies of the urinary tract (anatomic, neurologic, or functional) or compromised immune system [7]. Only a proper identification of the local pathogen, as well as information on the susceptibility patterns and any related risk factors, can provide appropriate treatment for UTIs [6]. Because of incorrect antibiotic use, the bacterial sensitivity pattern of common pathogens is gradually changing in all countries [8]. To decrease the morbidity rate of UTIs, proper treatment is required. The non-specific signs and symptoms of UTIs in children under the age of two years can make it difficult to diagnose UTIs [9]. Children with simple UTIs may respond to sulphonamides, amoxicillin, trimethoprim-sulfamethoxazole, or cephalosporins, with amoxicillin, sulphonamides, trimethoprim-sulfamethoxazole, or cephalosporins concentrating in the lower urinary tract [10]. In high-income countries suggest that bacteria that cause UTIs are more likely to form resistance to conventional antibiotics such as trimethoprim-sulfamethoxazole [11]. The fatality rate of S. aureus has been minimized with the help of antibiotics but S. aureus quickly develops resistance to antibiotics. Factors like toxins, adhering proteins, enzymes, antimicrobial peptides, and super-antigen make it a major pathogen for humans and animals [12]. Multidrug-resistant Escherichia coli has been a topic of concern in the current era because of its wide host range, elevation in its pathogenicity level, competency in survival, and many reported pandemics [13]. Multidrug resistance (MDR) in E. coli is a serious issue that poses a risk to human and animal health [14].
This study aims to collect and identify the isolates recovered from the clinical specimens from pediatric patients and the antimicrobial resistance of bacterial isolates as per CLIC guideline 2020.
Materials and Methodology
Ethical consideration
Before starting the study, ethical permission was obtained from the Ethical Review Committee, Government College University Faisalabad.
Consent forms
A consent form was designed that included name, gender, date and time of sampling, and permission from the patients/guardians to use their samples for research purposes. Consent forms were filled out by the patients/guardians at the time of the sampling. The data of the patients were kept secret and not shared with anyone.
Sample collection
A total of 200 catheter tips were collected from the pediatric patients of different wards (urology, surgery, medicine) at the Children’s Hospital Faisalabad. The clinical samples of catheter tips were collected by using sterile scissors and cutting catheter tips from the balloon side by 2cm and transferred into a sterile container.
Sample processing and staining
Samples were first kept in pre-prepared nutrient broth for 24 hours. The Broth was subcultured on Blood, nutrient, and MacConkey agar plates, and then incubation was done at 37 ºC overnight. Bacterial isolate colonies were preliminarily identified based on colony morphology, the color pigment of the isolates, size, and shape of the colonies.
Gram staining
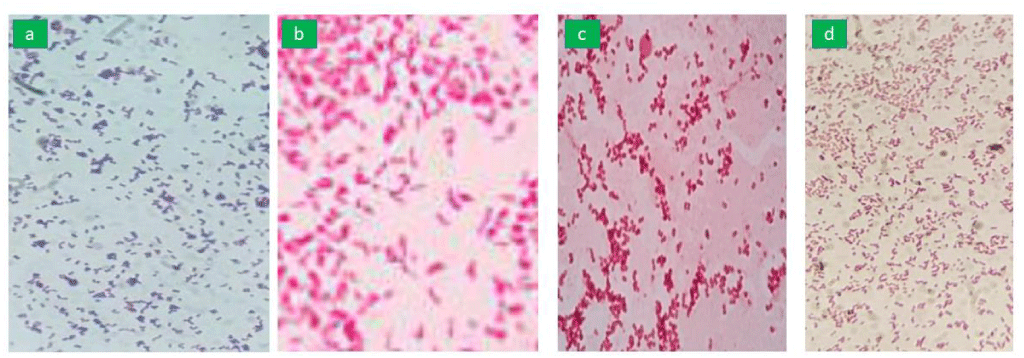
The basic principle of gram staining is to distinguish between gram-positive and gram-negative bacteria based on a cell wall. Gram staining of the isolates included smear preparation, Gram staining, and microscopy of the colonies. The gram staining observed at 100x, under the microscope; Gram-positive isolates appear to be purple-blue while Gram-negative isolates appear to be pink.
Biochemical profiling of isolates
Isolates were processed further for biochemical profiling for confirmation of biochemical characteristics. Oxidase, triple sugar iron, citrate, urease, indole, methyl red, and Voges Proskauer tests were conducted and results were noted for each of the processed isolates.
Antibiotic susceptibility testing
Hudzicki & Kirby-Bauer, 2016 method, measured the sensitivity of bacteria. Results were recorded while different zones appeared on antibiotic agar plates.
Statistical analysis
Data were analyzed by SPSS software; sheets were prepared for each of the tested samples. Statistical interpretations were performed for analysis of the results.
Results
A total of 200 samples were processed in this study and 76 (38%) of the samples showed growth on nutrient agar. Sample positivity has been presented in Tables 1,2 presents sample positivity for the tested samples.
Prevalence of bacteria in samples
76 samples marked positive were processed further for estimation of the prevalence of bacteria. In processed samples high prevalence was marked for P. aeruginosa (24/200; 12%) followed by E. coli (22/200; 11%) and S. aureus (19/200; 9.5%) while 11 K. pneumoniae isolates (5.5%) were identified in this study. The results for the prevalence of bacteria have been presented in Table 3.
Patients’ clinical demographic distribution for P. aeruginosa
In the study of demographic factors for P. aeruginosa, in overall sample distribution for investigation of gender, the high prevalence was found for males (15%), for investigation of sample location, the high prevalence was found for surgical wards and urological wards (12.12%) and for investigation of age group, the high prevalence was found for age group 1-4 (13.54%). The results for patients’ clinical demographic distribution for P. aeruginosa have been presented in Table 4.
Patients’ clinical demographic distribution for E. coli
In the study of demographic factors for E. coli, in overall sample distribution for investigation of gender, the high prevalence was found for males (13.33%), for investigation of sample location, the high prevalence was found for urological wards (18.18%) and for investigation of age group, the high prevalence was found for age group 5-9 (10.94%). The results for patients’ clinical demographic distribution for E. coli have been presented in Table 5.
Patients’ clinical demographic distribution for K. pneumoniae
In the study of demographic factors for K. pneumoniae, in overall sample distribution for investigation of gender, the high prevalence was found for males (7.5%), for investigation of sample location, the high prevalence was found for surgical wards (6.06%) and for investigation of age group, the high prevalence was found for age group 1-4 (7.30%). The results for patients’ clinical demographic distribution for K. pneumoniae have been presented in Table 6.
Patients’ clinical demographic distribution for S. aureus
In the study of demographic factors for S. aureus, in overall sample distribution for investigation of gender, the high prevalence was found for males (11.67%), for investigation of sample location, the high prevalence was found for surgical wards (10.61%) and for investigation of age group, the high prevalence was found for age group 1-4 (14.58%). The results for patients’ clinical demographic distribution for S. aureus have been presented in Table 7.
Confirmation of the isolates
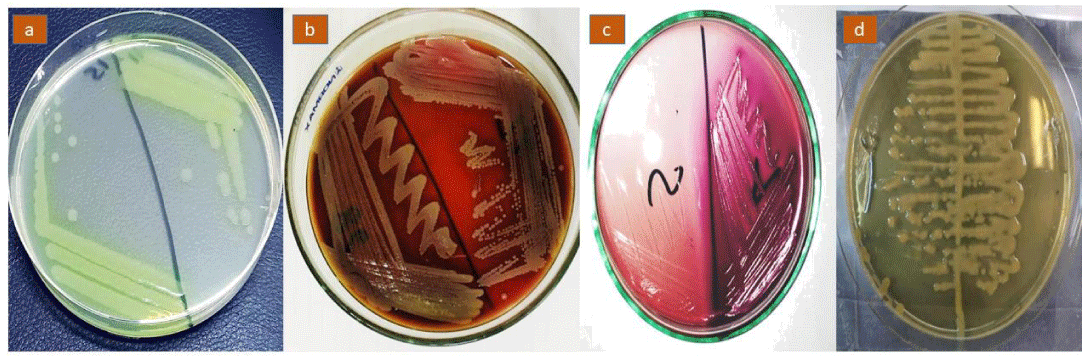
For confirmation of the isolates, identification of P. aeruginosa was carried out on cetrimide agar and smooth, convex colonies with greenish pigment and grape-like odor are the characteristic features of the P. aeruginosa isolates. For confirmation of the isolates, identification of E. coli was carried out on MacConkey agar and red-pinkish non-mucoid colonies are characteristic features for E. coli isolates. For the confirmation of the isolates, identification of S. aureus was carried on blood agar and convex, shiny white hemolytic colonies are characteristic features for S. aureus isolates. For the confirmation of the isolates, identification of K. pneumoniae was carried out on EMB agar and mucoid pinkish growth is a characteristic feature of K. pneumoniae isolates. Growth exhibiting the culture characteristics of P. aeruginosa, E. coli, K. pneumoniae, and S. aureus has been presented in Figure 1. The isolates were observed under microscope 100 X has been presented in Figure 2.
Biochemical profiling of isolates
Biochemical profiling of the isolates was carried out for the confirmation of the biochemical characteristics of the isolates. The results of the biochemical profiling of the isolates have been presented in Tables 8,9
Antibiotic susceptibility testing
Antibiotic susceptibility testing was carried out against the enlisted antibiotics and results were formulated according to the CLSI 2021 guidelines. The results of antibiotic susceptibility profiling of the isolates have been presented in Tables 10-13.
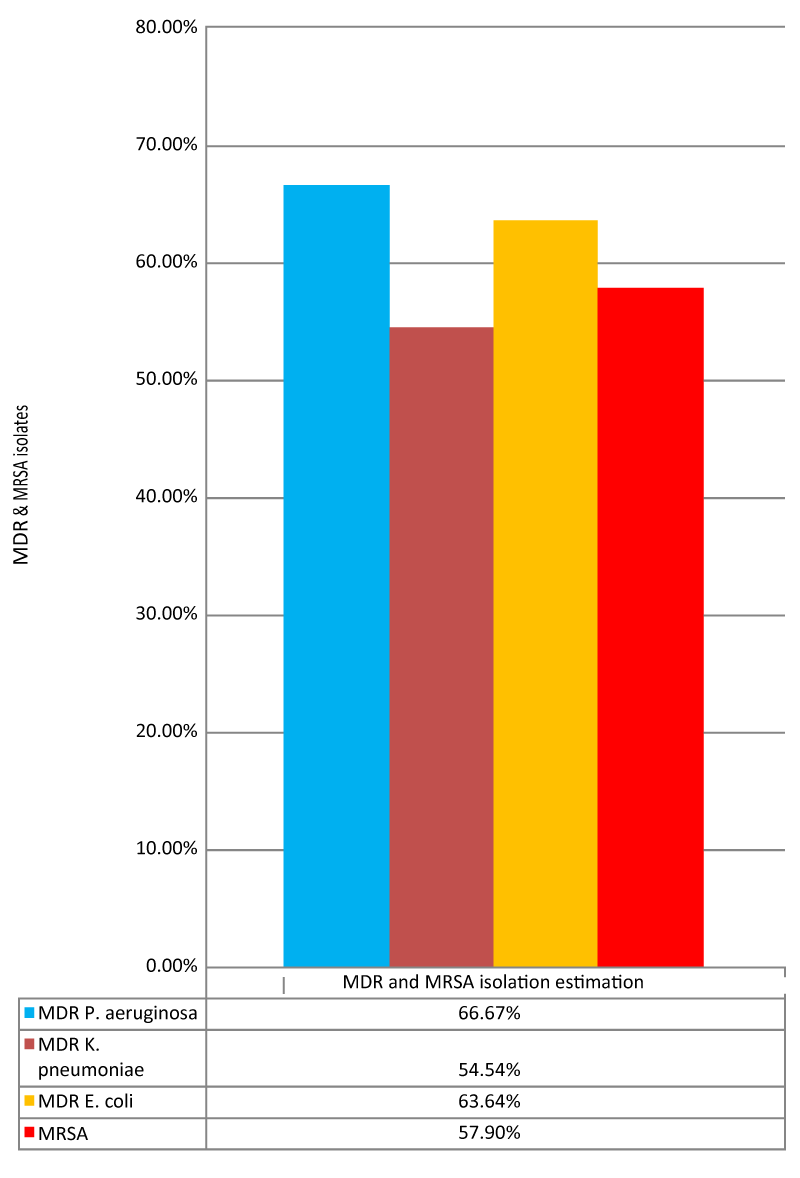
MDR and MRSA isolate estimation
For Gram-negative bacteria occurrence of MDR isolates was formulated on the basis of resistance in studied isolates while phenotypic detection of MRSA isolates was estimated by cefoxitin disk analysis see Table 14, Figure 3.
Discussion
The most frequent bacterial infection in children is urinary tract infection (UTI), which affects 8% of girls and 2% of boys under the age of 7. Also, 30% of people have a chance of developing a second UTI who have already developed a UTI in childhood [15]. Some diseases, such as congenital anomalies of the urinary tract, put some children at a high risk of having UTIs [16]. The upper urinary tract (pyelonephritis or kidney infection) or the lower urinary tract (cystitis or bladder infection) may be affected by UTI and it is very difficult, to differentiate cystitis-based clinical symptoms and indications of pyelonephritis, particularly in children and infants [17]. Proteus mirabilis is more frequent in males than in girls while in newborn infants Streptococcus agalactiae is more common than Haemophilus influenza, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus viridians, Streptococcus pneumoniae, and Streptococcus agalactiae may be responsible in children with anomalies of the urinary tract (anatomic, neurologic, or functional) or compromised immune system [17].
Only a proper identification of the local pathogen, as well as information on the susceptibility pattern and any related risk factors, can provide appropriate treatment for UTIs. Because of incorrect antibiotic use, the bacterial sensitivity pattern of common pathogens is gradually changing in all countries [18]. To decrease the morbidity rate of UTIs, proper treatment is required [19]. Keeping in view the above facts and figures and the importance of UTIs in pediatrics, the current study was designed with the objectives to isolate and identify catheter-associated bacterial pathogens in UTIs among pediatric patients and to estimate the prevalence and antibiotic susceptibility profiling of catheter-associated bacterial pathogens in UTIs among pediatric patients.
A total of 200 catheter tips were collected from the patients of different wards (surgery, urology, medicine) at the Children’s Hospital Faisalabad. Samples were first kept in pre-prepared nutrient broth for 24 hours and then streaked on nutrient agar plates and the positivity of the samples was noted after 24 hours. Positive samples were processed further for the identification of E. coli, K. pneumoniae, S. aureus, and P. aeruginosa using culture identification, microscopy, and biochemical profiling on the basis of culture characterization, microscopy, and biochemical profiling. Cultures were processed on selective agar, set for incubation at 37 ºC for 24 hours, and processed further for Gram-staining, microscopy, and biochemical profiling using oxidase, catalase, triple sugar iron, urease, indole, methyl red, and Voges Proskauer test. Antibiotic susceptibility testing was performed to determine the antibiotic resistance profile of each isolate by disc diffusion method. Antibiotics were selected based on clinical relevance which belongs to different antimicrobial groups. The zone of inhibition was interpreted according to Clinical and Laboratory Standards Institute guidelines (CLSI) 2021 and isolates were determined as resistant, intermediate, and susceptible according to CLSI guideline 2021.
A total of 200 samples were processed in this study and 76 (38%) of the samples showed growth on nutrient agar. In processed samples, the high prevalence was marked for P. aeruginosa (24/200; 12%) followed by E. coli (22/200; 11%) and S. aureus (19/200; 9.5%) while 11 K. pneumoniae isolates (5.5%) were identified in this study. This study showed relevance with the results presented by Mishra & Wadhai (2016) in research designed on P. aeruginosa in OT samples [20], Mohammad, et al. 2017 in research designed on K. pneumoniae in OT samples, Dhom, et al. (2017) in a method of research on E. coli in surgical sites [21]. These results were also supported by the results presented by Sapkota, et al. (2016) in the method of research on P. aeruginosa in ward samples [22], Baban, et al. (2019) in research designed on E. coli on surgical ward samples [23], and Yusuf, et al. (2017) in a study designed on OT samples. In a comparative study designed on clinical isolates, Habyarimana, et al. (2020) reported the prevalence of P. aeruginosa at 22.50%, E. coli at 7.5%, and K. pneumoniae isolates at 15% [24].
In antibiotic susceptibility profiling of P. aeruginosa, the highest susceptibility was found for colistin (100%) and imipenem (70.83%) followed by gentamicin (54.17%) while the highest resistance was found for tobramycin (54.17%) followed by meropenem, ceftazidime, and cefotaxime (50%). In a comparative study designed on catheter samples in the Czech Republic Olejnickova, et al. (2014) also reported more than 90% susceptibility to colistin however resistance to ciprofloxacin (56.6%) and gentamicin (42.9%) and a little susceptibility to amikacin (lesser than 10%) was reported in P. aeruginosa isolates [25]. Bizuayehu, et al. (2022) in Ethiopia also designed a comparative study on catheter samples and also reported imipenem as a susceptible antibiotic (85.3%) reported high resistance to ceftazidime (83.3%) and resistance to gentamicin (41.7%) and tobramycin (41.7%) were also reported in P. aeruginosa isolates [26]. The minor difference in results might be due to the difference in the demographic location of the study.
In antibiotic susceptibility profiling of K. pneumoniae highest susceptibility was found for colistin (100%) and imipenem (72.73%) followed by gentamicin and ciprofloxacin (45.45%) while the highest resistance was found for cefotaxime (63.63%) followed by meropenem, tobramycin and amikacin (54.54%). Hyun, et al. (2019) designed a study on clinical samples in Korea and reported high susceptibility to amikacin (94.4%), gentamicin (80.3%), ciprofloxacin (70.4%), and cefotaxime (53.5%) were reported [27]. The difference in results might be due to differences in sample type and location of the sampling.
In antibiotic susceptibility profiling of E. coli, the highest susceptibility was found for colistin (100%) and imipenem (63.64%) followed by ciprofloxacin (54.55%) while the highest resistance was found for gentamicin (54.55%) followed by tobramycin, meropenem, ceftazidime, and amikacin (50%). In a comparative study designed on clinical samples in Korea, Hyun, et al, (2019) reported 99.2% susceptibility to amikacin, 56% to ciprofloxacin, and 66.1% to gentamicin. These results were also supported by El-Mahdy, et al. (2021) in a study designed on catheter samples in Ethiopia in which 55.6% resistance to ceftazidime was reported [28]. Almost similar results were also reported by Vidyasagar & Nagarathnamma, (2018) in a study designed on E. coli isolates from catheter samples also reported high susceptibility to imipenem (95.7%), amikacin (58.7%), and tobramycin (58.7%) [29]. Bizuayehu, et al. (2022) in a study designed on catheter samples in Nepal in which 100% susceptibility to imipenem and 37.5% resistance to ceftazidime was reported however 100% susceptibility to meropenem and amikacin was also reported in E. coli isolates [30]. Ndomba, et al. (2022) in a study designed on catheter samples in Tanzania also reported 50.7% resistance to ceftazidime in E. coli isolates however a bit of fluctuation in resistance to gentamicin (43%) was also reported [31].
In antibiotic susceptibility profiling of S. aureus highest susceptibility was found for vancomycin (100%) clindamycin, cefoxitin, and trimethoprim-sulfamethoxazole (57.89%) while the highest resistance was found for erythromycin and ampicillin (47.37%). Vidyasagar & Nagarathnamma, (2018) in a study designed on S. aureus in catheter samples also reported 100% resistance to vancomycin however a little susceptibility to erythromycin (20%) and clindamycin (20%) was found in these isolates.
A high prevalence of pathogens in catheter samples has been alarming and has been worsened with the presence of resistant isolates that have not only been found resistant to antibiotics studied. Advance studies are needed to investigate the real investigations of bacterial contamination; resistance to treatment options and resistance to antibiotics are needed.
Conclusion
This study concluded that the high prevalence was determined for P. aeruginosa (24/200; 12%) and E. coli (22/200; 11%). In this study, the male patients were mostly infected as compared to females (3:2). The antimicrobial profile suggested that 54.17 % of P. aeruginosa were resistant to tobramycin, and the highly sensitive drug was colistin (100%). In antibiotic susceptibility profiling of K. pneumoniae, the highest susceptibility was found for colistin (100%) and the highest resistance was found for cefotaxime (63.63%).
In antibiotic susceptibility profiling of E. coli, the highest susceptibility was found for colistin (100%) while the highest resistance was found for gentamicin (54.55%). In antibiotic susceptibility profiling of S. aureus highest susceptibility was found for vancomycin (100%) while the highest resistance was found for erythromycin and ampicillin (47.37%). There should be public awareness of the use of antibiotics, there should be a stoppage of irrational use of antibiotics, people should not take self-antibiotics, over-the-counter availability of antibiotics should be banned, and continuous education on health care.
Advanced studies are needed to investigate the real investigations of bacterial contamination resistance to treatment options and resistance to antibiotics is needed.
Declaration
Availability of data and materials: Availability of data and materials on request by the corresponding author
Authors’ contributions: All authors contribute equally
I am grateful to all of those with whom I have had the pleasure to work during this and other related projects.
- Kanellopoulos TA, Salakos C, Spiliopoulou I, Ellina A, Nikolakopoulou NM, Papanastasiou DA. First urinary tract infection in neonates, infants and young children: a comparative study. Pediatr Nephrol. 2006 Aug;21(8):1131-7. doi: 10.1007/s00467-006-0158-7. Epub 2006 Jun 30. PMID: 16810514.
- Isac R, Basaca DG, Olariu IC, Stroescu RF, Ardelean AM, Steflea RM, Gafencu M, Chirita-Emandi A, Bagiu IC, Horhat FG, Vulcanescu DD, Ionescu D, Doros G. Antibiotic Resistance Patterns of Uropathogens Causing Urinary Tract Infections in Children with Congenital Anomalies of Kidney and Urinary Tract. Children (Basel). 2021 Jul 8;8(7):585. doi: 10.3390/children8070585. PMID: 34356564; PMCID: PMC8304885.
- González R, Ludwikowski BM. Editorial: Progress in Pediatric Urology in the Early 21st Century. Front Pediatr. 2019 Aug 20;7:349. doi: 10.3389/fped.2019.00349. PMID: 31482079; PMCID: PMC6710393.
- Harper L, Blanc T, Peycelon M, Michel JL, Leclair MD, Garnier S, Flaum V, Arnaud AP, Merrot T, Dobremez E, Faure A, Fourcade L, Poli-Merol ML, Chaussy Y, Dunand O, Collin F, Huiart L, Ferdynus C, Sauvat F. Circumcision and Risk of Febrile Urinary Tract Infection in Boys with Posterior Urethral Valves: Result of the CIRCUP Randomized Trial. Eur Urol. 2022 Jan;81(1):64-72. doi: 10.1016/j.eururo.2021.08.024. Epub 2021 Sep 22. PMID: 34563412.
- Buonsenso D, Sodero G, Mariani F, Lazzareschi I, Proli F, Zampino G, Pierantoni L, Valentini P, Rendeli C. Comparison between Short Therapy and Standard Therapy in Pediatric Patients Hospitalized with Urinary Tract Infection: A Single Center Retrospective Analysis. Children (Basel). 2022 Oct 28;9(11):1647. doi: 10.3390/children9111647. PMID: 36360375; PMCID: PMC9688884.
- Oumer Y, Regasa Dadi B, Seid M, Biresaw G, Manilal A. Catheter-Associated Urinary Tract Infection: Incidence, Associated Factors and Drug Resistance Patterns of Bacterial Isolates in Southern Ethiopia. Infect Drug Resist. 2021 Jul 24;14:2883-2894. doi: 10.2147/IDR.S311229. PMID: 34335034; PMCID: PMC8318706.
- Mirzaei R, Mohammadzadeh R, Alikhani MY, Shokri Moghadam M, Karampoor S, Kazemi S, Barfipoursalar A, Yousefimashouf R. The biofilm-associated bacterial infections unrelated to indwelling devices. IUBMB Life. 2020 Jul;72(7):1271-1285. doi: 10.1002/iub.2266. Epub 2020 Mar 9. PMID: 32150327.
- Ponvelil JJ, Gowda HN, Raj S. Prevalence of urinary tract infection and sensitivity pattern amongst children less than 3 years of age with fever in a tertiary care hospital in South Karnataka. Int J Basic Clin Pharmacol. 2020; 9(5):736-78.
- Aslam O, Aslam I. Urinary tract infection in childhood. InnovAiT. 2022; 15(5):280-5.
- Wang ME, Greenhow TL, Lee V, Beck J, Bendel-Stenzel M, Hames N, McDaniel CE, King EE, Sherry W, Parmar D, Patrizi ST, Srinivas N, Schroeder AR. Management and Outcomes in Children with Third-Generation Cephalosporin-Resistant Urinary Tract Infections. J Pediatric Infect Dis Soc. 2021 May 28;10(5):650-658. doi: 10.1093/jpids/piab003. PMID: 33595081.
- Sangeda RZ, Paul F, Mtweve DM. Prevalence of urinary tract infections and antibiogram of uropathogens isolated from children under five attending Bagamoyo District Hospital in Tanzania: A cross-sectional study [version 1; peer review: awaiting peer review]. 2021.
- Umoh HP. Characterization of staphylococcus aureus isolated from door handles in the college of humanities, management and social sciences, mountain top university. 2022.
- Davies R, Wales A. Antimicrobial Resistance on Farms: A Review Including Biosecurity and the Potential Role of Disinfectants in Resistance Selection. Compr Rev Food Sci Food Saf. 2019 May;18(3):753-774. doi: 10.1111/1541-4337.12438. Epub 2019 Apr 17. PMID: 33336931.
- Arbab S, Ullah H, Wang W, Zhang J. Antimicrobial drug resistance against Escherichia coli and its harmful effect on animal health. Vet Med Sci. 2022 Jul;8(4):1780-1786. doi: 10.1002/vms3.825. Epub 2022 May 24. PMID: 35608149; PMCID: PMC9297802.
- Millner RO, Preece J, Salvator A, McLeod DJ, Ching CB, Becknell B. Albuminuria in Pediatric Neurogenic Bladder: Identifying an Earlier Marker of Renal Disease. Urology. 2019 Nov;133:199-203. doi: 10.1016/j.urology.2019.08.013. Epub 2019 Aug 24. PMID: 31454657.
- Isac R, Stroescu R, Olariu C, Pop M, Farkas F, Ardelean M. Diagnosis and clinical consequences of urinary tract malformation in children. Journal of the Romanian Pediatric Surgery Society. 2019; 22(1):27-30.
- Leung AKC, Wong AHC, Leung AAM, Hon KL. Urinary Tract Infection in Children. Recent Pat Inflamm Allergy Drug Discov. 2019;13(1):2-18. doi: 10.2174/1872213X13666181228154940. PMID: 30592257; PMCID: PMC6751349.
- Kariyappa P, Reddy SC, Mavinahalli AS, Rao US. Urinary tract abnormalities in children with first urinary tract infection. Indian Journal of Child Health. 2020; 337-9.
- Sangeda RZ, Paul F, Mtweve DM. Prevalence of urinary tract infections and antibiogram of uropathogens isolated from children under five attending Bagamoyo District Hospital in Tanzania: A cross-sectional study. F1000Research. 2021; 10:449.
- Mishra AK, Wadhai VS. Sterility Testing of Operation Theatres in Hospitals. Int J Curr Microbiol App Sci. 2016; 5(5): 440-447. doi: http://dx.doi.org/10.20546/ijcmas.2016.505.046
- Dhom J, Bloes DA, Peschel A, Hofmann UK. Bacterial adhesion to suture material in a contaminated wound model: Comparison of monofilament, braided, and barbed sutures. J Orthop Res. 2017 Apr;35(4):925-933. doi: 10.1002/jor.23305. Epub 2016 Jun 14. PMID: 27208547.
- Sapkota B, Gupta GK, Shrestha SK, Pradhan A, Karki P, Thapa A. Microbiological burden in air culture at various units of a tertiary care government hospital in Nepal. Australas Med J. 2016 Jan 31;9(1):1-7. doi: 10.4066/AMJ.2015.2558. PMID: 26913084; PMCID: PMC4748305.
- Baban ST, Saeed PA, Jalal DM. Microbial contamination of operating theatres and intensive care units at a surgical specialty hospital in Erbil City. Med J Babylon. 2019; 16:150-5.
- Habyarimana T, Uwizeye C, Munyeshyaka E, Izere C, Mucumbitsi J, Yadufashije C. Bacteriological Study of Electronic Devices Used by Healthcare Workers at Ruhengeri Referral Hospital. BioMed Research International. 2020: 5872929; 6. https://doi.org/10.1155/2020/5872929
- Olejnickova K, Hola V, Ruzicka F. Catheter-related infections caused by Pseudomonas aeruginosa: virulence factors involved and their relationships. Pathogens and Disease. 2014; 72: 87-94. https://doi.org/10.1111/2049-632X.12188
- Bizuayehu H, Bitew A, Abdeta A, Ebrahim S. Catheter-associated urinary tract infections in adult intensive care units at a selected tertiary hospital, Addis Ababa, Ethiopia. PLoS One. 2022 Mar 22;17(3):e0265102. doi: 10.1371/journal.pone.0265102. PMID: 35316286; PMCID: PMC8939826.
- Hyun M, Lee JY, Kim HA, Ryu SY. Comparison of Escherichia coli and Klebsiella pneumoniae Acute Pyelonephritis in Korean Patients. Infect Chemother. 2019 Jun;51(2):130-141. doi: 10.3947/ic.2019.51.2.130. PMID: 31270992; PMCID: PMC6609746.
- El-Mahdy R, Mahmoud R, Shrief R. Characterization of E. coli Phylogroups Causing Catheter-Associated Urinary Tract Infection. Infect Drug Resist. 2021 Aug 16;14:3183-3193. doi: 10.2147/IDR.S325770. PMID: 34429618; PMCID: PMC8378909.
- Vidyasagar K, Nagarathnamma T. Study of catheter-associated urinary tract infection and biofilm production by Escherichia coli . Indian J Microbiol Res. 2018; 5(3):387-392.
- Bizuayehu H, Bitew A, Abdeta A, Ebrahim S. Catheter-associated urinary tract infections in adult intensive care units at a selected tertiary hospital, Addis Ababa, Ethiopia. PLoS One. 2022 Mar 22;17(3):e0265102. doi: 10.1371/journal.pone.0265102. PMID: 35316286; PMCID: PMC8939826.
- Ndomba ALM, Laisser RM, Silago V, Kidenya BR, Mwanga J, Seni J, Mshana SE. Urinary Tract Infections and Associated Factors among Patients with Indwelling Urinary Catheters Attending Bugando Medical Centre a Tertiary Hospital in Northwestern Tanzania. Microorganisms. 2022 Feb 21;10(2):473. doi: 10.3390/microorganisms10020473. PMID: 35208927; PMCID: PMC8879566.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
