Journal of Clinical Microbiology and Biochemical Technology
Microbiological controls in polyculture farming: A pilot case study in the Castellammare Gulf (Sicily)
Caruso G1*, Caruso R2 and Sarà G3
2Hospital Agency “G. Martino”, Messina, Italy
3Department of Earth and Marine Sciences, Laboratory of Experimental Ecology & Behaviour (LoEEB), University of Palermo, Palermo, Italy
Cite this as
Caruso G, Caruso R, Sarà G (2020) Microbiological controls in polyculture farming: a pilot case study in the Castellammare Gulf (Sicily). J Clin Microbiol Biochem Technol 6(1): 014-028. DOI: 10.17352/jcmbt.000039Background: Integrated multi-trophic aquaculture (IMTA) experiences, based on the co-cultivation of two or more organisms, have proliferated in recent years; nevertheless, studies regarding the microbiological implications of these farming systems are not available yet.
The context and purpose of the study: The microbiological conditions of shellfish and surrounding rearing environment were investigated during a pilot polyculture system (fish and shellfish farming) performed in the Castellammare Gulf (Sicily). The quantitative distribution of faecal coliforms and enterococci as faecal pollution indicators, together with that of environmental and potentially pathogenic halophilic Vibrios, was determined in two different seasons (spring and autumn). Samplings of surface waters and bivalves (oysters, Cassostrea gigas and Ostrea edulis; mussel, Mytilus galloprovincialis, and clam, Tapes philippinarum) were performed at stations where integrated polyculture was performed (Impact sites) compared to others (Control sites) where farming activities regarded shellfish only.
Results: Higher numbers of faecal pollution indicators were detected in waters at the Impact than at Control ones, generally in spring, although polyculture seemed to have only a low impact over the area. Shellfish products were characterized by Vibrios concentrations higher than in waters, due to their filter-feeding activity. They ranged in the order of- 102-103 CFU/g, with higher abundances in spring in the specimens reared at the Impact sites; conversely, in shellfish farmed at the Control sites, no significant quantitative variations were found between the two samplings. The qualitative study of Vibrios isolated from both shellfish and environment showed the ubiquitous distribution of V. alginolyticus, and the predominance of V. vulnificus at the Impact sites in both shellfish and waters, representing the 40% and 27% of the total isolates, respectively.
Main findings: Overall, the low levels of microbial contamination detected in the area showed that polyculture did not result in a heavy impact on the surrounding environment.
Conclusions: The results obtained in the polyculture experiment performed in Castellammare Gulf suggest the use of this system as a sustainable farming strategy for productive purposes.
Brief summary: During a pilot experiment of integrated aquaculture, the microbiological quality of the shellfish products was assessed, proving that this productive practice did not give negative results on the concentrations of total heterotrophic bacteria, faecal pollution indicators and potentially pathogenic Vibrios.
Any potential implications: Polyculture is suggested as an ecofriendly farming system, that allows the production of shellfish safe for consumers.
Introduction
Aquaculture (including fish, shellfish and macroalgae farming) represents a very important sector for the world economic growth, that can lead to social benefits in terms of new occupational perspectives [1]. Productive activities, however, have well-known impacts on the environment, mainly due to the release of feed wastes, chemical and pharmaceuticals (i.e. antibiotics), as well as to potential transmission of diseases, dispersal of non-native species, and destruction of habitats [2,3].
The severity of aquaculture impacts varies in relation to the reared species, local environmental conditions, farming and management techniques. Future developments in the aquaculture field depend on the selection of rearing practices able to meet the principles of environmental sustainability [3,4]. In this context, particular interest has recently been addressed to to Integrated Multi-Trophic Aquaculture (IMTA) systems, where organisms belonging to different trophic levels and inhabiting different ecological niches are co-cultured. IMTA includes several aquaculture practices based on the complementarity of different productive compartments. By IMTA, inorganic and organic wastes from finfish are assimilated respectively by autotrophic (i.e. phytoplankton, macroalgae, plants) and heterotrophic species (e.g. oysters, mussels, sea cucumbers) that are co-cultured with the target reared species. It can represent a key alternative in the evolution of aquaculture systems, allowing to face a double challenge: sustaining the growing demand of aquatic products and preserving the environment by reducing wastes [5,6]. IMTA has also been suggested as a possible ecofriendly strategy to mitigate the effects of multiple stressors [7].
In Asian Countries, the integrated cultivation of fish with organisms of different trophic levels, as well as the rearing of both shellfish and seaweed in lagoons or bays close to fish farming structures is an old practice [8,9]. In China, the polyculture systems with the macroalga Eucheuma gelatinae and the bivalve Gafrarium tumidum provided a suitable tool for the purification of eutrophic seawater and control of algal bloom [10]. In other Countries, such as Canada, Reid, et al. [11], reviewed shellfish production in the context of open-water IMTA; later, Chopin [12] underlined the advantages of IMTA systems, able to increase economic profitability per cultivation unit through co-cultivation of many species (fish, seaweeds, invertebrates), characterized by environmental sustainability and societal acceptability. More recently, Buck, et al. [13], have provided an in-depth review of the main variables affecting IMTA and the potential benefits/limitations of offshore mariculture plants. The feasibility of integrated fish, shellfish and/or seaweed aquaculture within offshore wind farming areas has been reported to be affected by factors such as biological feasibility, technological implementation, environmental sustainability and economic feasibility of the farming systems.
In Europe, Alexander, et al. [14], studied the main incentives and barriers to the development of IMTA, highlighting that moving from IMTA at a pilot scale to commercial scale developments requires changes in policy and legislation to promote innovation and technologies for aquaculture and simplification of the regulations regarding licensing or spatial planning for aquaculture. Although attention to IMTA systems is more recent compared to Asia, in Europe integrated polyculture production still faces inherent difficulties which limit the adoption of this practice across this Continent [15]. In Italy integrated polyculture of fish and low-trophic-level organisms such as shellfish is a still scarcely exploited practice [16]. Among shellfish, mussels, oysters and clams are organisms highly appreciated by consumers; oysters are commonly cultured in shallow waters or in intertidal zones near estuaries, while mussels and clams are cultured also in brackish inland ponds. Although the impacts on the environment of bivalve cultivation are well known in terms of local benthic (i.e. physical disturbance, changes in sediment topography and sedimentation, accumulation of debris, biodeposition) and water column effects (alteration in water quality and nutrient cycling) as well as of wider ecological effects (on fish, seabirds, transmission of diseases) [17], the effects of bivalve cultivation in polyculture have been underestimated [18]. The limited knowledge of growth performance of the reared organisms explains the delayed success of IMTA experiences and of the economic performance of this practice. Polyculture experiences have proliferated in recent years [15], nevertheless studies regarding the microbiological implications of polyculture systems are not available yet. Microbiological controls in aquaculture systems play a crucial role to prevent possible transmission to man of pathogenic bacteria or toxins via food consumption [19-23]. Compliance to high quality microbiological criteria is particularly important in shellfish farming areas, in relation to the filtering-feeding behavior of these organisms that results in the concentration of bacteria and polluting substances inside the animal body. Consequently, hygienico-sanitary monitoring of shellfish microbiological quality must be extended also to the waters of the farming environments [24-27].
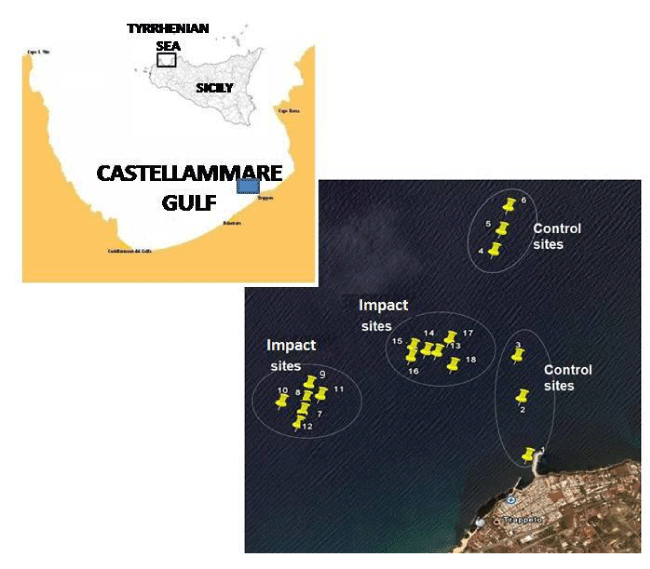
The Gulf of Castellammare (Trapani, Sicily) is a 70 Km wide coastal area that extends along the Tyrrhenian coast between Cape S. Vito and Terrasini. Thanks to its particular morphological and hydrological characteristics - which ensures an efficient water exchange and low urban and industrial loading - this area has traditionally been designed for mariculture purposes. Some reports on the microbiological conditions of this ecosystem are those available from Caruso, et al. [28-30]; nevertheless, knowledge of the health risks related to aquaculture farming is still scarce. In the framework of a pilot IMTA experiment of mussels and fish co-cultivation, an investigation of the bacteriological quality of shellfish products was undertaken. With the aim of assessing the microbiological quality of both shellfish and environment, the bacterial indicators of faecal pollution (faecal coliforms, Escherichia coli and
Materials and methods
Study site
The Castellammare Gulf was chosen as a polyculture site due to the intense aquaculture practiced in this area; the wastes released by productive plants such as faecal pellets and food residuals cause the organic enrichment of the waters [29]. In this context, shellfish farming could represent a suitable tool to convert the trophic inputs into biomass production. The study area was located 3 miles offshore Balestrate, where the polyculture pilot plant was set up (Figure 1). The geographical coordinates of the sampling sites are reported in Table 1.
The experimental design of integrated cultivation consisted of five submersible cages (Farmocean, Sweden; volume = 4,500 m3) and 6 smaller cages (volume = 1,000 m3) filled with seabass (Dicentrarchus labrax) and seabream (Sparus aurata) for a total annual production of about 600 tons of biomass. All the species selected for the study were bivalves highly appreciated due to their high nutritional value: Japanese oyster (Cassostrea gigas), European oyster (Ostrea edulis), common mussel (Mytilus galloprovincialis), Philippine clam (Tapes philippinarum), which belong to Ostreidae (Ostrea genus), Mytilidae (Mytilus genus) and Veneridae (Tapes genus) families, respectively. Mussel seed was cultivated in tight nylon net bags for 12 months in sites close to fish cages (hereafter indicated as Impact sites) and 1 Km far from fish cages (hereafter indicated as Controls).
Collection of shellfish and water samples
Shellfish and surface waters were collected at both Impact and Control sites, during two different samplings, performed in spring and autumn. Surface seawater samples were drawn using sterile 10 litres Niskin bottles. Both seawater and shellfish samples were stored at +5°C in fridge containers until their analysis at the CNR laboratory. They were examined within 4 hours of sampling.
Physico-chemical parameters
The main physico-chemical parameters, temperature and salinity, were recorded using a multiparametric probe.
Shellfish samples
The bacteriological analysis of shellfish was carried out on a sample constituted by 6-8 individuals for oysters, 15 for mussels and 20-30 for clams. The shellfish samples were carefully cleaned from mud, removing the epiphytes; the individuals showing open valves were excluded from analysis. To open shellfish, a sterile scalpel was used, then the tissues and the intravalvular liquid were collected in a sterile container and homogenised using a Stomacher. The homogenate was diluted in sterile saline solution in a 1:10 ratio (weight/volume), and treated differently depending on the target bacteria to be searched.
Each shellfish sample was examined for the quantitative determination of Faecal Coliforms (FC), Escherichia coli, faecal streptococci (ENT), culturable not marine and marine heterotrophic bacteria, and Vibrio spp. at 24°C and 35°C. The search for Salmonella spp. was carried out. The qualitative composition of Vibrio spp. was determined too.
For the determination of FC abundance, the Most Probable Number (MPN) method of fermentation in multiple tubes was applied; briefly, for each shellfish homogenate three dilutions (1:10, 1:100, 1:1000 in sterile saline solution, each dilution in five replicates) were prepared and inoculated into tubes of lactose broth - containing a Durham tube. The tubes showing turbidity and lactose fermentation with gas production after incubation at 35°C for 24 hours were counted.
The abundance of E. coli was estimated through indole production test; small volumes (0.1-0.2 ml) of the broth cultures positive for FC were inoculated in tryptone water, incubated at 44°C for 24 hours. The tubes showing a typical pink colouration after addition of the Kovacs’ reagent were considered as positive.
For the qualitative search of Salmonella spp., 25 g of shellfish were inoculated for 20 hours at 35°C into 225 ml of buffered peptone water as a pre-enrichment step. After further enrichment in Rappaport-Vassiliadis and Tetrathionate broths (Oxoid), incubated at 42°C for 20-24 hours, isolation was performed by sub-culturing on plates of Hektoen Enteric and SS (Salmonella-Shigella) agar (Oxoid), incubated at 35°C for 20-24 hours as selective media for Salmonella spp. Suspected colonies were identified according to their biochemical profiles as obtained by API 20E strips and confirmed by serological tests (Omni-O anti-Salmonella polyvalent serum, Sanofi Pasteur).
Culturable not marine bacteria were counted by spreading 0.1 ml of sample homogenate on the surface of Plate Count Agar plates, further incubated at 22°C for 24-48 hours. The quantification of heterotrophic marine bacteria was carried out by spreading of Marine Agar 2216 plates (Difco), incubated at 22°C for 7 days.
The abundance of halophilic Vibrio spp. was estimated by spreading 0.1 ml of sample homogenate on the surface of Thiosulfate Citrate Bile Salts (TCBS, Oxoid) agar plates added with 2% sodium chloride and incubated at 24°C for 48 hours and at 35°C for 24 hours, for the determination of total presumptive Vibrios (VP) - microorganisms autochthonous in the marine environment - and of the fraction of potentially pathogenic species (VPP) respectively.
For the qualitative study of Vibrio spp, bacterial strains were isolated “at random” from TCBS agar plates, by streaking on fresh plates of the same medium until axenic cultures were obtained. The phenotypic characteristics examined were: Gram staining, glucose fermentation on Triple Sugar Iron agar (Difco), cytocrome-oxidase activity, acid from sucrose on TCBS agar, sensitivity to the Vibriostatic agent O129 (150 µg), biochemical profiling on API 20 E (Biomerieux) strips.
Water samples
Quantitative analyses for the bacteriological characterization of seawater samples were performed by filtration through a sterile 0.45 µm pore size membrane (Millipore); the filter was further placed in culture media different depending on the target microorganism to be evaluated. Particularly, for FC counts, incubation was carried out on m-FC agar (Oxoid) plates at 44.5°C for 24 hours; for Escherichia coli, incubation was performed on TBX agar (Oxoid) plates at 35°C for 24 hours and colonies grown were confirmed by indole test. The abundance of ENT was determined on Slanetz-Bartley agar plates incubated at 35°C for 48 hours.
For the search of Salmonella spp., 300 ml of seawater were filtered and the membrane was pre-enriched in buffered peptone water incubated at 35°C for 20 hours. The further steps of the analytical procedure were similar to those described for shellfish samples.
For the determination of culturable non-marine bacteria and heterotrophic marine bacteria, 0.1 ml seawater samples were streaked on the same culture media as reported for shellfish samples; for VP and VPP counts, 100 ml of seawater were filtered through 0.45 µm pore size sterile membranes and the filters incubated on TCBS agar plates as for shellfish samples.
Assays for enzymatic activities as proxies of virulence factors
All the Vibrio spp. isolates were screened for the presence of enzymes involved in virulence. Before such assays, bacterial strains were grown overnight in Tryptic Soy agar with 2% NaCl at 35° C, and further inoculated into different solid culture media according to Garcıa Moreno and Landgraf [31]. In detail, protease (caseinolytic) activity was determined by incubation at 35°C in skim milk agar plates (2% final concentration). Bacterial isolates able to produce clearing zones into the medium containing the casein substrate were recorded as positive.
Lecithinolytic activity was determined by streaking bacteria onto a nutrient basal agar containing 10% (v/v) egg yolk emulsion, further incubated at 35°C for up to 7 days. Zones of precipitation surrounding the colonies indicated the production of lecithinase.
Lipase production was tested by inclusion into a nutrient basal agar of Tween 80 (polyoxyethylene sorbitan mono-oleate, 1% final concentration) as a substrate for bacterial growth.
The production of haemolysins (i.e. Kanagawa phenomenon) was assayed on plates of Columbia agar base added with a 5% suspension of sheep red blood cells. The colonies able to produce haemolysis after incubation at 35°C for 24 hours were considered as positive.
Expression of the results and statistical data elaboration
For the shellfish samples, the number of positive tubes in the MPN procedure was recorded and the results were reported as MPN index per 100 grams of homogenate after comparison with a Mc Grady probability table. Not marine and marine bacteria as well as Vibrio spp. were reported as Colony Forming Units (CFU) per gram of sample.
Bacteriological counts of the seawater samples were expressed as the mean value of duplicate plates, and reported as CFU per 100 ml of water, except for not marine and marine bacteria, which were reported in CFU per ml of water.
Analysis of Variance (ANOVA) test was performed by Excel software to evaluate the occurrence of significant spatial variations in the bacterial abundances among the Control and Impact sites. When data failed the assumption of normal distribution, logarithmic transformation was performed before ANOVA was applied.
Pearson’s correlation was used to assess whether the abundance of bacteria was related to the environmental parameters.
Results
Temperature values ranged from a minimum of 14.10 to a maximum of 27.84°C; salinity from 35.88 to 38.12 and oxygen from 50.55 to 91.25 %.
Shellfish samples
The results obtained in the examined shellfish are shown in Table 2a,b.
In spring (Table 2a) the values of FC were generally ≤20 MPN/100 g in all the samples. Only one sample of O. edulis harvested from the Impact site showed higher concentrations (130 MPN/100 g) that, however, were still lower than 300 MPN/100 g (i.e. the value fixed for shellfish coming from areas classified as A zone). Only in this species a significant difference between the Control and the Impact sites was found, with FC values higher at these latter. The distribution of E. coli reflected that of FC, showing peak values in the O. edulis sample collected from the Impact site (40 MPN/100 g). No positive results for Salmonella spp. were obtained in the examined samples. The biochemical profiling by API 20E strips of the bacterial isolates allowed the identification of a strain of Citrobacter freundii, an Enterobacterium with biochemical characteristics similar to Salmonella spp., that was isolated from one sample of C. gigas harvested from the Impact site.
Marine and not marine bacteria showed values in the range of 102-103CFU/g, with higher concentrations for the marine bacteria and a peak of 1.1 x 104 CFU/g in M. galloprovincialis collected at Impact sites.
VP concentrations varied between 2.0 x 101 and 1.0 x 104 CFU/g recorded in C. gigas from Control site and in M. galloprovincialis from Impact site, respectively. VPP values (data not shown) were in the order of 101-102 CFU/g (2.7 x 101- 1.2 x 102 CFU/g), peaking in the mussel sample collected from Impact sites.
Regarding the qualitative composition of Vibrios (Table 3), most of the isolates were assigned to Vibrio spp., while the species V. alginolyticus and V. vulnificus were predominant within the VPP fraction of shellfish collected from the Control and Impact sites, respectively.
FC, ENT, VP and VPP abundances correlated positively with temperature (Pearson correlation coefficients r= +0.55, +0.54, +0.58 and +0.64, P<0.05, respectively).
In autumn (Table 2b), FC concentrations in shellfish collected from the Control sites were ≤ 30 MPN/100g; only C. gigas and M. galloprovincialis harvested from the Impact sites showed high concentrations (130 MPN/100g and 110 MPN/100g respectively). E. coli distribution showed values not exceeding 20 MPN/100 g in all the examined samples. Shellfish samples were also negative for Salmonella spp. The biochemical profiling by API 20E strips of the bacterial isolates identified six strains classified as Proteus, isolated from O. edulis and M. galloprovincialis collected from the Impact sites (data not shown).
A decrease in the abundance of not-marine and marine bacteria was observed, compared to the spring numbers. The highest concentrations of not marine bacteria were found in O. edulis collected from the Impact site (1.1×103 CFU/g), while the lowest ones were recorded in one C. gigas sample collected from the Impact site (3.75×102 CFU/g).
Regarding marine bacteria, a peak value was measured in the O. edulis sample collected from the Impact site (2.7×103 CFU/g), while the minimum concentration was reached in C.gigas collected from the Control site (1.2×103 CFU/g).
VP counts showed the constant presence of these microorganisms with densities in the order of 102-103 CFU/g; the highest microbial concentrations were recorded in the mussel sample collected from the Impact sites, while the lowest ones occurred in one sample of the oyster C.gigas (102 CFU/g from both the Control and Impact sites). VPP were mostly absent in the shellfish collected during autumn (data not shown).
The qualitative analysis of Vibrios (Table 4) highlighted a more diversified composition compared to the spring, with the predominance in the shellfish collected at the Impact sites of Vibrio spp., V. vulnificus and small numbers of V. alginolyticus, V. mimicus and V. parahaemolyticus. Conversely, at the Control sites, strains of V. alginolyticus, followed by Vibrio spp., V. mimicus and V. vulnificus were isolated and V. parahaemolyticus was substituted by V. fluvialis.
Water samples
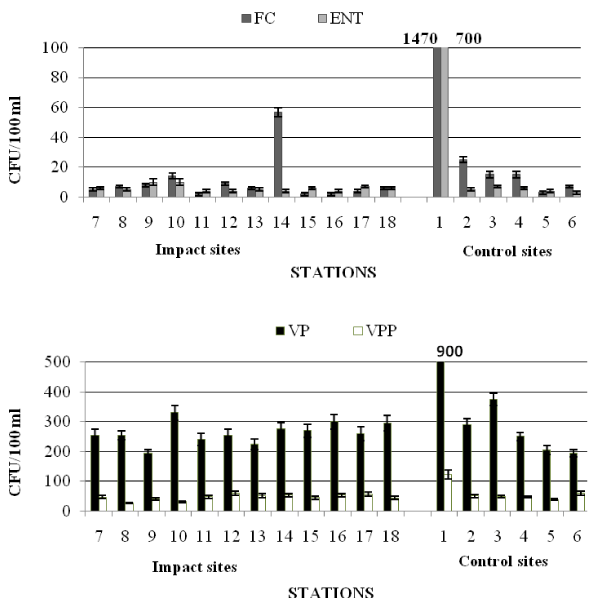
The results obtained from the seawater samples are reported in Table 5a,b and shown in Figures 2 and 3.
In spring (Table 5a and Figure 2) the examined area did not show significant faecal contamination inputs; the abundance of FC was comprised between 2×100 and 1.47×103 CFU/100 ml; the maximum value was recorded at the station 1.
E. coli was recorded at concentrations generally low, <7 CFU/100 ml, except for the station 1, where its abundance reached values two orders of magnitude higher than those of the other stations, similarly to FC distribution. No strains were identified as Salmonella spp. in the examined samples. A strain of Citrobacter freundii was isolated from the enrichment in Tetrathionate broth of the water sample drawn from station 1. Values of ENT were <10 CFU/100 ml, except for station 1.
The abundance of not marine bacteria ranged from 1.5×101 to 1.24×103 CFU/100 ml, recorded at stations 18 and 1, respectively.
Marine bacteria showed concentrations comprised between a minimum of 3.0×102 (station 9) and a maximum of 1.27×103 CFU/100 ml recorded at station 1.
VP showed the lowest concentrations at stations 6 and 9 (1.95 x 102 CFU/100 ml), while the peak value (9.0 x 102 CFU/100 ml) was found at station 1. The lowest value of VPP was measured at station 8 (2.7×101 CFU/100 ml), the highest one (1.22×102 CFU/100 ml) at station 1 (Figure 2).
The qualitative study of the Vibrios community highlighted that Vibrio spp., V. vulnificus and V. alginolyticus were predominant at the Impact sites, while V. vulnificus was not detected at the Control sites (Table 3).
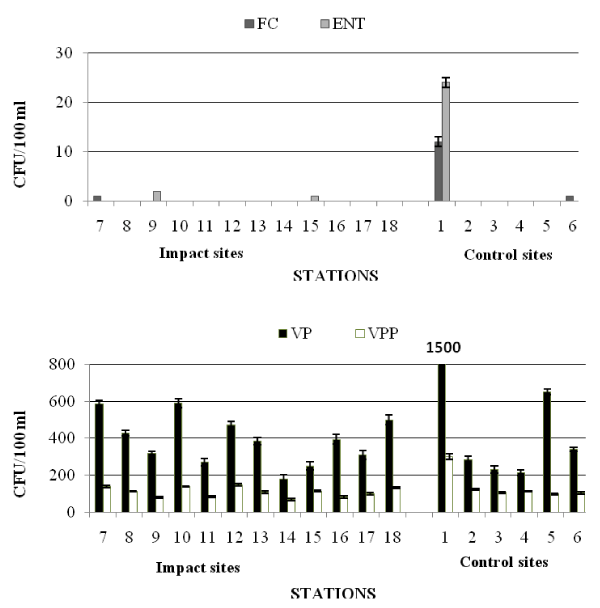
In autumn (Table 5b and Figure 3) the Castellammare Gulf exhibited very low faecal pollution levels. The highest densities of FC (1.2×101 CFU/100 ml) were detected at the station 1, while at the other stations FC were mostly absent or equal to 1 CFU/100 ml (stations 6 and 7).
E. coli was generally absent, except for the station 1, where this bacterium reached a maximum value of 10 CFU/100 ml, like FC distribution. All the examined water samples were negative for Salmonella spp. Values of ENT were generally absent except for station 1, where a maximum value of 2.4×101 CFU/100 ml was recorded.
Regarding not marine bacteria, the highest concentrations (1.44×103 CFU/100 ml) were found at the station 1; negative values were recorded at two stations (4 and 17). Station 1 was also characterized by the highest concentration of marine bacteria (7.85 x103 CFU/100 ml), while the lowest numbers were recorded at the station 14 (1.8×102 CFU/100 ml).
VP were everywhere detected at high densities, ranging around 102-103 CFU/100 ml, with the highest concentrations at the station 1 and lower values at the station 14. The quantitative distribution of VPP, with values one order of magnitude lower than VP, showed a similar course (Figure 3).
The qualitative study of Vibrios isolates (Table 4) showed that V. alginolyticus and V. vulnificus represented the predominant species within the Vibrio community at the Impact sites; at the Control ones, V. alginolyticus, V. parahaemolyticus and V. vulnificus were the most frequent isolates. No strains of V. fluvialis were isolated from the waters.
Statistical data elaboration
ANOVA calculated per each microbiological variable for shellfish and water samples showed that there were no statistically significant differences between Impact and Control sites, except for FC in shellfish sampled during the autumn (F= 7.52, P=0.033).
Virulence factors of Vibrio spp
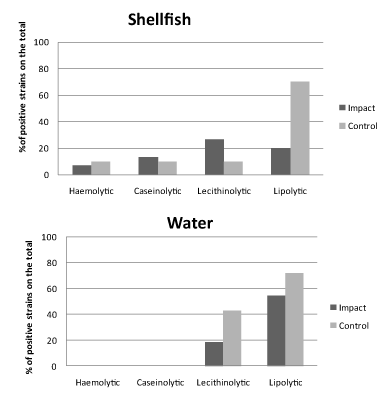
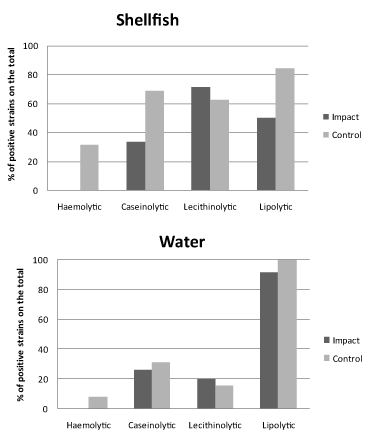
The results of the screening for enzymatic activities as proxies of potential virulence of Vibrio spp. isolates are shown in Figures 4 and 5 for spring and autumn samplings, respectively.
In spring (Figure 4), most of the strains exhibited lipolytic and lecithinolytic activities, reaching at the control sites percentages of 70% of the total for both shellfish and water samples. Conversely, for proteolytic and haemolytic activities bacterial isolates were almost negative (water origin) or slightly positive (shellfish origin, with positive strains accounting for < 13% of the total).
In autumn (Figure 5) there was an increase in the percentage of strains showing enzymatic abilities. The most evident increases in shellfish bacterial isolates were observed in the percentage of caseinolytic bacteria, which reached the 68.9% of total at the control sites, and in water bacterial isolates in the percentage of lipolytic bacteria, which reached values close to 100% at both impact and control sites. Both in shellfish and water samples the percentages of lecithinolytic bacteria increased too.
Globally, higher expression of enzymatic activities as virulence proxies were observed in bacterial strains isolated from water compared to shellfish samples. The bacterial strains isolated from shellfish samples showed higher ability to produce potential virulence factors at the impact sites compared to the control ones in summer, while an opposite trend was observed in autumn.
Discussion
The sustainable use and a correct management of resources, also through the application of eco-friendly production methods [32], could represent the winning strategies for future innovation in aquaculture sector, that is now considered a pillar for Blue Growth [33]. Accurate marine planning of the space dedicated to sea farming, together with the application of good practices for animal welfare, are priority measures to ensure sustainable aquaculture growth.
To date, European aquaculture relies on the farming of a few major species, such as sea bass, sea bream, oyster, mussel, salmon, and others minor productions, such as carp [34]. In Italy shellfish farming represents an important sector of aquaculture, with clams and mussels representing the 94.2% and 70.8% of the European aquaculture productions, respectively. In Sicily, however, the production of mussels (about 700 tons, equivalent to 0.5% of national production) is still low. Polyculture has now been regarded as an environmentally sustainable practice to increase local mussel production [16]. For shellfish farmers, however, microbiological quality of the products is often a cause of concern, causing severe economic losses. In this context, this study has aimed at determining the potential implications of a polyculture pilot experiment regarding shellfish bacteriological safety. The bacteriological quality of shellfish and of the waters surrounding the site, was assessed during two seasonal samplings by covering a wide range of parameters that included not only faecal pollution indicators, released through faecal pellets of farmed specimens, but also autochthonous microorganisms such as halophilic Vibrios. This has allowed to draw a more complete picture of the effects of IMTA on the quality of shellfish products and environment.
Faecal pollution indicators: spatial and temporal distribution
Faecal pollution indicators (FC, E.coli, ENT) do not represent by themselves pathogenic bacteria, but they provide information on the potential risk to consumers associated with the possible presence of enteric pathogens [35]. Furthermore, compared to FC - that may have different origins - E. coli is regarded as a more meaningful indicator of faecal contamination, being the main component of the intestinal flora of humans and warm-blooded animals [36]. E. coli concentrations recorded in the shellfish reared at the Impact stations were higher than those at the Control ones; nevertheless, their values fall within the threshold values (≤230 MPN/100 g of molluscs) prescribed by the Shellfish Hygiene Regulations for areas designed to aquaculture purposes (CE 853/2004 and 854/2004 [37,38]). Such low concentrations, together with the absence of Salmonella spp. in 25 g of shellfish, suggested the optimal hygienico-sanitary quality level of the production area, that could be classified as A zone. Therefore, IMTA did not have negative effects on the health quality of shellfish production. Also in the waters of the entire farming area, except for the station 1, faecal pollution indicators exhibited very low abundances, suggesting that terrigenous inputs of faecal origin were limited in the study area.
In the Castellammare Gulf waters, FC and ENT were more abundant during the spring period, however, compared to previous investigations in the same area [21,28,30], an improvement in the overall microbiological conditions was found, perhaps in relation to the start of efficient depuration systems. Moreover the absence of strains referable to Salmonella spp. confirmed the suitability of the area for shellfish farming, a productive activity which involves higher risks to human health compared to fish farming, due to the high filtering capacity of molluscs and their consequent concentration of pathogenic microorganisms.
The abundance of FC and ENT recorded in Castellammare fall in a magnitude order similar to the Mar Piccolo (Ionian Sea) by Cavallo and Stabili [27]; range: 2 - 94 MPN/ 100 ml), but significantly lower than those measured by Zaccone. et al. (log FC: 2.8 CFU/ml) [22]. Low concentrations of ENT (range: 0.55-10.70 CFU/g) were also found in the sediments of a mussel-farming area of the Gulf of Gaeta [26].
Distribution patterns of heterotrophic marine and not-marine bacteria
Bacteria have always been considered as the normal inhabitants of molluscan microflora; in oysters the presence of heterotrophic bacteria has implications not only for hatchery production but also for environmental and human health [39].
In the polyculture experiment, heterotrophic bacteria in shellfish collected at the Impact sites exhibited in both samplings almost doubled abundances than those at the Control sites, although these quantitative variations were not statistically significant. This finding could be explained by a stimulation of heterotrophic bacterial growth by feed wastes and excretions released by fish farming practices, that enriched waters with nutrients and organic substrates. At polyculture sites, mussel and clam were the species concentrating inside their body the highest bacterial abundance in spring, while in autumn O. edulis was more rich in heterotrophic bacteria.
At impact sites in spring allochthonous not-marine bacteria correlated with E. coli only weakly (r= 0.56, P<0.05), suggesting that microorganisms other than E. coli contributed to the bacterial flora present in these sites. In this season, inverse significant relationships between marine and not-marine bacteria were found, as expected (r= -0.72, P<0.01), while in autumn marine and allochtonous not-marine bacteria were directly correlated (r= 0.78, P<0.01), indicating that the reciprocal relationships between these microorganisms was modulated not only by fish farming wastes but also by other organic inputs, such as continental terrigenous matter.
Like in shellfish, in water heterotrophic bacterial abundances reached their highest values during the spring than the autumn period; bacterial growth was supported by the favorable temperature conditions (r= 0.54, P<0.05, between T and marine bacteria). In spring, marine and not-marine bacterial concentrations in water were unaffected by polyculture activities, as shown by the similar values recorded at both Impact and control sites. In autumn, higher bacterial abundances (marine and not-marine) measured at control than at impact sites were probably related to their different geographical location, being the control sites located in proximity of the coast, and therefore affected by organic inputs introduced into the marine environment.
Distribution patterns of Vibrio spp.
Members belonging to Vibrio genus are an ubiquitous component of the heterotrophic microflora autochthonous of marine and brackish environments. In shellfish collected from Castellammare, these microorganisms in spring accounted for an average percentage of the 43.2% and 11.5% of the heterotrophic bacteria at Impact and Control sites, respectively, while in autumn for the 37.0% and 29.3% of the heterotrophic bacteria at Impact and Control sites, respectively. Percentages close to 70% and 90% of heterotrophic bacteria were reached in C. gigas and M. galloprovincialis in spring, while in autumn in the same species they approached the 50% of the heterotrophic bacteria. These findings confirmed the widespread occurrence of pathogenic Vibrio species that has been documented in shellfish and water samples from several European Countries [24,27-28,40,41]. Bivalves have been regarded as an important ecological niche for Vibrios, since these bacteria can persist inside molluscan tissues even after their depuration process [42]. Indeed, molluscs normally host a diversified microbial community that varies in relation with environmental conditions, in agreement with the filtering feeding capacity of these organisms [43]. In the shellfish samples examined in our study, significantly higher concentrations than those recorded in water were observed for all microbial parameters (for VP as well as for FC, E. coli, not-marine and marine bacteria); in summer, this concentration ability was more relevant for E. coli, not-marine and marine bacteria (from 5.55 to 31.8 times), while in autumn for FC and marine bacteria (up to 5 orders of magnitude and 3 times, respectively).
Vibrio species are very frequent in aquatic ecosystems [21,44-49], and in coastal environments their concentrations can reach values higher than 103CFU/100 ml, supported by the availability of organic substrates. The abundance of Vibrios found in Castellammare waters was in the same range of magnitude as reported in a previous study in the same area [30], namely of 103 CFU/100 ml and 102 CFU/100 ml for VP and VPP respectively. Vibrio spp. concentrations of 104 CFU/100 ml were detected in seawater samples collected at a hatchery plant of Ostrea edulis located off the Mediterranean Spanish coast [50]. Higher concentrations of VP (up to 1.64x104 CFU/g) were reported in the sediments of a mussel farm in the Gulf of Gaeta (Tyrrhenian Sea) [22].
Spatial and temporal variations characterized the distribution of halophilic Vibrios in shellfish produced in the Castellammare Gulf; in spring Vibrios were about 6 times more abundant at impact than at control stations, while in autumn their spatial variations became more narrow (about 2 times between impact and control stations). In both samplings, however, ANOVA suggested that their quantitative variations in shellfish samples were not related to polyculture activities. Seasonal fluctuations in the abundance of halophilic Vibrios, with higher concentrations in summer compared to autumn, reflected the trends observed in the distribution of total heterotrophic bacteria and were consistent with the typical ecology of these bacteria, whose growth is supported by warm temperature and high nutrient availability [51]. The concentrations measured at the most coastal stations reflected the preferential distribution of Vibrios in coastal habitats rich in organic matter [28]. Moreover, in autumn at Impact sites Vibrio spp. abundances correlated significantly with FC (r= 0.92, P<0.01), indicating that both autochtonous and allochtonous bacteria were stimulated by organic fish wastes. On the other hand, the relatively low concentration of halophilic Vibrios found in waters confirmed the good health conditions of Castellammare Gulf area, already reported by Caruso, et al. [28,30]. The good bacteriological quality of farming waters is a basic requisite for productive activities, that is required also in order to limit the massive use of antibiotics and prevent possible related antibiotic resistance phenomena in aquaculture [52].
Qualitative study of Vibrio community and virulence factors
Several studies have addressed the study of Vibrio biodiversity in shellfish, given their relevance as aetiological agents of foodborne diseases [53]. In fact, other than the well-known pathogenic species V. cholerae, some Vibrios species such as V. parahaemolyticus, V. vulnificus, are primary or opportunistic pathogens responsible for severe gastroenteritis usually associated with the consumption of raw or under-cooked seafood that, through their filtering activity, concentrate these microorganisms inside their body [21,23-25,27,54]. Also, Vibrios species such as V. vulnificus are aetiological agents of wound infections and lethal septicemia [53,55] and other species (i.e. V. alginolyticus, V. anguillarum) are opportunistic pathogens responsible for animal diseases [56].
Vibrio species composition in Castellammare shellfish samples revealed that V. alginolyticus, V. vulnificus and V. parahaemolyticus were the most frequently found species. Even if a clear association was not found between these species and the site of isolation, V. vulnificus predominated at the Impact sites in spring season; conversely, in autumn the Vibrio community showed a more diversified composition, with the detection of other species such as V. mimicus and V. fluvialis.
V. alginolyticus is a common member of microflora of temperate and tropical marine environments and has been isolated from guts of different marine organisms and seafood [57]; however, it has been found to act as an opportunistic pathogen for aquatic organisms being the aetiological agent of infectious diseases in marine fish cultured in the Mediterranean Sea and of high mortality episodes in bivalve larvae [56]. V. alginolyticus was the dominant Vibrio species in several Mediterranean waters [44,45]; it is favored by haline conditions, but it can tolerate up to salinity levels of 11 [58,59].
In the Castellammare waters V. alginolyticus and V. vulnificus predominated within the Vibrio community, especially in autumn. V. vulnificus, probably favored by the low temperature compared to spring, includes three biotypes, all able to cause human infection; biotype 1 is of greatest importance to oyster producers and consumers, biotype 2 infects eels, while biotype 3 has been isolated in Israel in association with tilapia fish [60]. Infection by V. vulnificus produces pathological changes of bacterial hemorrhagic septicaemia similar to V. anguillarum in Japanese eels in Japan, cobia, seabass, rainbow trout and European eels in England, Spain, Denmark and the Netherlands [61].
V. vulnificus is often recovered from Mediterranean seawaters [62]; temperatures outside the range of 13 to 22°C and salinities greater than 25 ppt reduce the survival of this bacterium in seawater [63,64]. In Mexico, it was isolated from seawater and sediments of the Gulf of Mexico estuary [65] as well as in 31% of oysters and water samples collected from Pueblo Viejo Lagoon, Veracruz [66]; the detection of vvhA gene and some putative virulence factors suggested that this microorganism posed a health risk to both oyster consumers and fishermen.
V. parahaemolyticus is a natural inhabitant of coastal marine and estuarine environments; there is no correlation between its distribution and faecal pollution [67-69]. This species is involved in the outbreak of acute gastroenteritis associated with the consumption of raw contaminated seafood [53]. V. parahaemolyticus is also responsible for serious infections in fish species [61]. High incidence of V. parahaemolyticus was documented in oyster specimens from various Countries, including Brazil [70], Mexico [71] and USA [72]. It was also commonly isolated from shellfish (i.e. oyster and clam) collected from several Asian [73-77] and European Countries [41]. In shellfish collected from coastal areas of Southern Italy strains of V. parahaemolyticus accounted for percentages of 5.2-6.2% of the total Vibrio community [27, 78].
Another emerging human foodborne pathogen, responsible for sporadic extraintestinal diseases [79], found in the shellfish samples from Castellammare was V. fluvialis. This microorganism was first detected in oysters from Pacific Northwest during warmer seasons [80]; Chan, et al. [73] also reported V. fluvialis among the most important pathogenic Vibrios in seafood sold in the Hong Hong markets.
V. fluvialis was isolated from mussels from Brazil [58] and from bivalves from Costa Rica [81]; in Italy, Ripabelli, et al. [45] found that 11%-27% of the shellfish and shrimps contained V. fluvialis without any association between this pathogen and conventional fecal pollution indicators. Commonly found in coastal marine, estuarine and brackish environments [82], V. fluvialis was isolated in high percentages (29% of the total flora) in the Toulon harbor (France) [83], as well as in suburban effluents of South Africa (41.4% of the total [84]), where a positive relationship with seawater temperature, salinity and dissolved oxygen was observed.
The Vibrio community biodiversity found in shellfish samples collected in Castellammare polyculture area reflected that of the waters. Indeed, in oysters, V. parahaemolyticus has been found to concentrate by up to 104 times compared to the surrounding water [60], even if the mechanism of association (i.e. specific gut microbionts, resulting from selective enrichment, or aspecific uptake of transient microbiota from the aquatic environment) remains unclear [56]. Also in water the distribution of this species, together with V. alginolyticus, is closely related to temperature and salinity [71,85]. V. parahaemolyticus prefers water at temperature higher than 20°C [86], while below 16°C V. parahaemolyticus disappears from waters, surviving in the sediments [87]. V. parahaemolyticus and V. alginolyticus growth is also favoured by organic matter, therefore these species usually prefer coastal and brackish eutrophic temperate environments [51].
Through a meta-analysis of environmental variables affecting Vibrios distribution, temperature and salinity were found to be the main variables explaining the variance of total Vibrio abundance in the water, while other variables were only marginally predictive of Vibrios proliferation in the environment [56].
Seasonal variability in the Vibrios composition was found in Cape Peloro lakes by Zaccone, et al. [49], who reported high percentages (approaching 70% of the total) of V. vulnificus in winter, at temperatures of 16.11 oC, while V. parahaemolyticus was detected in autumn, winter and early spring seasons with percentages of 10-14% of the total.
The predominance of the species V. alginolyticus in Castellammare waters confirmed the ubiquitous distribution of this facultative pathogen in coastal marine waters. Similarly to this result, V. alginolyticus was the predominant component of the total culturable Vibrios in the Mar Piccolo of Taranto (Ionian Sea, Italy) [27].
The study of the expression of virulence factors as determinants of virulence has been suggested as a support tool for a better surveillance of the environmental spread of pathogens such as Vibrios. Indeed, previous studies showed the existence of a relationship between the expression of different enzymatic activities and the virulence mechanisms of bacterial organisms [31]. Particularly, virulence factors of V. parahaemolyticus are a thermostable direct hemolysin (TDH) and aTDH related hemolysin (TRH) [88], while proteases and hemolysins are considered to be relevant for the pathogenesis of V. vulnificus [53] and V. fluvialis [79]. In the Vibrios isolated from Castellammare Gulf shellfish and water, proteases, hemolysins, lipases and lecithinases were widespread. In the bacteria isolated from shellfish, higher percentages of positive responses were found in spring at impact sites, while in autumn the polyculture practice was associated to a reduction in the expression of enzymes related to proteolysis and haemolysis.The expression of enzymatic activities -as a proxy of virulence- in spring was higher in the bacterial strains isolated from water than from shellfish. In Mexico, strains of V. vulnificus isolated from oysters were found to be 100% proteolytic, 97.8% were lecithinase-positive and 79.8% lipase positive [66].
Conclusions
To our knowledge, only a few papers have dealt with the microbiological quality of shellfish produced in polyculture experiments and knowledge of bacterial communities growing in these conditions is still scarce [89]. Therefore, our study aimed at contributing to this topic in marine environments.
The analysis of whole quantitative data obtained in Castellammare Gulf confirmed that, due to their filterfeeding behaviour, shellfish were able to concentrate bacteria inside their body, compared to the surrounding waters. The simultaneous increase of faecal pollution indicators and marine heterotrophic bacteria close to polyculture activities suggested that the inputs of organic matter (such as from food residuals and faecal pellets) stimulated the bacterial growth. In spite of this, no detrimental effects due to the polyculture experiment were observed in the bacteriological quality of both shellfish and waters that fell within the standards required by current Directives for shellfish farming sites. Within the Vibrio community, V. alginolyticus and V. vulnificus displayed a mostly ubiquitous distribution in both the examined biological matrices (water and shellfish), similarly to other marine ecosystems. Future specific environmental research could be proposed to improve current understanding of shellfish-Vibrio ecological interactions.
In the light of these considerations, IMTA systems could represent an attractive, ecofriendly, practice to diversify local production, promoting the competitiveness of aquaculture and reducing the weight of importation from other Countries.
- Science for Environment Policy (2015) Sustainable Aquaculture. Future Brief 11. Brief produced for the European Commission DG Environment by the Science Communication Unit, UWE, Bristol. Link: https://bit.ly/2YNXiUv
- Karakassis I, Tsapakis M, Hatriyanni E, Papadopoulou KN, Placiti W (2000) Impact of cage farming of fish on the seabed in three Mediterranean coastal areas. ICES J Mar Sci 57: 1462-1471. Link: https://bit.ly/2SK49dJ
- Holmer M (2010) Environmental issues of fish farming in offshore waters: perspectives, concerns and research needs. Aquacult Environ Interact 1: 57-70. Link: https://bit.ly/2WELAsD
- Chopin T, Robinson SMC, Troell M, Neori A, Buschmann AH, et al. (2008) Multitrophic integration for sustainable marine aquaculture. In The Encyclopedia of ecology, ecological engineering, Edited by Jørgensen SE, Fath BD Oxford: Elsevier 3: 2463-2475. Link: https://bit.ly/3ced1Qq
- Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MC, et al. (2000) Effects of aquaculture on world fish supplies. Nature 405: 1017-1024. Link: https://go.nature.com/2yvE8bf
- Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, et al. (2004) Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231: 361-391. Link: https://bit.ly/2xNzHIp
- Sarà G, Reid G, Rinaldi A, Palmeri V, Troell M, et al. (2012) Growth and reproductive simulation of candidate shellfish species at fish cages in the southern Mediterranean: Dynamic Energy Budget (DEB) modelling for integrated multi-trophic aquaculture. Aquaculture 324-325: 259-266. Link: https://bit.ly/2Lgrqjo
- Neori A, Shpigel M, Ben-Ezra D (2000) A sustainable integrated system for culture of fish, seaweed and abalone. Aquaculture 186: 279-291. Link: https://bit.ly/3bk1ZI0
- Sorgeloos P, Olsen Y, Verreth JAJ (2011) Integrated multi-trophic aquaculture in coastal bays in China: a potential model for application in European seas? In: Proceedings of the European Aquaculture Society, Rhodes, Greece, 18-21. Link: https://bit.ly/2WD86lu
- Li C, Yu X, Peng M (2015) The roles of polyculture with Eucheuma gelatinae and Gafrarium tumidum in purification of eutrophic seawater and control of algae bloom. Mar Pollut Bull 101: 750-757. Link: https://bit.ly/3bhq8yY
- Reid GK, Cranford PJ, Robinson SMC, Filgueira R, Guyondet T (2011) Open-water Integrated Multi-Trophic Aquaculture (IMTA): Modelling the Shellfish Component. Bull Aquacul Assoc Canada 109-2: 1-13. Link: https://bit.ly/2ztaRxJ
- Chopin T (2015) Marine aquaculture in Canada: well-established monocultures of finfish and shellfish and an emerging integrated multi-trophic aquaculture (IMTA) approach including seaweeds, other invertebrates, and microbial communities. Fisheries 40: 28-31. Link: https://bit.ly/3dsyBRt
- Buck BH, Troell MF, Krause G, Angel DL, Grote B, et al. (2018) State of the art and challenges for offshore Integrated Multi-Trophic Aquaculture (IMTA). Front Mar Sci 5: 165. Link: https://bit.ly/3dpYNvS
- Alexander KA, Potts TP, Freeman S, Israel D, Johansen J, et al. (2015) The implications of aquaculture policy and regulation for the development of integrated multi-trophic aquaculture in Europe. Aquaculture443: 16-23. Link: https://bit.ly/2A83YlP
- Kleitou P, Kletou D, David J (2018) Is Europe ready for integrated multi-trophic aquaculture? A survey on the perspectives of European farmers and scientists with IMTA experience. Aquaculture 490: 136-148. Link: https://bit.ly/2SP7EQ2
- Sarà G, Zenone A, Tomasello A (2009) Growth of Mytilus galloprovincialis (Mollusca, Bivalvia) close to fish farms: a case of integrated multi-trophic aquaculture within the Tyrrhenian Sea. Hydrobiologia 636: 129-136. Link: https://bit.ly/2SRvL0m
- Forrest BM, Keeley NB, Hopkins GA, Webb SC, Clement DM (2009) Bivalve aquaculture in estuaries: Review and synthesis of oyster cultivation effects. Aquaculture 298: 1-15. Link: https://bit.ly/2SRvJFM
- Sarà G (2007) Ecological effects of aquaculture on living and non-living suspended fractions of the water column: A meta-analysis. Water Res 41: 3187-3200. Link: https://bit.ly/3dH2qOv
- Christensen NO, Larsen JL (1983) Microbiological and hygienic problems in marine aquaculture: experience and recommendation. Rapp et procès-verbaux des Réunions Cons Int pour l'Explor Mer Médit 182: 49-53. Link:
- Reilly A, Kaferstein F (1999) Food safety and products from aquaculture. J Appl Microbiol Symp 85 (Suppl): 249S-257S. Link:
- Caruso G, Maimone G, Mancuso M, Modica A, Genovese L (2004) Microbiological controls across the productive cycle of Dicentrarchus labrax L. and Sparus aurata L.: a study from the environment to the final product. Aquacult Res 35: 184-193. Link: https://bit.ly/2A83qfL
- Zaccone R, Mancuso M, Modica A, Zampino D (2005) Microbiological indicators for aquaculture impact in Mar Piccolo (Taranto, Italy). Aquacult Int 13: 167-173. Link: https://bit.ly/3bi08n5
- Caruso G, La Ferla R, Azzaro M, Zoppini A, Marino G, et al. (2015) Microbial assemblages for environmental quality assessment: Knowledge, gaps and usefulness in the European Marine Strategy Framework Directive. Crit Rev Microbiol 46: 883-904. Link: https://bit.ly/2YTHSOB
- Maugeri TL, Caccamo D, Gugliandolo C (2000) Potentially pathogenic vibrios in brackish waters and mussels. J Appl Microbiol 89: 261-266. Link: https://bit.ly/35KYXLL
- Croci L, Serratore P, Cozzi L, Stacchini A, Milandri S, et al. (2001) Detection of Vibrionaceae in mussels and in their seawater growing area. Lett Appl Microbiol 32: 57-61. Link: https://bit.ly/2YPfvkq
- La Rosa T, Mirto S, Marino A, Alonzo V, Maugeri TL, et al. (2001) Heterotrophic bacteria community and pollution indicators of mussel-farm impact in the Gulf of Gaeta (Tyrrhenian Sea). Mar Environ Res 52: 301-321. Link: https://bit.ly/35RVAmw
- Cavallo RA, Stabili L (2002) Presence of vibrios in seawater and Mytilus galloprovincialis (Lam.) from the Mar Piccolo of Taranto (Ionian Sea). Water Res 36: 3719-3726. Link: https://bit.ly/3dujLK9
- Caruso G, Zaccone R, Genovese L, Crisafi E (1998) Microbiological monitoring of Castellammare Gulf (TP) waters for their suitability in marine aquaculture. Microbiologica 21: 169-182. Link: https://bit.ly/3bhF4x9
- Caruso G, Genovese L, Mancuso M, Modica A (2003) Effects of fish farming on microbial enzyme activities and densities: comparison between three Mediterranean sites. Lett Appl Microbiol 37: 324-328. Link: https://bit.ly/2YUjdsS
- Caruso G, Genovese L, Zaccone R, Caruso R, Modica A, et al. (2016) Temporal evolution of the microbiological conditions of a Sicilian area designed for aquaculture (Castellammare Gulf, Southern Tyrrhenian Sea). Oceanography 4: 1. Link: https://bit.ly/2Wi0LJl
- Garcia Moreno ML, Landgraf M (1998) Virulence factors and pathogenicity of Vibrio vulnificus strains isolated from seafood. J Appl Microbiol 84: 747-751 Link: https://bit.ly/3blVBQC
- UNEP/CBD (2000) The Ecosystem Approach. Decision V/6. UNEP/CBD/COP/5/23. Decisions Adopted by The Conference of The Parties To The Convention On Biological Diversity At Its Fifth Meeting. Nairobi 66. Link: https://bit.ly/2SP6d46
- European Commission (2013) Strategic guidelines for the sustainable development of EU aquaculture. COM 229. Link:
- FAO (Food and Agriculture Organization of the United Nations) (2016) The state of world fisheries and aquaculture. Contributing to food security nutrition for all. Rome 200. Link: https://bit.ly/2WglA7F
- Caruso G, Zaccone R, Crisafi E (2000) Use of the indirect immunofluorescence method for detection and enumeration of Escherichia coli in seawater samples. Lett Appl Microbiol 31: 274-278. Link: https://bit.ly/35IZlui
- Odonkor ST, Ampofo JK (2013) Escherichia coli as an indicator of bacteriological quality of water: an overview. Microbiol Res 4: e2. Link: https://bit.ly/2SQgT2j
- European Community (2004b) Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for food of animal origin. Off J Eur Community L 226: 22-82. Link: https://bit.ly/2WEMSUq
- European Community (2004) Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Off J Eur CommunityL 226: 83-127. Link: https://bit.ly/35VMtBn
- Cottrell MT, Kirchman DL (2004) Single-cell analysis of bacterial growth, cell size and community structure in the Delaware estuary. Aquat Microb Ecol 34: 139-149. Link: https://bit.ly/3dvWp6S
- Su YC, Liu C (2007) Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol 24: 549-558. Link: https://bit.ly/2YPdG74
- Ottaviani D, Leoni F, Rocchegiani E, Canonico C, Potenziani S, et al. (2010) Prevalence, serotyping and molecular characterization of Vibrio parahaemolyticus in mussels from Italian growing areas, Adriatic Sea. Environ Microbiol Rep 2: 192-197. Link: https://bit.ly/2Lft3xy
- Pruzzo C, Gallo G, Canesi L (2005) Persistence of vibrios in marine bivalves: the role of interactions with haemolymph components. Environ Microbiol 7: 761-772. Link: https://bit.ly/2znSzxY
- Schmitt P, Rosa RD, Duperthuy M, Lorgeril JD, Bachere E, et al. (2012) The antimicrobial defense of the Pacific oyster, Cassostrea gigas. How diversity may compensate for scarcity in the regulation of resident/pathogenic microflora. Front Microbiol 3: 1-17. Link: https://bit.ly/2xR0K5C
- Barbieri E, Falzano L, Fiorentini C, Pianetti A, Baffone W, et al. (1999) Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl Environ Microbiol 65: 2748-2753. Link: https://bit.ly/3dBasIF
- Ripabelli G, Sammarco ML, Fanelli I, Grasso GM (2004) Detection of Salmonella, Listeria spp., Vibriospp., and Yersinia enterocolitica in frozen seafood and comparison with enumeration for faecal indicators: implication for public health. Ann Ig16: 531-539. Link: https://bit.ly/3fuW1HT
- Stabili L, Cavallo RA (2004) Biodiversity of culturable heterotrophic bacteria in the Southern Adriatic Sea Italian coastal waters. Sci Mar 68: 31-41. Link: https://bit.ly/35OFytE
- Baffone W, Tarsi R, Pane L, Campana R, Repetto B, et al. (2006) Detection of free-living and plankton-bound vibrios in coastal waters of the Adriatic Sea (Italy) and study of their pathogenicity-associated properties. Environ Microbiol 8: 1299-1305. Link: https://bit.ly/2xR0rYw
- Covazzi Harriague A, Brino MD, Zampini M, Albertelli G, Pruzzo C, et al. (2008) Vibrios in association with sedimentary crustaceans in three beaches of the northern Adriatic Sea (Italy). Mar Pollut Bull 56: 574-579. Link: https://bit.ly/2xKvWDo
- Zaccone R, Azzaro M, Azzaro F, Bergamasco A, Caruso G, et al. (2014) Seasonal dynamics of prokaryotic abundance and activities in relation to environmental parameters in a transitional aquatic ecosystem (Cape Peloro, Italy). Microb Ecol 67: 45-56. Link: https://bit.ly/2SPXBKs
- Pujalte MJ, Ortigosa M, Macián MC, Garay E (1999) Aerobic and facultative anaerobic heterotrophic bacteria associated to Mediterranean oysters and seawater. Int Microbiol 2: 259-266. Link: https://bit.ly/2WhZEcD
- Colwell RR (1984) Vibrios in the environment. Wiley, New York 1-634.
- Laganà P, Caruso G, Minutoli E, Zaccone R, Delia S (2011) Susceptibility to antibiotics of Vibrio spp. and Photobacterium damsela spp. piscicida strains isolated from Italian aquaculture farms. New Microbiol 34: 53-63. Link: https://bit.ly/2Wiy6E0
- Nair GB, Faruque SM, Sack DA (2006) Vibrios. In Emerging food pathogens, Edited by Motarjemi Y, and Adams M, Woodhead Publishing Series in Food Science, Technology and Nutrition 332-372. Link:
- Decker SD, Reynaud Y, Saulnier D (2013) First molecular evidence of cross-species induction of metalloprotease gene expression in Vibrio strains pathogenic for Pacific oyster Cassostrea gigas involving a quorum sensing system. Aquaculture 392-395: 1-7. Link: https://bit.ly/2YNPu4Q
- Oliver JD, Kaper JB (2007) Vibrio species. In Food microbiology: fundamentals and frontiers, Edited by Doyle MP, Beuchat LR, Washington, DC, USA: ASM Press 343-379. 3rd ed.
- Takemura AF, Chien DM, Polz MF (2014) Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front Microbiol 5: 38. Link: https://bit.ly/3fxfojm
- Böer SI, Heinemeyer EA, Luden K, Erler R, Gerdts G, et al. (2013) Temporal and spatial distribution patterns of potentially pathogenic Vibrio spp. at recreational beaches of the German North Sea. Microb Ecol 65: 1052-1067. Link: https://bit.ly/2YUhtQm
- Matté GR, Matté MH, Sato MI, Sanchez PS, Rivera IG, et al. (1994) Potentially pathogenic vibrios associated with mussels from a tropical region on the Atlantic coast of Brazil. J Appl Bacteriol77: 281-287. Link: https://bit.ly/2zjt0OB
- Amirmozafari N, Forohesh H, Halakoo A (2005) Occurrence of pathogenic vibrios in coastal areas of Golestan province in Iran. Arch Razi Inst 60: 35-44. Link: https://bit.ly/2SN9DUQ
- Froelich B, Oliver JD (2013) The interactions of Vibrio vulnificus and the oyster Cassostrea virginica. Microb Ecol 65: 807-816. Link: https://bit.ly/3cjqJ4G
- Austin B, Austin DA (Eds, 1993) Bacterial fish pathogens. Chichester: Ellis Horwood, 265-307. 2nd edition. Link:
- Maugeri TL, Carbone M, Fera MT, Gugliandolo C (2006) Detection and differentiation of Vibrio vulnificus in seawater and plankton of a coastal zone of the Mediterranean Sea. Res Microbiol 157: 194-200. Link: https://bit.ly/2yx32aw
- Randa MA, Polz MF, Lim E (2004) Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl Environ Microbiol 70: 5469-5476. Link: https://bit.ly/2zkFx4v
- FAO/WHO [Food and Agriculture Organization of the United Nations/World Health Organization] (2005) Risk assessment of Vibrio vulnificus in raw oysters: interpretative summary and technical report. Microbiological risk assessment series 1-135. Link: https://bit.ly/2xOdSbJ
- Lipp EK, Rodriguez-Palacios C, Rose JB (2001) Occurrence and distribution of the human pathogen Vibrio vulnificus in a subtropical Gulf of Mexico estuary. Hydrobiologia 460: 165-173. Link: https://bit.ly/2Lk02kd
- Quiñones-Ramírez E, Natividad Bonifacio I, Betancourt-Rule M, Ramirez-Vives F, Vázquez Salinas C (2010) Putative virulence factors identified in Vibrio vulnificus strains isolated from oysters and seawater in Mexico. Int J Environ Health Res 20: 395-405. Link: https://bit.ly/3cks4rI
- Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, et al. (2000) Vibrio parahaemolyticus infections in the United States, 1973-1998. J Infect Dis181: 1661-1666. Link: https://bit.ly/2WEkEsN
- Normanno G, Parisi A, Addante N, Quaglia NC, Dambrosio A, et al. (2006) Vibrio parahaemolyticus, Vibrio vulnificus and microorganisms of fecal origin in mussels (Mytilus galloprovincialis) sold in the Puglia region (Italy). Int J Food Microbiol 106: 219-222. Link: https://bit.ly/2zt6UsT
- FAO/WHO Food and Agriculture Organization of the United Nations/World Health Organization (2011) Risk assessment of Vibrio parahaemolyticus in seafood: Interpretative summary and Technical report. Microbiological Risk Assessment Series No. 16. Rome. 193. Link: https://bit.ly/35L2rhm
- Sobrinho P de S, Destro MT, Franco BD, Landgraf M (2010) Correlation between environmental factors and prevalence of Vibrio parahaemolyticus in oysters harvested in the southern coastal area of Sao Paulo State, Brazil. Appl Environ Microbiol 76: 1290-1293. Link: https://bit.ly/2WJhRPd
- Zimmerman AM, DePaola A, Bowers JC, Krantz JA, Nordstrom JL, et al. (2007) Variability of total and pathogenic Vibrio parahaemolyticus densities in northern Gulf of Mexico water and oysters. Appl Environ Microbiol 73: 7589-7596. Link: https://bit.ly/2WhUHjS
- Parveen S, Hettiarachchi KA, Bowers JC, Jones JL, Tamplin ML, et al. (2008) Seasonal distribution of total and pathogenic Vibrio parahaemolyticus in Chesapeake Bay oysters and waters. Int J Food Microbiol 128: 354-361. Link: https://bit.ly/2WBArbU
- Chan KY, Woo ML, Lam LY, French GL (1989) Vibrio parahaemolyticus and other halophilic vibrios associated with seafood in Hong Kong. J Appl Bacteriol66: 57-64. Link: https://bit.ly/2LaptVs
- Elhadi N, Radu S, Chen CH, Nishibuchi M (2004) Prevalence of potentially pathogenic Vibrio species in the seafood marketed in Malaysia. J Food Prot 67: 1469-1475. Link: https://bit.ly/2YMPceH
- Deepanjali A, Kumar HS, Karunasagar I, Karunasagar I (2005) Seasonal variation in abundance of total and pathogenic Vibrio parahaemolyticus bacteria in oysters along the southwest coast of India. Appl Environ Microbiol 71: 3575-3580. Link: https://bit.ly/2WK89Mi
- Yu WT, Jong KJ, Lin YR, Tsai S, Tey YH, et al. (2013) Prevalence of Vibrio parahaemolyticus in oyster and clam culturing environments in Taiwan. Int J Food Microbiol 160: 185-192. Link: https://bit.ly/3fBTcVd
- Kang CH, Shin YJ, Jang SC, Yu JS, Kim SK, et al. (2016) Characterization of Vibrio parahaemolyticus isolated from oysters in Korea: resistance to various antibiotics and prevalence of virulence genes. Mar Pollut Bull 118: 261-266. Link: https://bit.ly/3bmqHaF
- Di Pinto A, Ciccarese G, De Corato R, Novello L, Terio V (2008) Detection of pathogenic Vibrio parahaemolyticus in southern Italian shellfish. Food Control 19: 1037-1041. Link: https://bit.ly/2SRhZLj
- Ramamurthy T, Chowdhury G, Pazhani GP, Shinoda S (2014) Vibrio fluvialis: an emerging human pathogen. Front Microbiol 5: 91. Link: https://bit.ly/2YPSwWm
- Kelly MT, Stroh EM (1988) Occurrence of Vibrionaceae in natural and cultivated oyster populations in the Pacific Northwest. Diagn Microbiol Infect Dis9: 1-5. Link: https://bit.ly/3chWxXH
- García Cortés V, Antillón F (1990) Isolation of enteropathogenic Vibrio in bivalves and mud from the Nicoya Gulf, Costa Rica. Rev Biol Trop38: 437-440. Link: https://bit.ly/35ODj9I
- Uchiyama H (2000) Distribution of Vibriospecies isolated from aquatic environments with TCBS agar. Environ Health Prev Med4: 199-204. Link: https://bit.ly/3biJIe4
- Martin YP, Bonnefont JL (1990) Annual variations and identification of Vibrios growing at 37 degrees C in urban sewage, in mussels and in seawater at Toulon harbour (Mediterranean, France). Can J Microbiol 36: 47-52. Link: https://bit.ly/3ck0smC
- Igbinosa EO, Obi CL, Okoh AI (2011) Seasonal abundance and distribution of Vibrio species in the treated effluent of wastewater treatment facilities in suburban and urban communities of Eastern Cape Province, South Africa. J Microbiol49: 224-232. Link: https://bit.ly/2LfVnQA
- Caburlotto G, Bianchi F, Gennari M, Ghidini V, Socal G, et al. (2012) Integrated evaluation of environmental parameters influencing Vibrio occurrence in the coastal Northern Adriatic Sea (Italy) facing the Venetian lagoon. Microb Ecol 63: 20-31. Link: https://bit.ly/2xM5JnZ
- Martinez-Urtaza J, Lozano-Leon A, Varela-Pet J, Triñanes J, Pazos Y, et al. (2008) Environmental determinants of the occurrence and distribution of Vibrio parahaemolyticus in the Rias of Galicia, Spain. Appl Environ Microbiol74: 265-274. Link: https://bit.ly/2yvydmx
- Kaneko T, Colwell RR (1977) The annual cycle of Vibrio parahaemolyticus in Chesapeake Bay. Microb Ecol 4: 135-155. Link: https://bit.ly/3chW7AB
- Honda T, Iida T (1993) The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev Med Microbiol 4: 106-113. Link: https://bit.ly/2yxana3
- Zheng X, Tang J, Zhang C, Qin J, Wang Y (2017) Bacterial composition, abundance and diversity in fish polyculture and mussel-fish integrated cultured ponds in China. Aquacult Res 48: 3950-3963. Link: https://bit.ly/2SP1TSq

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
