Journal of Clinical Microbiology and Biochemical Technology
Secondary Metabolites, Anti-Diabetic, Antioxidant, Anti-Arthritic and Antimicrobial Potential of Justicia secunda for Health Benefits
Zacchaeus S Ololade1,2*, Iyadunni A Anuoluwa3, Aanuoluwa J Salemcity4, Olayinka F Onifade5, Funmilayo J Gbenga-Fabusiwa6, Oluwatimilehin G Salemcity2 and Seyi P Balogun2
2Department of Chemistry, Medicinal and Organic Chemistry Unit, University of Medical Sciences, Ondo, Nigeria
3Department of Microbiology, University of Medical Sciences, Ondo, Nigeria
4Department of Biochemistry, University of Medical Sciences, Ondo, Nigeria
5Department of Chemical and Food Sciences, Biochemistry Unit, Bells University of Technology, Ota, Nigeria
6Department of Food Science, University of Medical Sciences, Ondo, Nigeria
Cite this as
Ololade ZS, Anuoluwa IA, Salemcity AJ, Onifade OF, Gbenga-Fabusiwa FJ, Salemcity OG, et al. Secondary Metabolites, Anti-Diabetic, Antioxidant, Anti-Arthritic and Antimicrobial Potential of Justicia secunda for Health Benefits. J Clin Microbiol Biochem Technol. 2024;10(1): 009-021. DOI: 10.17352/jcmbt.000057Copyright
© 2024 Ololade ZS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The study was designed to investigate the ameliorative effect of phytochemicals in the extract of Justicia secunda (JS) on hyperglycaemia, the antioxidant status of alloxan-induced diabetic rats, and inhibitory potential on two important diabetes mellitus-associated proteins-alpha-amylase and alpha-glucosidase. At the same time to evaluate the anti-arthritic and antibacterial of the sample. The extract at doses of 200 mg/kg and 400 mg/kg was used to evaluate hyperglycaemia and antioxidants using wistar rats model. There was a significant decrease (p < 0.05) in blood insulin concentration levels observed in the diabetic control group relative to normal control, while treated groups showed relatively normal concentration levels. A significant increase (p < 0.05) in blood glucose concentration levels was observed in the diabetic control group relative to the normal control. There was a reversal of the elevated blood glucose upon treatment with JS when compared to the diabetic control. There was a significant decrease (p < 0.01) in Malondialdehyde (MDA) levels in all test groups relative to the diabetic control group. Glutathione (GSH) status, Glutathione S-transferase (GST) and catalase activities showed a significant increase in all test groups, relative to diabetic control. It was observed that 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one, and 3-amino-4-methoxybutyric acid are potential inhibitors of alpha-amylase while 1-(1-adamantyl)-3-(dimethylamino)-1-propanone, 2-(2-hydroxypropyl)-1,4-benzenediol, and 3-amino-4-methoxybutyric acid are potential inhibitors of alpha-glucosidase. Interestingly, phytochemicals in JS were shown to be a stable dual inhibitor of both α-amylase and α-glucosidase. Anti-arthritic potential values were ranged between 24.50-80.00%. The Zone of inhibition in the antibacterial assay was between 0.7-30.0 mm. Galvinoxyl and DPPH IC50 values of the extract were 50.0 µgml-1 each. The results of this study showed that the leaf of Justicia secunda possesses phytochemicals that significantly reduce blood glucose and ameliorate oxidative stress evidenced in diabetic rats. This plant may therefore serve as an excellent alternative therapy for the management of diabetes mellitus, arthritic and bacterial-related health problems and be formulated food or drug supplement.
Introduction
Phytochemicals provide an alternative approach for the control and treatment of diseases and health problems, due to the various side effects or resistance associated with conventional drugs [1,2]. Edible medicinal plants have been used by man from the beginning of human civilization as health remedies for human and animal diseases because they contain a moiety of therapeutic value [3,4]. Most of these natural products from plants are also used for prophylactic purposes [5,6]. Recently, it has been observed on a global scale that medically there is an increase in the use of natural products as remedies for different therapeutic purposes and most of the secondary metabolites have been incorporated into modern medicinal drugs [7].
Diabetes mellitus, a metabolic disorder, has been associated with elevated blood glucose and oxidative stress which play a major role in its pathogenesis and progression. Several attempts to manage the condition with orthodox medicine have been limited because of side effects observed with the use of these drugs [8,9].
Justicia secunda Vahl belongs to the family Acanthaceae [10]. The aerial parts (leaves and stems) of the plant are used to prepare natural purple tisane or tea for the treatment of amenorrhea and dysmenorrhoea in African nations [11,12]. The plant is also used traditionally to treat anaemia, abdominal pain, shortage of blood, healing infected wounds, pharyngitis, gingivitis, bronchitis, topical ulcers, gastrointestinal disorders, respiratory tract infection, fever, pain, diarrheal, liver diseases, rheumatism, arthritis [13,14].
To the best of our knowledge, there is a paucity of information on the phytochemical and therapeutic potential of the leaf extract of J. secunda (JS). Therefore, the present study was undertaken with the aim of looking into the phytochemical analysis, anti-diabetic, antioxidant, anti-arthritic, and antimicrobial potential of the leaf extract of J. secunda (JS) grown in Nigeria.
Materials and methods
Collection and identification of the sample
The leaves of the plant were collected at Harmony Estate, Ondo, Nigeria where it was grown as a vegetable. The researchers obtained permission to collect and scientifically investigate the medicinal plant. The sample was identified and authenticated as Justicia secunda Vahl (Acanthaceae) by Mr. Esimehhuai Donatus of the Department of Botany, University of Ibadan, Nigeria. The voucher specimen of the sample was deposited in the herbarium. The herbarium number for the sample is UIH-22999.
Preparation of leaf extract
Air-dried pulverised leaves were macerated and extracted with methanol and ethyl acetate (2:1) for at least 3 days with intermittent shaking after which it was subjected to filtration and the concentrated extract was refrigerated until use [15].
Evaluation of pH of the Leaves
2 g of the fresh leaves of JS was ground to paste and then extracted using 20 cm3 of distilled water. The pH of the aliquot leaves solution was measured using a digital pH meter [15].
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
The GC-MS analysis of the leaf extract of JS was carried out by means of a GCMS-QP2010 Plus (Shimadzu) system equipped with an AOC-20i auto sampler. The separations were carried out using Restek Rtx-5MS fused silica capillary column (5%-diphenyl-95%-dimethylpolysiloxane) of 30 m× 0.25 mm internal diameter (di) and 0.25 mm in film thickness [16].
Experimental animals
The animals used for this study were locally bred male Wistar rats from the University of Medical Sciences (UNIMED), Ondo Animal House. The animals were acclimatized for 1 week and maintained under the standard environmental conditions on 12 h-day/night cycle and given rat feed and water ad libitum. They were then pre-treated every morning through the oral route of administration with the JS leaf extract for 2 weeks [3].
Experimental design
In this experiment, a total of twenty-five (25) rats with body weights ranging from 80-120g were used in the experiment. The Wistar rats were divided into five groups of five rats each and were distributed in cages. The experimental animals were grouped as follows:
Group 1: Normal control (Distil water)
Group 2: Diabetic control (Distil water)
Group 3: Diabetic sterile rats treated with 200mg/kg body weight aqueous fraction of JS extract.
Group 4: Diabetic sterile rats treated with 400mg/kg body weight aqueous fraction of JS extract.
Group 5: Diabetic sterile rats treated with 5mg/kg Gibenclamide.
Induction of diabetes mellitus
Diabetes was induced by administering a single dose of 100mg/kg of alloxan via the intraperitoneal route. After three days, their blood glucose level was measured using a glucometer with the threshold being 250mg/dl glucose level [17].
Blood samples collection
The animals were euthanized and sacrificed by cervical dislocation and blood samples were collected. The pancreas was also excised and homogenized in phosphate buffer and centrifuged at 10,000 rpm for 5 minutes to obtain the supernatant preserved at 4 °C for insulin and antioxidant assays [18].
Insulin test
Insulin assay is a blood test that is used to evaluate insulin production by the beta cells in the pancreas.
Procedure: Serum insulin obtained from the whole blood was used for this assay. The microplate was coated with a capture antibody specific to insulin. The capture antibody solution (25 µl) was then added and the plate was washed with a buffer (400 µl). It was then blocked with Bovine serum albumin (BSA) to avoid non-specific binding and then incubated for 1-2hrs. An insulin standard was prepared and the samples and standards were diluted in a dilution buffer. A detection antibody labelled with (100 µl) HRP (Horseradish peroxidase) was added so as to bind to the captured insulin. It was then incubated and the capture and detection antibodies were allowed to bind the insulin in the samples. The plates were then washed with a suitable wash buffer to remove unwanted/unbound material. A substrate solution (200 µl) was then added. The reaction produced a colour change proportional to the amount of insulin present. A stop solution (50 µl) using an acidic solution was added to stop the colour development reaction. A plate reader was used to measure the absorbance of each well at 450nm wavelength [19].
Blood glucose test
This works with the principle of amperometry, where the peak current obtained during an electrochemical reaction, maintaining a constant potential between the electrodes is taken as an indicator of the concentration of the analyte. The glucometer determines the concentration of glucose in the solution.
Assessment of lipid peroxidation
Using the pancreas as a lipid-rich medium, lipid peroxidation was determined by measuring the formation of Thiobarbituric Acid Reactive Substances (TBARS) present in the test sample. The absorbance of the pink-coloured complex formed upon reaction with the chromogenic reagent, 2-thiobarbiturate, was read at 532nm with the use of a spectrophotometer [3].
Estimation of reduced glutathione (GSH) level
The method described by Onifade, et al. [3] was followed in estimating GSH level. The absorbance of the yellow colour formed upon the addition of Ellman’s reagent was read within 30 minutes at 412 nm with the use of a spectrophotometer.
Alpha-amylase and alpha-glucosidase anti-diabetic assays
Phytochemicals and protein crystal structures retrieval and preparation: The 2D structures of twenty-six (26) compounds that have been characterized from our GC-MS analysis of leaf extract J. secunda (JS) were mined from PubChem online database (https://pubchem.ncbi.nlm.nih.gov/). Using the LigPrep tool in the Maestro, Schrödinger suite, the structures were prepared into 3D structures and dockable formats by adding hydrogen atoms, ionizing at pH (7.2 0.2), and removing salt with Epik. For ionization and tautomeric state creation, the OPLS3 force field was used [20].
The X-ray crystallographic structures of two diabetes mellitus-associated proteins-alpha-amylase and alpha-glucosidase-in complex with their inhibitors (PDB ID: 4GQR and 3A4A) were retrieved from the Protein Data Bank (https://www.rcsb.org/). The structures were prepared using the Protein Preparation Wizard tool of Maestro, Schrodinger Suite; hydrogen bonds were added and optimized at pH 7.0 and minimized using the OPLS3 force field. For each protein target, the inhibitor-present at the active site of the protein-provided insight into the dockable binding regions of the protein to screen for potential inhibitors from the JS phyto-compound library generated herein above. The docking grid was generated around the regions using the Glide Receptor Grid Generation tool of the Maestro, Schrodinger suite by selecting the co-crystalized ligand [21].
Molecular docking
The molecular docking was performed using the Glide Ligand Docking tool on Maestro 11.1 [22].
MM/GBSA
The Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) continuum solvent model was used to determine the docked protein-ligand complex binding free energy. To complete this project, rotamer search techniques from the prime were used in conjunction with the OPLS3 force field and the VSGB solvent model [23].
ADMET study
To establish the pharmacokinetic profile, drug-likeness, and toxicity, the hit compounds from docking were subjected to Absorption, Distribution, Metabolism, Excretion, and Toxicity using the SwissADME (http://www.swissadme.ch) and the Pro-Tox II web servers (https://tox-new.charite.de/protox_II/), respectively [24].
Determination of anti-arthritic protein denaturation (Egg albumin assay)
The anti-arthritic activity of JS extract was determined using the fresh hen’s egg albumin assay; where aspirin was used as a reference compound [25].
Determination of In vitro Antibacterial Potential
The in vitro antibacterial potential of JS extract was determined using Agar-well diffusion assay [25].
In vitro Antioxidant Activities
- Galvinoxyl radical scavenging assay: The Galvinoxyl antioxidant and free radical scavenging of JS extract were measured according to the method described by Ololade and Anuoluwa [26] with slight modifications.
- Evaluation of antioxidant using 2,2’-Diphenyl-1-picrylhydrazil (DPPH): The DPPH antioxidant and free radical scavenging of the JS extract was determined according to the method of Ololade and Anuoluwa, et al. [26].
- Evaluation of Total Antioxidant Capacity (TAC): The TAC of JS leaf extract was determined by the phosphomolybdenum assay; where ascorbic acid was used as a reference compound [27].
Evaluation of total lycopene and beta-carotene contents
The evaluation of total lycopene and beta-carotene contents of the JS leaf were evaluated following the method described by Iheagwam, et al. [28].
Evaluation of total chlorophyll content
The evaluation of the total chlorophyll content of the JS leaf was estimated following the method described by Zhang, et al. [29].
Evaluation of Polyphenol Content (PC)
Folin-Ciocalteu reagent was used to measure the PC of the JS extract [25].
Evaluation of Total Flavonoid Content (TFC)
Aluminium chloride (AlCl3) solution was used to determine the TFC of JS extract [25].
Evaluation of Tannin Content (TC)
FeCl3/gelatine test was used to estimate the TC of the extract using tannic acid as standard [25].
Evaluation of Total Ascorbic Acid (TAA)
2,4-dinitrophenylhydrazine (2,4-DNPH) was used to estimate the TAA of JS extract using ascorbic acid as standard [25].
Results and Discussions
pH of the leaves of J. secunda (JS)
The aliquot obtained from JS leaf had moderate acidic properties with a pH of 5.8. This is within the threshold limit range of pH 3.40-6.10 which assures consumption of the plant materials [30].
Chemical constituent of the leaf extract of J. secunda (JS)
From the GC-MS analysis of JS leaf extract, twenty-six (26) therapeutically active organic compounds were identified. This accounted for 99.6% of the secondary metabolites in the extract (Table 1), and the principal phytochemicals identified were: 1-(1-adamantyl)-3-(dimethylamino)-1-propanone (15.0%), olean-12-en-28-al (14.5%), trans-phyto (12.0%), i-propyl-9-tetradecenoate (7.5%), oleic acid (5.5%), 2-amino-1-nitro-3,3,3-trifluoropropane (5.0%) and Z-10-pentadecen-1-ol(5.0%). The secondary metabolites identified in this study were entirely different from what was obtained from related species and other members of the Acanthaceae family [31].
Result of anti-diabetic biochemical assays
This study revealed that JS was able to alleviate alloxan-induced diabetes mellitus depicted by the reduced blood glucose levels at the end of the treatment period. The dose-dependent reduction in blood glucose concentration also showed that JS is a more effective anti-hyperglycaemic agent at a higher dose of 400mg/kg body weight. The result of the serum insulin concentration levels in alloxan-induced diabetic male Wistar rats treated with JS extract for a period of two weeks. There was a significant decrease (p < 0.01) in insulin concentration levels in the diabetic control group relative to normal control. However, the treated groups showed no significant changes in insulin concentration levels relative to normal control (Figure 1). Figure 2 shows the result of the blood glucose concentration levels in alloxan-induced diabetic male Wistar rats treated with JS extract for a period of two weeks. There was a significant increase (p < 0.0001) in glucose concentration levels in diabetic control relative to normal control. Conversely, there was a dose-dependent decrease in blood glucose levels in the JS-treated groups relative to the diabetic control rats, although the decrease was not as in normal rats. Figure 3 shows the result of the pancreatic MDA concentration in alloxan-induced diabetic male Wistar rats treated with JS extract for a period of two weeks. There was a significant increase (p < 0.001) in MDA levels in diabetic untreated rats relative to normal control. On the contrary, there was a dose-dependent decrease in MDA level in the group treated with doses of JS extract relative to normal control. Moreover, there was no observable difference in MDA concentration level in the group treated with higher doses (400mg/kg) of JS relative to normal control. Figure 4 shows the result of the pancreatic GSH concentration level in alloxan-induced diabetic male wistar rats treated with JS extract for a period of two weeks. A significant decrease was observed (p < 0.001) in pancreatic GSH levels in the diabetic control group relative to normal control. Dose-dependent elevation of GSH was observed in the rats administered doses of JS extract compared to the diabetic control. In Figure 5, GST activity was significantly low in the diabetic control in contrast to the normal control. Meanwhile, upon treatment with JS extract doses, there was a significant improvement in enzyme activity when compared with the diabetic control. Figure 6 showed that catalase activity was significantly reduced in the diabetic control when compared to the normal. Whereas, treatment with doses of JS extract showed significant dose-dependent enhancement of the enzyme activity compared to normal control.
Increased level of malondialdehyde (MDA) observed in the diabetic control group relative to the normal control may be indicative of alloxan-induced oxidative stress which resulted from the excessive generation of free radicals (Figure 3). The increased GSH level observed in groups treated with JS relative to diabetic control is indicative of its anti-oxidant properties which help prevent GSH depletion, thus increasing GSH levels (Figure 4). This decrease was also seen to be dose-dependent, thus showing that the anti-oxidants properties of the extract were more effective at a higher dose concentration. The increase in activities of other antioxidant parameters such as GST and catalase (Figures 5,6) in the JS-treated rats relative to the diabetic control, is in tandem with the elevated level of GSH and reduction in MDA levels in JS-treated animals in previous results.
Result of alpha-amylase and alpha-glucosidase anti-diabetic assays
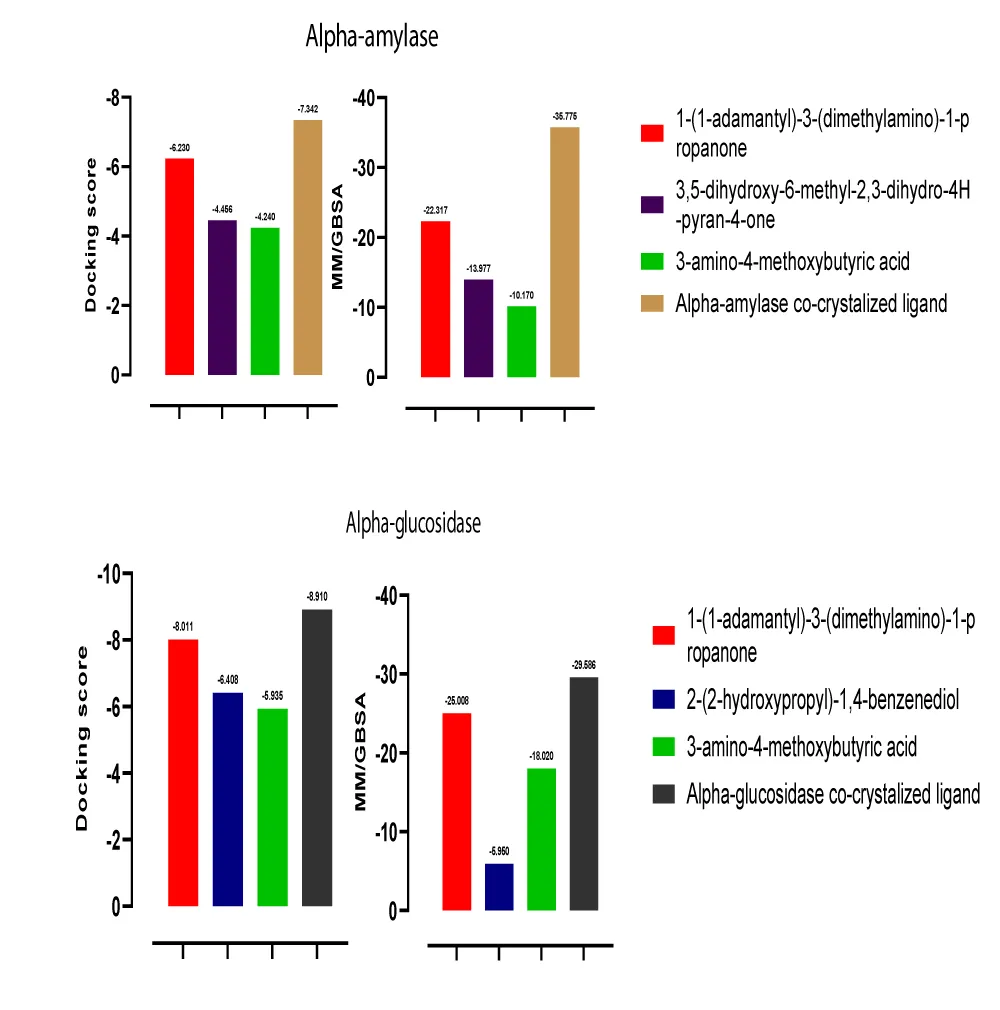
The molecular docking result showed that 1-(1-adamantyl)-3-(dimethylamino)-1-propanone, 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one, and 3-amino-4-methoxybutyric acid are potential inhibitors of alpha-amylase with their docking scores close to that of alpha-amylase co-crystalized ligand. The docking scores of the hit compounds compared with the co-crystalized ligand are; -6.230, -4.456, -4.240, and -7.342 respectively (Figures 7 and 8). In the same vein, 1-(1-adamantyl)-3-(dimethylamino)-1-propanone, 2-(2-hydroxypropyl)-1,4-benzenediol, and 3-amino-4-methoxybutyric acid are predicted potential inhibitors of alpha-glucosidase with docking scores close to the co-crystallized ligand docking score. The docking scores of these compounds compared with the co-crystallized ligand docking score are; -8.011, -6.408, -5.935, and – 8.910 respectively. The differences in the docking scores were pictorially represented in Figure 9.
The MM-GBSA method is a more precise methodology to estimate the free binding energies (∆G) of protein-ligand complexes [32]. A negative ∆G value means the complexes generated were stable in the target’s binding pocket [33]. All hit compounds show a negative ∆G value, however, 1-(1-adamantyl)-3-(dimethylamino)-1-propanone tends to be more stable at the active site of the targets by having a ∆G value very close to the co-crystalized ligands values. The ∆G values of the hit compounds and the co-crystalized ligands are represented pictorially in Figure 9. This suggests that 1-(1-adamantyl)-3-(dimethylamino)-1-propanone could be a stable dual inhibitor for both alpha-amylase and alpha-glucosidase.
Moreover, the interactions between a ligand and a combination of several amino acid residues at a target’s active site determine significant inhibition in a typical inhibitory potential study like this [34]. The interaction which contributes greatly to the inhibitory potentials of hit compounds against alpha-amylase as represented in Figures 7 and 8 showed that; 1-(1-adamantyl)-3-(dimethylamino)-1-propanone shows a hydrogen bond with GLU 233 and salt bridges with ASP 197 and GLU 233; 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one has a hydrogen bond with GLN 63; and 3-amino-4-methoxybutyric acid exhibits hydrogen bonds with ARG 195, ASP 197 and GLU 233 and additionally shows salt bridges with ASP 197 and GLU 233. Nonetheless, the alpha-amylase co-crystallized ligand shows a hydrogen bond with GLU 233 and Pi-pi stacking with TRP 59 and TRP 58.
The interaction which contributes greatly to the inhibitory potentials of the hit compounds against alpha-glucosidase as represented in Figure 7 also showed that; 1-(1-adamantyl)-3-(dimethylamino)-1-propanone has a hydrogen bond with GLU 277, pi-cation with TYR 72, and salt bridges with ASP 352, ASP 69 and ASP 215; 2-(2-hydroxypropyl)-1,4-benzenediol have pi-pi stacking with PHE 178 and hydrogen bonds with GLU 277, ASP 352, and ASP 69; and 3-amino-4-methoxybutyric acid has hydrogen bonds with GLU 277, ASP 215, and ARG 422, a pi-cation bond with PHE 178 and salt bridges with ASP 69, ASP 215, and ARG 213. Notably, alpha-glucosidase co-crystallized ligand has hydrogen bonds with ASP 352, ARG 213, ARG 442, ASP 215, ASP 69, and HIS 112.
Furthermore, the Absorption, Distribution, Metabolism, Excretion, and Toxicity screening of the hit compounds reveals that 1-(1-adamantyl)-3-(dimethylamino)-1-propanone and 2-(2-hydroxypropyl)-1,4-benzenediol tends to be more lipophilic than other compounds including the co-crystalized ligands. The ESOL model of water solubility shows that all the compounds including the co-crystalized ligands are soluble in water. This means the compounds can move well in systemic circulation [35]. The Lipinski rule of five for drug-likeness shows that all the compounds are drug-like (Table 2). The rule is valid when mol. MW < 500, QPlogPo/w < 5, donor HB ≤ 5, acceptor HB ≤ 10 [36]. Also, any drug molecules satisfying the ‘rule of five’ with a BA score of 0.55 are considered sufficiently absorbable via the oral route;[37] all the compounds except 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one also showed a positive bioavailability score (Table 2). Most of the hit compounds showed a higher level of GI absorption than the co-crystalized ligands, and the compounds with high GI absorption tend to cross the lumen of the intestine better (Table 3). Although the pharmacokinetic studies showed that 1-(1-adamantyl)-3-(dimethylamino)-1-propanone and 2-(2-hydroxypropyl)-1,4-benzenediol are drug-like, they are predicted to cross the blood-brain barrier which may render them ineffective at their target tissue (Table 4). Further study showed that alpha-glucosidase co-crystallized ligand is a substrate of P-glycoprotein, this will eventually lead to low efficacy due to the protein drug efflux action. Interestingly, none of the compounds inhibit the selected CYP isoforms; this implies that the compounds would not cause drug-drug interaction [38].
Generally, ProTox-II places chemical compounds into 6 classes of toxicity (Table 3), with Class 1 being the most toxic and Class 6 being the least toxic. The toxicity test showed that our hit compounds are less toxic with LD50 ranging from 400mg/kg to 5000mg/kg, being placed in Class 4 while alpha-amylase co-crystallized ligand falls into Class 3, hence, more toxic with LD50 of 158mg/kg (Table 4). Nevertheless, alpha-glucosidase co-crystallized ligand is neither toxic, carcinogenic, nor hepatotoxic. The toxicity test also showed that alpha-amylase co-crystallized ligand is carcinogenic, whereas our hit compounds are not carcinogenic and hepatotoxic (Tables 3, 4).
Anti-arthritic and anti-inflammatory potential of JS
Leaf extract of JS showed significantly high (IC50 of 80.00 µgml-1) anti-arthritic/anti-inflammatory potential against protein denaturation in a dose-dependent manner, 80.00±0.01, 78.00±0.10, 51.70±0.03 and 24.50±0.02 at various concentrations C1-C4 (500, 250, 125, 62.5 µgml-1), respectively (Figure S1), as compared to the aspirin with percentage inhibition of 90% at concentration of 3 mgml-1. The arthritic/anti-inflammatory activities may be due to the synergistic effects of the chemical composition of the leaf extract. Rheumatoid arthritis is a chronic disease that causes serious and painful irreversible joint damage decreased expectancy of life and workability, considerable disability, and may even increase mortality [39,40]. It was reported in previous studies that drugs derived from plants that can interact with the mediators of inflammation are used in the treatment of rheumatoid arthritis [41,42].
Antibacterial potential
The antibacterial screening of the leaf extract of JS gave a wide range of zones of inhibition against the tested strains of fourteen (14) multi-drug resistant Gram-positive and Gram-negative bacteria. The zones of inhibition of the leaf extract of JS showed high activities from sensitive to ultra-sensitive as compared to gentamicin antibiotics. The extract was active against all the tested bacteria with high ZI, AI, and RPI% values; 07-30.0 mm, 0.6-15.0, and 36.0-311.4, respectively (Figure S2-S6). The antibacterial evaluations were rated as resistant (--), not sensitive (<8 mm), sensitive (9–14 mm), very sensitive (15–19 mm) and ultrasensitive (>20 mm). Among the tested bacteria, the extract had zones of inhibition of 30 mm, respectively on K. pneumoniae and S. marcescens which indicated that K. pneumoniae and S. marcescens were highly susceptible compared to the other tested bacteria within the concentration of 1000 µgml-1 of JS in this study. As depicted in Figures 4-8, other highly susceptible bacterium at 1000 µgml-1 were Bacillus sp (20 mm), E. coli(20 mm), M. varians (20 mm), P. mirabilis (18 mm), P.stuartii (18 mm), S. dysenteriae (18 mm), S. agalactiae (18 mm), E. faecalis (15 mm), S. aureus (15 mm), S. saprophyticus (15 mm), S. typhimurium (14 mm) and P. aeruginosa (11 mm). At the concentration of 500 µgml-1 of the leaf extract, the bacteria inhibition activities were very high in K. pneumoniae (30 mm) and S. marcescens (30 mm), E. coli (20 mm), M. varians (18 mm), P. stuartii (18 mm), S. dysenteriae (18 mm), Bacillus sp (16 mm), E. faecalis (15 mm), P.mirabilis (15 mm), S. aureus (15 mm), S. saprophyticus (15 mm), S. typhimurium (14 mm) and P. aeruginosa (11 mm). The zone of inhibition of the extract at the concentration of 250 µgml-1 was significantly different when compared to 1000 and 500 µgml-1 of the extract for the tested bacteria. At a lower concentration of 250 µgml-1 of the extract, K. pneumoniae (30 mm) and S. marcescens (20 mm), M. varians (18 mm), E. faecalis (15 mm), P. mirabilis (15 mm), S. aureus (15 mm), S. dysenteriae (15 mm), P. stuartii (14 mm), S. typhimurium (14 mm), S. saprophyticus (12) and P. aeruginosa (11 mm) were more susceptible to the activities of the synergic activities of the secondary metabolites in the leaf extract, most especially the phenolic compound and the terpenoids. The activity index (AI) values are useful in determining the potential of the extract compared to the respective standards. The leaf extract investigated in this study had a higher antibacterial activity than the extract of a related species such as the essential oil obtained from the leaf of Justicia schimperiana (Acanthaceae) which has no obvious activity against E. coli and B. subtilis from Northern-Central Ethiopia [43]. Moreover, synergistic actions of all phytochemicals in the extract also contributed tremendously to the antibacterial activities observed in this study. The development of resistance to conventional antimicrobials has been a serious issue in the invention of new drugs for both acute and chronic diseases. Therefore, scientists globally are now seeking natural antimicrobial drugs from plant origins with no or very low side effects due to bacteria resistance to common synthetic and conventional drugs, malicious use of antimicrobial drugs, and high rates of allergies coupled with side effects [44,45].
Results of phytochemicals antioxidant potential
- In vitro galvinoxyl free radical scavenging assay: The galvinoxyl percentage radical scavenging of the leaf extract of JS at varying concentrations (1000. 500, 250, 125, and 62.5 µgml-1) were 96.00, 96.00, 88.62, 62.68, and 44.93% respectively. The JS gave IC50 and AAI values of 50.0 µgmL-1 and 0.8 respectively (Table 5). The extract had similar galvinoxyl antioxidant activity close to that of ascorbic acid (reference compound), which had an IC50 value of 15.0 μgml-1 and 2.8. Comparatively, the leaf extract of J. secunda investigated in this study gave a similar and promising free radical scavenging and antioxidant activity comparable with the rhizome methanolic extract of Curcuma longa from Nigeria with galvinoxyl IC50 and AAI values of 25 µgml-1and 1.68, respectively (Ololade, et al. 2020). Likewise, the seed extract of Annona cinerea grown in Nigeria exhibited a low inhibition concentration (IC50) of 100.0 µgml-1 and an antioxidant activity index (AAI) of 0.4 [46].
- In vitro DPPH free radical scavenging assay: The DPPH percentage inhibitions by JS at different concentrations (1000, 750, 500, 250, 125, 100 and 62.5 µgml-1) were 78.95, 59.44, 50.35, 48.25, 44.10, 30.17 and 26.00%, respectively. JS gave an IC50 value of 50.0 µgml-1 while the ascorbic acid (reference compound) had an IC50 value of 9.0 μgml-1 and the AAI of the extract was 0.8 (Table 5). This reaction occurs when the electron of nitrogen in the DPPH molecule is reduced by receiving a hydrogen atom or single electron from antioxidants [47]. The in vitro antioxidant potential of the leaf of the plant used in this study is greater than those of the related species such as the leaves, the stem, and root extracts of some other members of the Acanthaceae family such as B. lineriifolia, D. verticillata, D. perrotteii, H. auriculata, L. anobrya and N. canescens from Burkina Faso which have DPPH IC50 between 16.33-785.67 μgmL-1 [48]. The lower the IC50, the higher the free radical scavenging potential [49]. Comparatively, the extract investigated in this study was observed to be a good natural radical scavenger and antioxidant agent even at very low concentrations. The results showed that the extract was able to act effectively on a strong free radical molecule such as galvinoxyl with the steric hindrance among adjacent bulky groups within the galvinoxyl, the extract of the plant was able to actively scavenge galvinoxyl radicals effectively in a similar manner to DPPH.
Total Antioxidant Capacity (TAC)
The total antioxidant of the leaf extract of JS was found to be moderately high (531.54±0.00 µgmg-1 AAE) as shown in Table 5. Previous studies showed that increment of intake of natural antioxidants from plants would ameliorate the damage caused by reactive oxygen species, through scavenging the initiation or propagation of oxidative chain reaction, acting as free radical scavengers, quenchers of singlet oxygen, and reducing agents. Moreover, natural antioxidants from vegetables exhibit broad therapeutic activities, such as anti-arthritic, anti-inflammatory, anti-malaria, antibacterial, anti-viral, anti-aging, and anti-cancer [50,51].
Evaluation of lycopene and β-carotene content
The β-carotene, lycopene, and total carotenoid content of the JS extract were 0.14, 0.06, and 4.86 mgg-1, respectively as shown in Figure S7. Carotenoids have been shown to play an important role in human health [52]. Moreover, it was reported that β-carotene ameliorates the problem of erythropoietic protoporphyria and X-linked protoporphyria which are characterized by sharp pain, itching, tingling, burning sensation, erythema and swelling (oedema) resulting from exposure and hypersensitivity of the body to UV radiation, sunlight or some other artificial light [53].
Evaluation of total pigment content
Figure S7 showed that the quantitative amount of chlorophyll a, b, a+ b, and total chlorophyll in the JS extract varied significantly with the following values 21.11, 26.21, and 417.83 mgg-1, respectively. There is an increased interest of researchers in pigments such as carotenoids from edible vegetables due to the popular use and brilliant bioactivity of these natural products in foods. These plant pigments exhibited significant natural antioxidant potential against hydroperoxide generation [54,55].
Polyphenol Content (PC)
The total phenolic content (TPC) of the JS extract was 2,313.58±0.00 µgmg-1 GAE (Figure S8). The presence of these phenolic compounds such as 2-(2-hydroxypropyl)-1,4-benzenediol in the leaf extract enhanced the bioactivity of the leaf extract. The quantitative amount of polyphenol in the leaf extract investigated in this study is higher than those reported in other Acanthaceae families such as the methanolic leaf and stem extracts of L. keralensis (Acanthaceae) with TPC of139.76 and 66.98mg GAE/g [31].
Flavonoid Content (FC)
The quantitative amount of flavonoids in JS leaf extract evaluated in this study was 53.55±0.00 µgmg-1 QE (Figure S8), this is comparable to the flavonoid concentration in the methanolic leaf and stem extracts of L. keralensis (Acanthaceae) in which flavonoid contents were 258.33 and 61.67mg RE/g [31].
Tannin Content (TC)
The evaluated amount of tannin in JS extract was104.90±0.00 µgmg-1 AAE (Figure S8). Proanthocyanidins or other condensed tannins have beneficial effects on animal and human health [15].
Ascorbic acid concentration
The leaf of JS had a high amount (43.25±0.02 µgmg-1 AAE) of vitamin C and its derivatives present in the leaves of the plant (Figure S8). Ascorbic acid is an essential nutrient with potent immunity enhancement, antioxidant, bacteriostatic, anti-infection anti-atherosclerotic, anti-inflammatory, organ protection, and anti-toxic properties. High-dose of ascorbic acid improved oedema and respiratory function in critically ill patients with severe burn injury and decreased organ failure and rapid healing in patients after major surgery. Any food that is high in phenolic content and antioxidants serves as promising functional food, thus, Justicia secunda (JS) serves as a functional vegetable and food supplement among Africans [56].
Conclusion
In this study, J. secunda (JS) was found to have notable pharmacological activities. The leaves of the plant indeed possessed valuable phytochemicals, which could be exploited to control several diseases. The possible mechanism of the medicinal activities of the leaf of this plant may be related to its terpenoids and phenolic compounds. It was observed that 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one, and 3-amino-4-methoxybutyric acid are potential inhibitors of alpha-amylase while 1-(1-adamantyl)-3-(dimethylamino)-1-propanone, 2-(2-hydroxypropyl)-1,4-benzenediol, and 3-amino-4-methoxybutyric acid are potential inhibitors of alpha-glucosidase when compared to the previously reported inhibitor of each target. Interestingly, 1-(1-adamantyl)-3-(dimethylamino)-1-propanone from J. secunda was shown to be a stable dual inhibitor of both alpha-amylase and alpha-glucosidase. These results showed that the leaves of the plant studied are a potential source of dietary anti-diabetic, antioxidant, anti-arthritic, anti-inflammatory, analgesic, and antimicrobial agents and demonstrate the importance of this plant in medicine and in assisting primary health care in this part of the world. The study showed that phenolic compounds such as flavonoids, flavanols, tannins, etc are part of the major phytochemicals that played major roles in the therapeutic activities of the leaf extract of the plant investigated scientifically.
Data availability statement
Data supporting the findings of this study are available within the article and supplementary materials.
Authors contribution
ZSO: Conceptualization, Design, Recourses, Investigation, Data collection, Analysis, Writing, Edited and Approved the final manuscript.
IAA, AJS, OFO, FJGF, OGS and SPB: Investigation, Data collection, Analysis, Writing, Edited and Approved the final manuscript.
We really appreciate Dr. Rachel A. Ololade for her support in making the research a reality, most especially during the growth of the plant sample and running of the antibacterial test. Likewise, Mr. Esimehhuai Donatus is well appreciated for the botanical identification and authentication of the sample.
- Gbenga-Fabusiwa FJ, Jeff-Agboola YA, Ololade ZS, Akinrinmade R, Agbaje DO. Waste-to-wealth; nutritional potential of five selected fruit peels and their health benefits: A review. Afr J Food Sci. 2022;16(7):172-83. Available from: http://dx.doi.org/10.5897/AJFS2021.2138
- Ololade ZS, Onifade OF, Eze JC, Oyebanji OT, Olaniran AC, Anuoluwa IA, et al. Integrative phytochemical, ligand structure based drug design nephroprotective potential of Annona muricata flower-petals. Nat Prod Res. 2024a;1-6. Available from: https://doi.org/10.1080/14786419.2024.2342554
- Onifade OF, Ayodele PF, Ololade ZS, Balogun DO. Mitigative effects of coconut oil and its water on the lungs of male albino rats exposed to petrol vapour. Biomed J Sci Tech Res. 2020;25(1):18865-73. http://dx.doi.org/10.26717/BJSTR.2020.25.004155
- Adesina AR, Ogunmoyela OAB, Arisa NU, Ololade ZS. Optimization of the production of local cheese from cow milk processed with the seed of Moringa oleifera. J Food Process Preserv. 2022a;46(1). Available from: https://doi.org/10.1111/jfpp.16189
- Milutinovici R-A, Chioran D, Buzatu R, Macasoi I, Razvan S, Chioibas R, et al. Vegetal compounds as sources of prophylactic and therapeutic agents in dentistry. Plants. 2021;10(10):2148. Available from: https://doi.org/10.3390/plants10102148
- Farinacci P, Mevissen M, Ayrle H, Maurer V, Sørensen Dalgaard T, Melzig MF, et al. Medicinal plants for prophylaxis and therapy of common infectious diseases in poultry - a systematic review of in vivo studies. Planta Med. 2022;88(3-04):200-17. Available from: https://doi.org/10.1055/a-1543-5502
- Chaachouay N, Zidane L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates. 2024;3(1):184-207. Available from: https://doi.org/10.3390/ddc3010011
- Ololade ZS, Onifade OF, Olugboye BO, Alabi TE, Faleye BC, Jimson FE. Hepatoprotective efficacy of phytochemicals in Clerodendrum volubile as potential natural inhibitor on CYP2E1 protein implicated for toxicity in liver. J Toxicol Risk Assess. 2024d;10(1):1-11. Available from: https://clinmedjournals.org/articles/ijtra/international-journal-of-toxicology-and-risk-assessment-ijtra-10-057.php?jid=ijtra
- Martemucci G, Fracchiolla G, Muraglia M, Tardugno R, Dibenedetto RS, D’Alessandro AG. Metabolic syndrome: A narrative review from the oxidative stress to the management of related diseases. Antioxidants. 2023;12(12):2091. Available from: https://doi.org/10.3390/antiox12122091
- Świątek Ł, Sieniawska E, Sinan KI, Zengin G, Boguszewska A, et al. Chemical characterization of different extracts of Justicia secunda Vahl and determination of their anti-oxidant, anti-enzymatic, anti-viral, and cytotoxic properties. Antioxidants (Basel). 2023;17;12(2):509. Available from: https://doi.org/10.3390/antiox12020509
- Onochie AU, Oli AH, Oli AN, Ezeigwe OC, Nwaka AC, Okani CO, et al. The pharmacobiochemical effects of ethanol extract of Justicia secunda Vahl leaves in Rattus norvegicus. J Exp Pharmacol. 2020;2;12:423-37. Available from: https://doi.org/10.2147/jep.s267443
- Kamlo Kamso VF, Dongmo Melogmo YK, Tchegnitegni BT, Tchatat Tali MB, Dize D, Ngansop CN, et al. New lignan glycosides from Justicia secunda Vahl (Acanthaceae) with antimicrobial and antiparasitic properties. Heliyon. 2023;27;9(12). Available from: https://doi.org/10.1016/j.heliyon.2023.e22897
- Carneiro MRB, Sallum LO, Martins JLR, Peixoto JdC, Napolitano HB, Rosseto LP. Overview of the Justicia genus: Insights into its chemical diversity and biological potential. Molecules. 2023;28(3):1190. Available from: https://doi.org/10.3390/molecules28031190
- Ajayi EB, Adeyeni EG, Igereh BL, Bamgbose JT, Majolagbe ON, Odinakaose EL. Organotoxic effect of aqueous extract of Justicia secunda Vahl. leaf in Wistar rats. Int Res J Pure Appl Chem. 2023;24(5):16-23. Available from: https://doi.org/10.9734/irjpac/2023/v24i5821
- Ololade ZS, Abam EO, Anuoluwa IA, Abiona OO. Secondary metabolites, pharmacognostic and therapeutic activities of the rhizome extract of Curcuma longa grown in South-West, Nigeria. J Phytopharmacol. 2020;9(1):30-7. Available from: http://dx.doi.org/10.31254/phyto.2020.9106
- Ogagaoghene AJ. pH level, ascorbic acid, proline and soluble sugar as bio-indicators for pollution. ChemSearch J. 2017;8(2):41-9. Available from: https://africanstudieslibrary.org/en/discovery/record/base-ftjafricanj-oai-ojs-ajol-info-article-166247/
- Kuyoro SE, Akinloye OA, Ololade ZS, Kayode OT, Badejo OT. Anti-diabetic properties of methanolic extract of Launaea taraxacifolia (Wild Lettuce). Niger J Biochem Mol Biol. 2017;32(1):67-77. Available from: https://www.nsbmb.org.ng/journals/index.php/njbmb/article/view/115
- Begum N, Nasir A, Parveen Z, Muhammad T, Ahmed A, Farman S, et al. Evaluation of the hypoglycemic activity of Morchella conica by targeting protein tyrosine phosphatase 1B. Front Pharmacol. 2021;12:661803. Available from: https://doi.org/10.3389/fphar.2021.661803
- Cai D, Fraunfelder M, Chen SY. Blood serum analysis: A modified sandwich enzyme-linked immunosorbent assay protocol. MethodsX. 2022;22;9:101923. Available from: https://doi.org/10.1016/j.mex.2022.101923
- Ololade ZS, Oyebanji OT, Onifade OF, Olaniran AC, Idowu OO, Quadri-Oloye MT, et al. Inhibitory potential of Struchium sparganophora phytochemicals on cyclooxygenases and their health implications on inflammations and pains. Int Arch Addict Res Med. 2024c;9(1):1-8. Available from: https://clinmedjournals.org/articles/iaarm/international-archives-of-addiction-research-and-medicine-iaarm-9-039.php?jid=iaarm
- Ogunyemi OM, Gyebi GA, Saheed A, Paul J, Nwaneri-Chidozie V, Olorundare O, et al. Inhibition mechanism of alpha-amylase, a diabetes target, by a steroidal pregnane and pregnane glycosides derived from Gongronema latifolium Benth. Front Mol Biosci. 2022;10;9:866719. Available from: https://doi.org/10.3389/fmolb.2022.866719
- Schrödinger LLC. Schrödinger Suite, 2. New York, NY: Schrödinger, LLC; 2017.
- Ololade ZS, Ojo OA, Onifade OF, Akinnawo CA, Orodepo GO, Oyebanji OT, et al. Chemoinformatic study on phytochemicals from Melissa officinalis for ligand based drug design inhibition of aflatoxins synthesis. Toxin Rev. 2024b;43(2):1-17. Available from: https://doi.org/10.1080/15569543.2024.2344472
- Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46(W1): :W257-W263. Available from: https://doi.org/10.1093/nar/gky318
- Ololade ZS, Ogunmola OO, Kuyooro SE, Abiona OO. Stachytarpheta jamaicensis leaf extract: Chemical composition, antioxidant, anti-arthritic, anti-inflammatory and bactericidal potentials. J Sci Innov Res. 2017;6(4):119-25. Available from: https://www.jsirjournal.com/Vol6_Issue4_01.pdf
- Ololade ZS, Anuoluwa IA. Compositional analysis, antioxidant and antimicrobial potential of the seed extract of Annona cinerea Dunal grown in Nigeria. Global J Med Res (C) Microbiol Pathol. 2020;20(3):17-25. Available from: https://medicalresearchjournal.org/index.php/GJMR/article/view/2131
- Encarnacao S, Lima K, Malu Q, Caldeira GI, Duarte MP, Rocha J, et al. An integrated approach to the anti-inflammatory, antioxidant, and genotoxic potential of Portuguese traditional preparations from the bark of Anacardium occidentale L. Plants. 2024;13(3):420. Available from: https://doi.org/10.3390/plants13030420
- Iheagwam FN, Israel EN, Kayode KO, DeCampos OC, Ogunlana OO, Chinedu SN. Nauclea latifolia Sm. leaf extracts extenuates free radicals, inflammation, and diabetes-linked enzymes. Oxid Med Cell Longev. 2020;2020:5612486. Available from: https://doi.org/10.1155/2020/5612486
- Zhang H, Ge Y, Xie X, Atefi A, Wijewardane NK, Thapa S. High throughput analysis of leaf chlorophyll content in sorghum using RGB, hyperspectral, and fluorescence imaging and sensor fusion. Plant Methods. 2022;18(1):60. Available from: https://doi.org/10.1186/s13007-022-00892-0
- El-Sohaimy SA, Masry SHD, Shehata MG. Physicochemical characteristics of honey from different origins. Ann Agric Sci. 2015;60(2):279-87. Available from: https://doi.org/10.1016/j.aoas.2015.10.015
- Palakkal L, Hukuman NHZ, Mullappally J. Antioxidant activities and chemical composition of various crude extracts of Lepidagathis keralensis. J Appl Pharm Sci. 2017;7(06):182-9. Available from: https://japsonline.com/admin/php/uploads/2312_pdf.pdf
- Bandyopadhyay S, Abiodun OA, Ogboo BC, Kola-Mustapha AT, Attah EI, Edemhanria L, et al. Polypharmacology of some medicinal plant metabolites against SARS-CoV-2 and host targets: Molecular dynamics evaluation of NSP9 RNA binding protein. J Biomol Struct Dyn. 2021;1-17. Available from: https://chemrxiv.org/engage/chemrxiv/article-details/60c74fd7ee301c9693c7a779
- Bathula R, Muddagoni N, Lanka G, Dasari M, Potlapally SR. Glide docking, Autodock, binding free energy and drug-likeness studies for prediction of potential inhibitors of cyclin-dependent kinase 14 protein in Wnt signaling pathway. 2021; 12:2473-24788. Available from: https://biointerfaceresearch.com/wp-content/uploads/2021/06/20695837122.24732488.pdf
- Olubode SO, Omotuyi OI, Fadipe DO. Computational prediction of HCV RNA polymerase inhibitors from alkaloid library. Letter Appl NanoBioScience. 2021;11(3):3661-71. Available from: https://nanobioletters.com/wp-content/uploads/2021/09/22846808113.36613671.pdf
- Akinnusi PA, Olubode SO, Salaudeen WA. Molecular binding studies of anthocyanins with multiple antiviral activities against SARS-CoV-2. Bull Natl Res Cent. 2022;46:102. Available from: https://doi.org/10.1186/s42269-022-00786-0
- Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337-41. Available from: https://doi.org/10.1016/j.ddtec.2004.11.007
- Mazumder K, Hossain ME, Aktar A, Mohiuddin M, Sarkar KK, Biswas B, Aziz MA, Abid MA, Fukase K. In silico analysis and experimental evaluation of ester prodrugs of ketoprofen for oral delivery: With a view to reduce toxicity. Processes. 2021;9(12):2221. Available from: https://doi.org/10.3390/pr9122221
- Omoboyowa DA, Balogun TA, Saibu OA, Chukwudozie OS, Alausa A, Olubode SO, et al. Structure-based discovery of selective CYP17A1 inhibitors for castration-resistant prostate cancer treatment. Biol Methods Protoc. 2021;7(1): bpab026. Available from: https://doi.org/10.1093/biomethods/bpab026
- Oberemok VV, Andreeva O, Laikova K, Alieva E, Temirova Z. Rheumatoid arthritis has won the battle but not the war: How many joints will we save tomorrow? Medicina. 2023;59(10):1853. Available from: https://doi.org/10.3390/medicina59101853
- Sharma V, Shukla SS, Gidwani B, Pandey RK. Antiarthritic activity and inflammatory mediators modulation effect of traditional Ajmodadi Churna on arthritis experimental model. J Pharmacopuncture. 2023;26(3):257-64. Available from: https://doi.org/10.3831/kpi.2023.26.3.257
- Zhao X, Kim Y-R, Min Y, Zhao Y, Do K, Son Y-O. Natural plant extracts and compounds for rheumatoid arthritis therapy. Medicina. 2021;57(3):266. Available from: https://doi.org/10.3390/medicina57030266
- Faustino C, Pinheiro L, Duarte N. Triterpenes as potential drug candidates for rheumatoid arthritis treatment. Life. 2023;13(7):1514. Available from: https://doi.org/10.3390/life13071514
- Abebe W, Zhang W, Zhang S, Xie G. Chemical composition and antimicrobial activity of essential oil from Justicia schimperiana. J Pharmacogn Nat Prod. 2018;4(2):1-3. Available from: https://www.hilarispublisher.com/open-access/chemical-composition-and-antimicrobial-activity-of-essential-oil-from-emjusticia-schimperianaem-2472-0992-1000154.pdf
- Alao FO, Ololade ZS, Fagge YA. Evaluation of the antibacterial potential of the phytochemicals in leaf extracts of Newboudia laevis on uropathogens. J Bacteriol Parasitol. 2021;S10(003):1-3. Available from: https://www.walshmedicalmedia.com/open-access/evaluation-of-the-antibacterial-potential-of-the-phytochemicals-in-leaf-extracts-of-emnewboudia-laevisem-on-uropathogens-78296.html
- Oyelese OJ, Olawore NO, Ololade ZS. Comparative study of the phytochemical and bioactivities of the essential oils from ripe and unripe seeds of Azadirachta indica. J Med Res. 2020;6(5):219-24. Available from: https://www.medicinearticle.com/JMR_20205_10.pdf
- Menon A, Soman R, Rodrigues C, Phadke S, Agashe VM. Careful interpretation of the wound status is needed with use of antibiotic impregnated biodegradable synthetic pure calcium sulfate beads: Series of 39 cases. J Bone Jt Infect. 2018;3(2):87-93 https://doi.org/10.7150/jbji.22684
- Habermann E, Imatomi M, Pontes FC, Gualtieri SCJ. Antioxidant activity and phenol content of extracts of bark, stems, and young and mature leaves from Blepharocalyx salicifolius (Kunth) O. Berg. Braz J Biol. 2016;76(4):898-904. Available from: https://doi.org/10.1590/1519-6984.03815
- Sawadogo WR, Meda A, Lamien CE, Kiendrebeogo M, Guissou IP, Nacoulma OG. Phenolic content and antioxidant activity of six Acanthaceae from Burkina Faso. J Biol Sci. 2006;6(2):249-52. Available from: https://doi.org/10.3923/jbs.2006.249.252
- Anokwuru C, Sigidi M, Boukandou M, Tshisikhawe P, Traore A, Potgieter N. Antioxidant activity and spectroscopic characteristics of extractable and non-extractable phenolics from Terminalia sericea Burch. ex DC. 2018;23:1303. Available from: https://doi.org/10.3390/molecules23061303
- Losada-Barreiro S, Sezgin-Bayindir Z, Paiva-Martins F, Bravo-Díaz C. Biochemistry of antioxidants: Mechanisms and pharmaceutical applications. Biomedicines. 2022;10(12):3051. Available from: https://doi.org/10.3390/biomedicines10123051
- Chaudhary P, Janmeda P, Docea AO, Yeskaliyeva B, Abdull Razis AF, Modu B, Calina D, Sharifi-Rad J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front Chem. 2023;11:1158198. Available from: https://doi.org/10.3389/fchem.2023.1158198
- Crupi P, Faienza MF, Naeem MY, Corbo F, Clodoveo ML, Muraglia M. Overview of the potential beneficial effects of carotenoids on consumer health and well-being. Antioxidants. 2023;12(5):1069. Available from: https://doi.org/10.3390/antiox12051069
- Leaf RK, Dickey AK. How I treat erythropoietic protoporphyria and X-linked protoporphyria. Blood. 2023;141(24):2921-31. Available from: https://doi.org/10.1182/blood.2022018688
- Saini RK, Prasad P, Lokesh V, Shang X, Shin J, Keum YS, Lee JH. Carotenoids: Dietary sources, extraction, encapsulation, bioavailability, and health benefits—a review of recent advancements. Antioxidants (Basel). 2022;11(4):795. Available from: https://doi.org/10.3390/antiox11040795
- Serino I, Squillaci G, Errichiello S, Carbone V, Baraldi L, La Cara F, et al. Antioxidant capacity of carotenoid extracts from the haloarchaeon Halorhabdus utahensis. Antioxidants. 2023;12(10):1840. Available from: https://doi.org/10.3390/antiox12101840
- Bako B. Applications of Justicia secunda extracts in functional foods and natural products: A review. Adv J Chem B. 2023;6:1-10. Available from: https://doi.org/10.48309/ajcb.2024.412568.1192

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.









 Save to Mendeley
Save to Mendeley
