Journal of Clinical Microbiology and Biochemical Technology
Intestinal microbiota and related metabolites are essential mediators for adoptive γδT cell antitumor immunotherapy
Jiajia Han*, Xichen Zheng and Meichun Xing
Cite this as
Han J, Zheng X, Xing M (2022) Intestinal microbiota and related metabolites are essential mediators for adoptive γδT cell antitumor immunotherapy. J Clin Microbiol Biochem Technol 8(1): 016-017. DOI: 10.17352/jcmbt.000050Copyright
© 2022 Han J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Intestinal microbiotas modulate multiple biochemical reactions and immune hemostasis of the host, numerous pieces of evidence have revealed that they are also tightly involved in the efficacy of antitumor immunotherapy. However,s which way local intestinal microbiota influences the activity of distant organs is still unknown. In this review, we highlighted the importance of metabolites produced by intestinal microbiotas which disorder prompted cytotoxic capability of adoptive γδT cells. The microbiota-metabolites-γδT cell axis is dominant in the antitumor immune response of adoptive γδT cell immunotherapy.
γδT cells are characterized by γ and δ chains and MHC unrestricted antigen recognition, thus being activated rapidly by various non-peptide antigens. Therefore, Vγ9Vδ2 cells expanded from human peripheral blood were applied for plenty of clinical research which focused on cancer therapy, for instance, CAR-T, TCR-T, and other genetic engineering T cells based on the advantage of γδT cell. Since adaptive immune cells are not always working well when encountering complex circumstances inside the human body, further improvements are required to enhance the cytotoxicity of those cells.
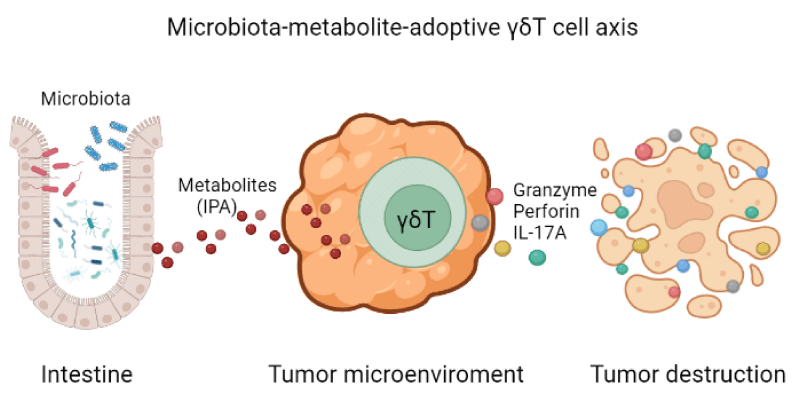
Microbiota, especially intestinal bacteria, which modulating various immune responses [1]. Studies have revealed that specific bacteria species that determine anti-PD-1 immunotherapy responses of melanoma patients, such as Akkermansia muciniphila and Ruminococcaceae substantially increased the immune response of patients against tumors by regulating the ratio of cytotoxic CD8+T and Treg cells [2,3], but how they communicate with tumor microenvironment where outside of intestinal area hasn’t been elucidated. In recent studies, the importance of microbiota-related metabolites was highlighted in γδT cells’ antitumor capability [4,5]. The dysbiosis of intestinal microbiota induced by antibiotics ahead of adoptive γδT cell therapy enhanced cytotoxicity of γδT cell against HepG2 hepatocellular carcinoma cells, metabolites produced by microbiota are essential mediators to facilitate intestinal bacteria commune with γδT cell that infiltrated within the tumor microenvironment.
The diversity and abundance of bacteria determine the characteristics of the intestinal environment, including the concertation of various microbiota-related metabolites which influence the antitumor responses of transferred immune cells. Besides in vivo evidence that antibiotic-treated mice generated higher concertation of 3-into propionic acid (IPA) according to the analysis of metabolites from cecum content by gas chromatography/mass spectrometry, thus better responded with γδT cell therapy with more granzyme B, perforin production (Figure 1). Moreover, in vitro cytotoxicity assay of γδT cell in the presence of IPA also be significantly improved against various tumor cell lines without causing damage to the tumor cell itself. But it is still debatable which specific bacteria species are the main resource of IPA production in the gut. Since IPA are from tryptophan metabolism under the assistance of numerous bacteria, we conduct that there are many microbiotas that may involve in IPA production and eventually make this happen after antibiotics treatment. Additionally, it needs to be determined in which way IPA induces cytotoxicity molecules release of γδT cells, such as granzyme B and perforin. In sum, we tend to believe that metabolites are functional operators for intestinal microbiota to modulate the immune response in the distant tumor site. Therefore, it would be better to pay more attention to the alteration of specific metabolites instead of focusing on microbiotas only in future studies.
We appreciate all the contributors to this study, the financial supports are from the Shanghai Sailing Program (20YF1437300), the National Natural Science Foundation of China (81903167).
- Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012 Apr 17;21(4):504-16. doi: 10.1016/j.ccr.2012.02.007. PMID: 22516259; PMCID: PMC3332000.
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018 Jan 5;359(6371):97-103. doi: 10.1126/science.aan4236. Epub 2017 Nov 2. PMID: 29097493; PMCID: PMC5827966.
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018 Jan 5;359(6371):91-97. doi: 10.1126/science.aan3706. Epub 2017 Nov 2. PMID: 29097494.
- Han J, Zhang S, Xu Y, Pang Y, Zhang X, Hu Y, Chen H, Chen W, Zhang J, He W. Beneficial Effect of Antibiotics and Microbial Metabolites on Expanded Vδ2Vγ9 T Cells in Hepatocellular Carcinoma Immunotherapy. Front Immunol. 2020 Jul 22;11:1380. doi: 10.3389/fimmu.2020.01380. PMID: 32849498; PMCID: PMC7396509.
- Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S, Whary MT, Meyerson M, Germain R, Blainey PC, Fox JG, Jacks T. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell. 2019 Feb 21;176(5):998-1013.e16. doi: 10.1016/j.cell.2018.12.040. Epub 2019 Jan 31. PMID: 30712876; PMCID: PMC6691977.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
