Journal of Clinical Microbiology and Biochemical Technology
Phytomediated synthesis of silver nanoparticles using Lepidium sativum L. and their antifungal and cytotoxic potential
Miran A El-Haggar1*, Lobna S El-Hosseiny2, Nabila M Ghazy3, Fathy K El-Fiky4 and Amr M El-Hawiet3
2Department of Microbiology and Immunology, Institute of Graduate Studies and Research, Alexandria University, Alexandria, Egypt
3Department of Pharmacognosy, Faculty of Pharmacy, Alexandria University, Alexandria, Egypt
4Department of Pharmacognosy, Faculty of Pharmacy, Delta University for Science and Technology, Dakhliya, Egypt
Cite this as
El-Haggar MA, El-Hosseiny LS, Ghazy NM, El-Fiky FK, El-Hawiet AM (2024) Phytomediated synthesis of silver nanoparticles using Lepidium sativum L. and their antifungal and cytotoxic potential. J Clin Microbiol Biochem Technol 10(1): 001-008. DOI: 10.17352/jcmbt.000056Copyright
© 2024 El-Haggar MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Bio-inspired synthesis of nanoparticles has received immense attention recently due to their vast applications in the biomedical field. Herein, a facile process using aqueous extracts of Lepidium sativum was used for the synthesis of nanosilver. The phytosynthesized silver nanoparticles showed characteristic silver surface plasmon absorption peaks at 420 and 440 nm for nanoparticles synthesized by Lepidium sativum seeds and leaves, respectively. Moreover, the spherical, non-aggregated nanoparticles exhibited a particle size of 150 nm and a zetapotential of -15.2 mv countering 111 nm and a zeta potential of -20 mv for those phytosynthesized by Lepidium sativum seeds and leave aqueous extract correspondingly. Both phytofabricated silver nanoparticles exhibited a potential antifungal effect against Candida albicans with a minimum inhibitory concentration of 1.75 and 2.08 ppm and a promising cytotoxic effect against MCF-7 breast cancer cell line with an IC50 of 20.1 and 9.3 ppm for nanosilver phytosynthesized by seeds and leaves extracts respectively. The current work provided a simple, environmentally friendly approach for the green synthesis of silver nanoparticles with a potential anticandidal and cytotoxic action against the MCF-7 breast cancer cell line. However, more investigation is required to clarify their safety and precise mode of action.
Graphical abstract
Abbreviations
AgNPs: Silver Nanoparticles; C. albicans: Candida albicans; IC50: Concentration of the sample required to inhibit 50%; IZ: Inhibition Zone; MIC: Minimum Inhibitory Concentration; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NanoZS: NanoZetasizer; SEM: Scanning Electron Microscope; UV-vis: Ultraviolet-Visible Spectroscopy
Introduction
With the advent of nanotechnology, nanoparticles have attained strong scientific space opening a vast spectrum of applications and research contexts [1]. Nanoparticles exhibit unique physico-chemical properties that are significantly different from their bulk counterparts. Silver nanoparticles (AgNPs), in particular, are heading the present scenario owing to their exceptional physical, chemical, and biological properties, which are of immense potential in various applications including biomedical, pharmaceutical, environmental, optical, and electronic fields [2].
Cancer and antibiotic resistance are amongst the top global health concerns. In the recent past, silver nanoparticles have received growing attention due to their renowned antimicrobial properties [3]. Although the antibacterial effect of AgNPs has been widely described in the literature, their antifungal effect has not received comparable attention [4-6]. Simultaneously, nanoparticles are emerging as new prospects for the detection, diagnosis, and treatment of cancer with a special interest in the anticancer potential of silver nanoparticles [7].
Different approaches have been employed for nanoparticle synthesis including mainly physical and chemical methods. Despite the fact that these methods have effective yields, they are associated with several shortcomings. Physical methods require costly equipment, high temperatures, and pressure and chemical methods involve the extensive use of toxic chemicals, non-polar solvents, and synthetic capping agents hence limiting their application in the biomedical domain [8]. Accordingly, research has recently turned towards bio-inspired methods for the synthesis of nanoparticles in the essence of the distinguished advantages they offer in terms of simplicity, accessibility, cost, and eco-friendliness [9]. The natural resources for the green biosynthesis of nanoparticles include bacteria, yeast, algae, and plants. The utilization of plants for the biosynthesis of metallic nanoparticles has spurred numerous investigations presumably due to their omnipresence and phytochemical versatility [10,11]. Moreover, it is thought that phytogenic metallic nanoparticles may be more suited to clinical approaches than those fabricated by physical or chemical methods.
The use of plants for the biosynthesis of metallic nanoparticles is based on the ability of plant systems to uptake, accumulate, utilize, and recycle different mineral species. As aforementioned, plants are known to harbor a wide range of secondary metabolites that act as bio-reducing agents to produce nanoparticles from their metal salts [12]. Many researchers reported plant-based green synthesis of silver nanoparticles using extracts of different plant parts such as leaves, roots, stems, peels, and fruits, and the diverse phytochemical composition of the extracts used entailed their complex role as reducing, capping, and stabilizing agents in the synthesis process [13-16]. Accordingly, the phytofabricated silver nanoparticles have unique properties enhancing their potential in biomedical applications meanwhile decreasing their hazardous implications on human health and the environment.
Garden cress (Lepidium sativum), belonging to the family Brassicaceae, is a traditional herb native to Egypt and is presently cultivated globally. Its folk curative claims have encouraged several pharmacological investigations. Meanwhile, phytochemical investigations on L. sativum revealed the presence of various constituents including flavonoids, glycosides, polyketides, vitamins, minerals, proteins, fats, carbohydrates, as well as, mucilage, sterols, carotene, volatile and fixed oils. Other chemical compounds identified in L. sativum included isorhamnetin, quercetin, kaempferol, protocatechuic acid, and staphylionosides A [17].
Amid the cropping methods of nanosynthesis, green-mediated synthesis of nanoparticles is evolving as one of the active areas of current nano-biotechnological research. Hence, the present study aims to investigate the potentiality of L. sativum for green-mediated synthesis of silver nanoparticles and evaluate the antifungal and anticancer potential of the phytofabricated nanosilver.
Methods
Plant material and extract preparation
Seeds and leaves of L. sativum were purchased from the local market, in Alexandria, Egypt. The plant was identified by Dr. Salama Aldarier, Faculty of Science, Alexandria University. Voucher specimens (LSS 2018/11 and LSL 2018/11) were deposited at the Herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Alexandria University, Egypt. The plant material was thoroughly washed several times with deionized water. A 10% aqueous extract was prepared and heated at 60 ºC for 15 minutes, cooled, and then filtered thrice using Whatman filter paper No. 1. The respectively collected filtrate of seeds/leaves extract was used for the subsequent phytosynthesis of nanoparticles.
Phytosynthesis of silver nanoparticles
For the synthesis of silver nanoparticles, 100 ml of 1 mM silver nitrate solution (AgNO3, Sigma Aldrich®, UK) was mixed separately with 10 ml of the aqueous extract of Lepidium sativum seeds or leaves. The mixture was left at room temperature and observed for color change as a preliminary indication of nanosilver formation. The resulting nanoparticles in the colloidal solution were characterized and the concentration of nanosilver was determined using inductively coupled plasma optical emission spectroscopy (ICP-OES 5100, Agilent Technologies®, Australia).
Characterization of phytosynthesized silver nanoparticles
The formation of AgNPs was scanned for characteristic plasmon resonance by UV-Vis spectrophotometry (PG Instruments Ltd®, UK) in the range of 300-650 nm. The surface morphology of the phytosynthesized AgNPs was characterized by scanning electron microscopy (JSM 5300, JEOL®, Japan). The particle size distribution and surface charge of the prepared nanosilver were assessed by zetapotentiometry using a zetasizer (NanoZS Ver. 6.20, Malvern®, UK).
Assessment of the antifungal activity of phytosynthesized nanosilver
The antifungal activity of phytosynthesized silver nanoparticles was assessed against Candida albicans (ATCC 18804) using the agar diffusion method [18]. Overnight culture of C. albicans in Potato Dextrose Broth (PDB, Oxoid®) was adjusted to 0.5 McFarland turbidity standard and was then surface plated onto Müller Hinton agar (MHA, Oxoid®). Ten μl of the respective phytosynthesized AgNPs were added into wells of 6 mm diameter. MHA plates were incubated at 35 oC for 24 hr. Following incubation, the zone of inhibition was measured in mm and expressed as mean ± standard error of the triplicate set. In all tests, the aqueous extract of seeds or leaves was used as a control.
Assessment of the cytotoxic activity of phytosynthesized nanosilver
Dimethyl thiazolyltetrazolium bromide (MTT) assay was carried out using the previously reported method by, [19] in which cells were seeded at a density of 7 x 103 cells/well in a 96-well culture plate and incubated at 37 0C for 24 h in a humidified atmosphere of 95% air and 5% carbon dioxide. Human breast MCF-7 cancer cells were treated with different concentrations of phytosynthesized AgNPs. After 24 h, 10 µl of MTT stock solution (5 mg/ml) were added to each well and the cultures were further incubated for 4 h and then 100 µl of DMSO was added to each well. There are several methods by which tumor cells can be exposed to phytosynthesized Silver Nanoparticles (AgNPs) extracts using L. sativum L. for a prolonged duration. Such as 1. Intravenous Administration: One common method is to administer the Phytosynthesized AgNP extracts intravenously into the animal model. This allows the nanoparticles to circulate within the bloodstream, enabling them to reach the tumor site and exert their effects over an extended period. 2. Slow-Release Systems: Researchers can develop drug delivery systems that provide a sustained release of the Phytosynthesized AgNP extracts. This can be achieved by encapsulating the nanoparticles within biocompatible materials or using nanoparticles with inherent slow-release properties. These systems can be implanted near the tumor site, allowing a controlled and prolonged exposure of the tumor cells to the nanoparticles. 3. Localized Administration: Another approach is to directly administer the phytosynthesized AgNP extracts at the tumor site. This can be done through methods such as intratumoral injection or implantation of nanoparticles near the tumor. By delivering the nanoparticles directly to the tumor, it is possible to achieve localized and sustained exposure to the tumor cells. 4. Repetitive Dosing: Instead of a single 24-hour exposure, we chose to administer multiple doses of the phytosynthesized AgNP extracts over a period of time. This can be done by administering the treatment at regular intervals, allowing the tumor cells to experience a cumulative effect of the nanoparticles over the study duration. The absorbance was then measured at 490 nm with reference at 630 nm using a plate reader. The percentage of cell cytotoxicity was calculated with respect to control cells.
Results and discussion
Plant material and extract preparation
In the diverging fields of nanotechnology, silver nanoparticles, in particular, gained superior interest seemingly due to their genuine physico-chemical and biological properties compared to their bulk forms [20]. In the present study, the bioreduction of silver ions by aqueous Lepidium sativum extracts was confirmed by visual color change to brown indicating that elemental silver was formed [21].
Characterization of phytosynthesized silver nanoparticles
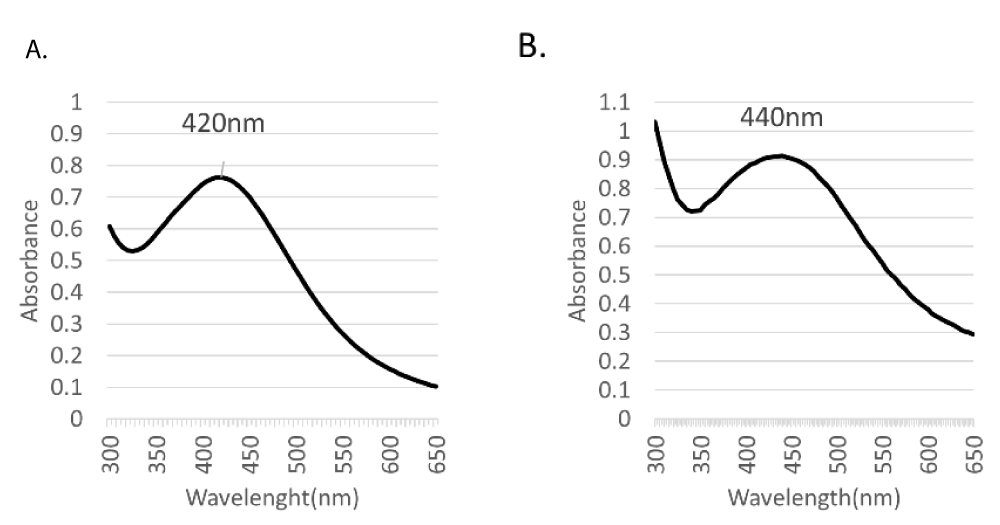
Scanning UV-Vis spectrophotometry, which demonstrated a narrow absorption peak at a wavelength range of 350-450 nm (Figure 1), correlated to the characteristic surface plasmon resonance of silver [22]. No additional peaks were detected in the UV-Vis spectrum, conveying the purity of the biosynthesized nanosilver.
The absorption intensity in UV-Vis spectra of AgNPs phytosynthesized by leaves was relatively higher than that of seeds phytofabricated nanosilver as revealed by an absorbance of 0.913 at 440 nm vs. 0.763 at 420 nm, respectively. The difference in the chemical composition of the respective plant part may account for the difference in absorption intensity where polyphenols, sterols, alkaloids, and fixed oils are reported as the main constituents in L. sativum seeds [23,24], countering proteins, fats, carbohydrates, and vitamins in leaves [25]. Morphological examination of the phytosynthesized nanoparticles demonstrated spherical, non-aggregated nanoparticles as shown in SEM micrographs (Figure 2).
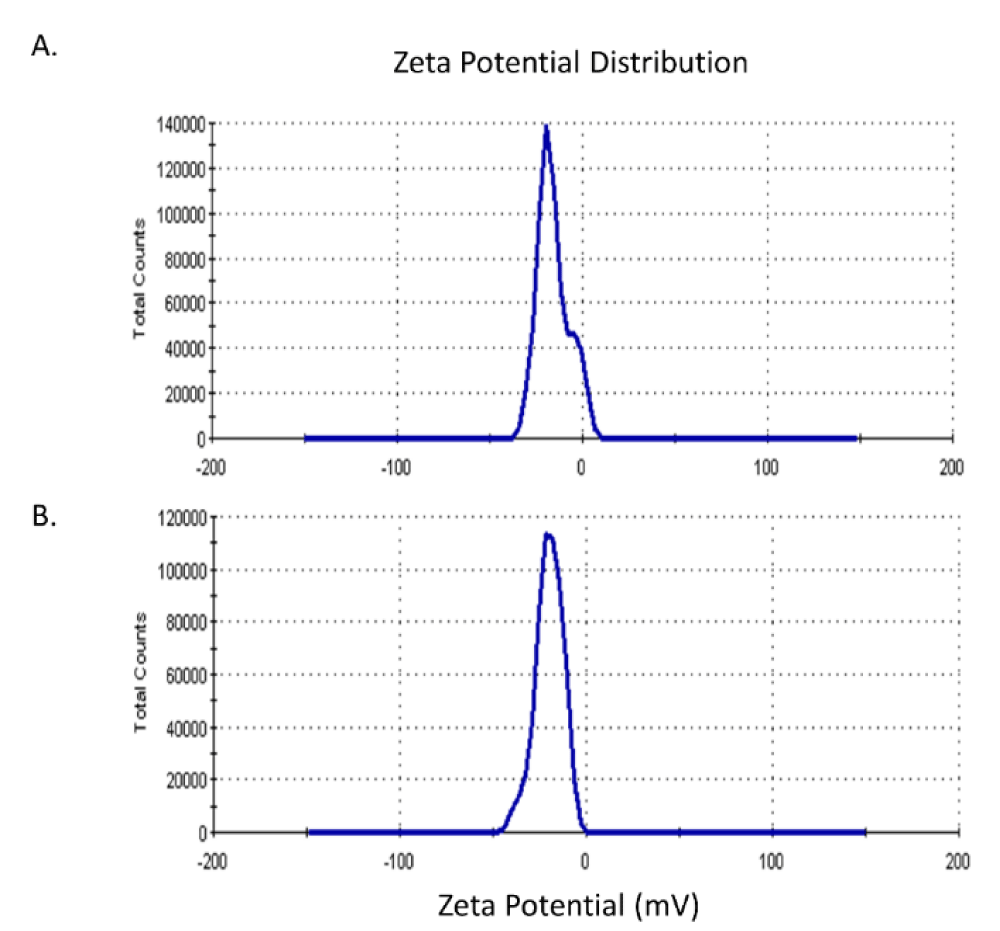
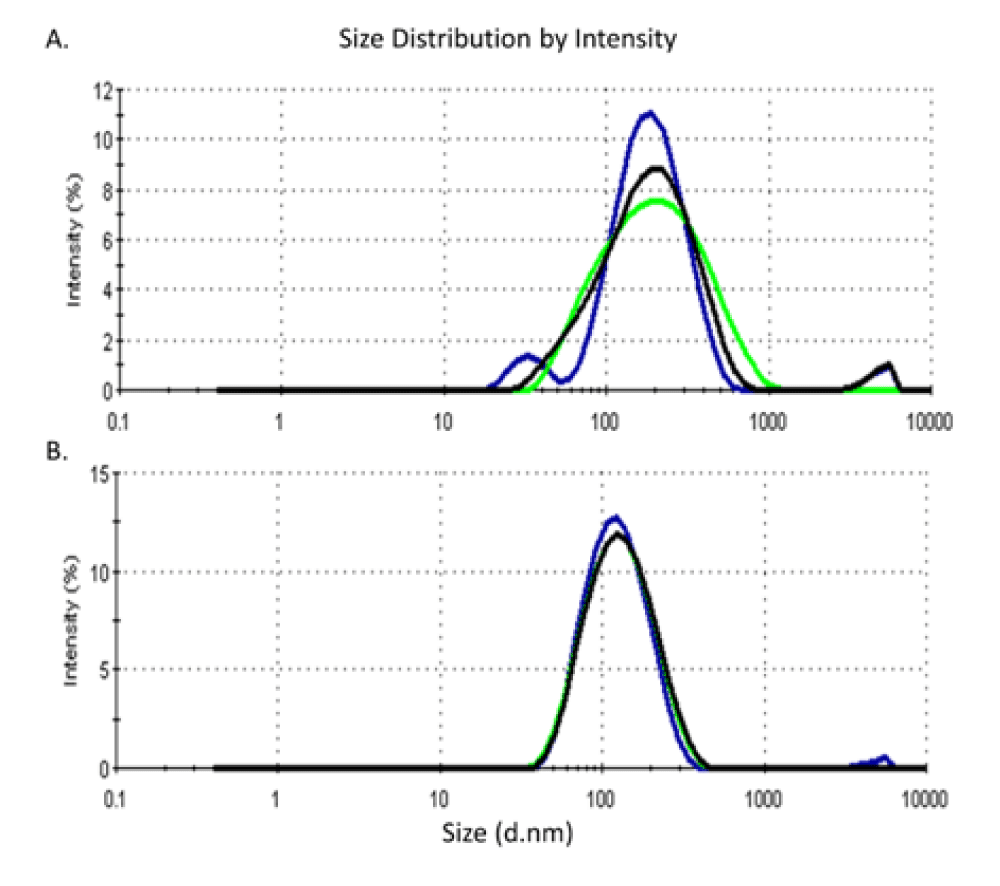
Zeta potentiometric analysis for AgNPs phytofabricated by L. sativum seeds and leaves revealed a zeta potential (ZP) of -15.6 mV and -20 mV and an average size of 150 and 111 nm, respectively (Figures 3,4).
In the context of the shape, size, and surface charge characteristics of nanoparticles, it is reported that the variation in functional structure and bioreducing potential of phytochemical compounds in different parts of plant system attributes for the morphological variation and polydispersity amongst phytofabricated nanoparticles [26]. Zetapotential and Polydispersity Index (PDI) are crucial indicative criteria for nanoparticle stability and particle size distribution. In this context, it is reported that ZP values ranging from ± 0-10 mV show a highly unstable colloid, whereas a ZP value of ± 10 - 20 mV, ± 20 - 30 mV, and > ± 30 mV shows relatively, moderately, and highly stable colloid in the respective order [27]. Accordingly, nanoparticles phytosynthesized by either L. sativum seeds or leaves aqueous extracts are stable in terms of their ZP values. Furthermore, a nanoparticle system with a PDI value < 0.1 is considered highly monodisperse, while that with PDI values in the range of 0.1 - 0.4 and > 0.4 is a moderately and highly polydisperse system respectively [28]. The presently phytosynthesized nanoparticles assumed PDI values of 0.347 and 0.206 respectively, indicating moderate dispersity. Countering the use of both reducing and capping agents in chemical nanosilver synthesis, L. sativum extracts exhibited a dual role as reducing and capping agents which grants green methods simplicity and uniqueness compared to physico-chemical routes of synthesis.
Assessment of the antifungal activity of phytosynthesized nanosilver
Candida albicans has been cited as the most common pathogenic candida species in several epidemiological surveys with a growing prevalence of resistance to conventional antifungal agents [29]. Albeit the documented reports on the antibacterial activity of nanosilver, its antifungal effect has received marginal attention [3,30,31]. In the present study, the antifungal activity of phytosynthesized nanosilver was evaluated against Candida albicans (ATCC 18804). As shown in Table 1, AgNPs synthesized using L. sativum seeds extract exhibited a relatively higher antifungal effect with an inhibition zone of 14 ± 0.57 mm and an MIC of 1.75 ppm versus an IZ of 11.7 ± 0.33 mm and an MIC of 2.08 ppm for nanosilver fabricated by L. sativum leaves extract.
Meanwhile, neither the aqueous extracts of L. sativum leaves nor seeds displayed any antifungal activity implying that the detected significant inhibitory effect is attributed to nanosilver. Few studies assessed the antifungal effect of nanosilver on Candida albicans. In one study, nanosilver prepared by chemical methods, exhibited a potent inhibitory effect, against pathogenic Candida spp. as compared to conventional antifungal agents [30,32]. Moreover, nanosilver-based drug combinations were found to inhibit multidrug-resistant fungal strains [33]. The cellular and molecular mechanisms of nanosilver against fungal pathogens have not been fully elucidated; its activity is postulated to occur via disruption of cell membrane integrity, inhibition of normal budding process, altering lipid composition, and induction of apoptosis due to increased production of reactive oxygen species [33-35].
Assessment of the cytotoxic activity of phytosynthesized nanosilver
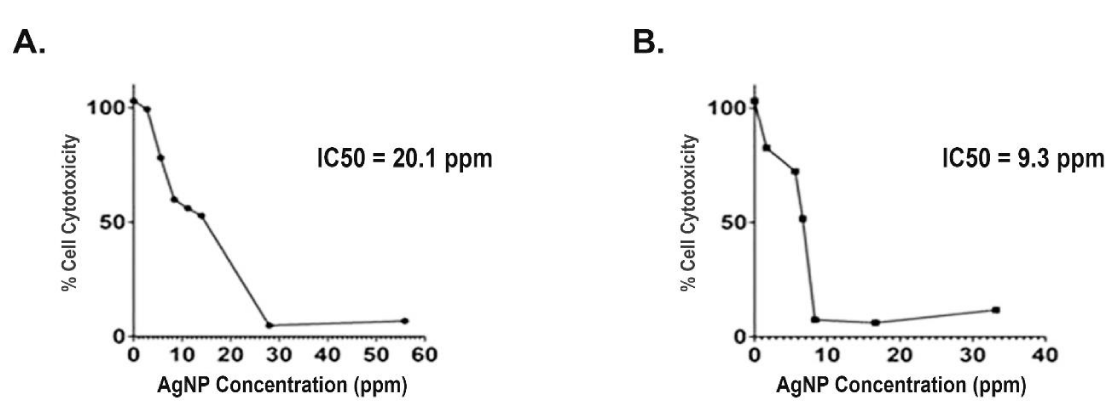
Upon investigating the cytotoxic effect of Lepidium sativum phytosynthesized nanosilver on MCF-7 cell line using MTT assay, the cytotoxicity of the cells was found to decrease upon increasing the concentration of silver nanoparticles (Figure 5). The relevant IC50 values were 20.1 ppm and 9.3 ppm for nanoparticles synthesized by aqueous extract of L. sativum seeds and leaves, respectively. Interestingly, the extract itself merely had no effect on the cells. The current cytotoxic results are in accordance with studies reporting that the in vitro cytotoxic effect of biosynthesized nanosilver against different cancer cell lines responded positively with different AgNP concentrations in a dose-response manner [36,37]. The biological role of silver nanoparticles on cancer cells could be related to their capacity to induce DNA damage, oxidative stress, apoptosis, and mitochondrial damage [7].
The anticandidal and cytotoxic effects are attributed to the presence of unique chemical constituents including apigenin, quercetin, kaempferol, luteolin, 7-hydroxy-4’,5,6-trimethoxyisoflavone, sinapic acid, chlorogenic acid, p-coumaric acid, ascorbic acid, α-tocopherol and 6-prenylnaringenin [38]. All these constituents along with colloidal silver are responsible for the effects observed. The variation in the percentage of the previously mentioned chemical composition of each plant part could explain the difference in biological effects. The observed effects in L. sativum can be attributed to its chemical constituents, which have implications in the fields of nanotechnology and biomedical applications.
L. sativum polyphenols have been of interest in nanotechnology due to their potential as natural antioxidants and their ability to stabilize nanoparticles and enhance their stability and bioavailability in drug delivery systems. They can serve as surface modifiers for nanoparticles, improving their biocompatibility and enhancing therapeutic efficacy [39]. Sterols, another important constituent of L. sativum, have been studied for their potential in nanotechnology and biomedical applications. These natural compounds have shown promise as biomaterials for drug delivery systems and tissue engineering due to their biocompatibility, biodegradability, and unique physicochemical properties. Sterols can be incorporated into nanocarriers to enhance their stability, solubility, and drug-loading capacity, thereby improving their performance as therapeutic delivery systems [40].
Alkaloids, which are present in L. sativum, have been of interest in both nanotechnology and biomedical research. Some alkaloids exhibit antimicrobial, anticancer, and anti-inflammatory properties, making them potential candidates for developing novel nanomedicines and drug-delivery systems. Additionally, alkaloids can be utilized as building blocks for the synthesis of nanomaterials with specific properties and functions, such as nanoparticles with controlled size, shape, and surface properties. Fixed oils, also known as vegetable oils, are another significant constituent of L. sativum seeds. These oils are rich in essential fatty acids, such as omega-3 and omega-6 fatty acids, which have been extensively studied for their health benefits. In the field of nanotechnology, vegetable oils have been used as carriers for the encapsulation and delivery of hydrophobic drugs. They can form stable nanoemulsions and lipid-based nanoparticles, providing a biocompatible and biodegradable platform for drug delivery applications [41].
The relevance of these chemical constituents of L. sativum to nanotechnology and biomedical applications lies in their potential to be utilized as natural materials, surface modifiers, and building blocks for the development of nanocarriers, nanomedicines, and drug delivery systems. Their biocompatibility, bioactivity, and unique physicochemical properties make them attractive candidates for various applications in these fields. Furthermore, the use of natural compounds from L. sativum aligns with the growing interest in green and sustainable approaches in nanotechnology and biomedicine.
The synthesized nanoparticles demonstrate potential mechanisms of action in both antifungal and cytotoxic activities.
1. Antifungal activity [42]:
The antifungal activity of nanoparticles can be attributed to various mechanisms:
- Disruption of fungal cell membrane: Nanoparticles can interact with the fungal cell membrane, leading to its disruption. This disruption can result in increased permeability and leakage of cellular components, ultimately leading to cell death. The small size and high surface area of nanoparticles facilitate their interaction with the cell membrane, enhancing their antifungal efficacy.
- Reactive Oxygen Species (ROS) generation: Some nanoparticles have the ability to generate reactive oxygen species, such as superoxide radicals and hydrogen peroxide, upon interaction with fungal cells. ROS can induce oxidative stress, causing damage to cellular components like proteins, lipids, and DNA. This oxidative stress can lead to fungal cell death and inhibition of fungal growth.
- Interference with fungal enzymes and proteins: Nanoparticles may interact with specific fungal enzymes or proteins, disrupting their normal function and inhibiting essential metabolic pathways. For example, nanoparticles can inhibit key enzymes involved in cell wall synthesis or disrupt fungal biofilm formation, impairing the ability of fungi to establish and maintain an infection.
- Metal ion release: Certain nanoparticles, particularly those containing metal ions like silver or copper, can release these ions in the presence of fungi. Metal ions have inherent antimicrobial properties and can interfere with crucial cellular processes within fungal cells, resulting in growth inhibition and cell death.
2. Cytotoxic activity [43]:
Nanoparticles can exhibit cytotoxic activity against cancer cells through multiple mechanisms:
- Induction of apoptosis: Nanoparticles can trigger apoptotic cell death in cancer cells. They can modulate signaling pathways involved in apoptosis, leading to the activation of apoptotic cascades and subsequent cell death. This can involve the upregulation of pro-apoptotic factors or downregulation of anti-apoptotic factors, ultimately promoting programmed cell death in cancer cells.
- Reactive Oxygen Species (ROS) generation: Similar to their antifungal activity, nanoparticles can generate ROS in cancer cells, causing oxidative stress-induced cytotoxicity. ROS can damage cellular components, disrupt mitochondrial function, and trigger cell death pathways specific to cancer cells.
- Targeted drug delivery: Nanoparticles can be functionalized to carry anticancer drugs specifically to tumor sites, enhancing drug delivery efficiency and reducing systemic toxicity. This targeted approach allows for higher drug concentrations at the tumor site, leading to increased cytotoxicity against cancer cells while minimizing adverse effects on healthy tissues.
- Enhanced cellular uptake: The small size and surface properties of nanoparticles can facilitate their internalization by cancer cells. This increased cellular uptake enhances the intracellular concentration of nanoparticles and associated therapeutic agents, leading to enhanced cytotoxic effects.
- Photothermal or photodynamic therapy: Nanoparticles can be designed to possess photothermal or photodynamic properties. Photothermal nanoparticles can convert absorbed light energy into heat, causing localized hyperthermia and thermal ablation of cancer cells. Photodynamic nanoparticles can generate reactive oxygen species upon activation by specific wavelengths of light, leading to cytotoxic effects in cancer cells.
It is important to note that the exact mechanisms of action may vary depending on the nature, composition, and surface characteristics of the synthesized nanoparticles. Further research and characterization are necessary to fully understand and optimize the antifungal and cytotoxic activities of these nanoparticles for potential therapeutic applications.
All these mechanisms justify the role of colloidal silver along with the attributed active constituents present in L. sativum seeds and leaves.
Conclusion
In the context of the present results, the current study offered a facile and environment-friendly method for the phytogenic synthesis of silver nanoparticles with promising anticandidal and cytotoxic effects against the MCF-7 breast cancer cell line. Nevertheless, further research is needed to elucidate their safety and exact mechanism of action. This is the first comparative work to report the anticandidal and cytotoxic activities against human MCF-7 breast cancer cells of AgNPs synthesized using the extract of Lepidium sativum L.
- Barhoum A, García-Betancourt ML, Jeevanandam J, Hussien EA, Mekkawy SA, Mostafa M, Omran MM, S Abdalla M, Bechelany M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials (Basel). 2022 Jan 6;12(2):177. doi: 10.3390/nano12020177. PMID: 35055196; PMCID: PMC8780156.
- Verma P, Maheshwari SK. Applications of Silver nanoparticles in diverse sectors. International Journal of Nano Dimension. 2019; 10:18-36.
- Sofi MA, Sunitha S, Sofi MA, Pasha SK, Choi D. An overview of antimicrobial and anticancer potential of silver nanoparticles. J King Saud Univ Sci. 2022;34(2):101791.
- Gong X, Jadhav ND, Lonikar VV, Kulkarni AN, Zhang H, Sankapal BR, Ren J, Xu BB, Pathan HM, Ma Y, Lin Z, Witherspoon E, Wang Z, Guo Z. An overview of green synthesized silver nanoparticles towards bioactive antibacterial, antimicrobial and antifungal applications. Adv Colloid Interface Sci. 2024 Jan; 323:103053. doi: 10.1016/j.cis.2023.103053. Epub 2023 Nov 26. PMID: 38056226.
- Gibała A, Żeliszewska P, Gosiewski T, Krawczyk A, Duraczyńska D, Szaleniec J, Szaleniec M, Oćwieja M. Antibacterial and Antifungal Properties of Silver Nanoparticles-Effect of a Surface-Stabilizing Agent. Biomolecules. 2021 Oct 7;11(10):1481. doi: 10.3390/biom11101481. PMID: 34680114; PMCID: PMC8533414.
- Vanlalveni C, Lallianrawna S, Biswas A, Selvaraj M, Changmai B, Rokhum SL. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv. 2021 Jan 13;11(5):2804-2837. doi: 10.1039/d0ra09941d. Erratum in: RSC Adv. 2022 May 31;12(25):16093. PMID: 35424248; PMCID: PMC8694026.
- Yesilot S, Aydin C. Silver nanoparticles; a new hope in cancer therapy? Eastern Journal of Medicine. 2019; 24:111-116. DOI: 10.5505/ejm.2019.66487.
- Ijaz I, Gilani E, Nazir A, Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chemistry Letters and Reviews. 2020; 13(3):223-245.
- Gour A, Jain NK. Advances in green synthesis of nanoparticles. Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):844-851. doi: 10.1080/21691401.2019.1577878. PMID: 30879351.
- Sharma N, Vuppu S. In Silico Study of Enzymatic Degradation of Bioplastic by Microalgae: An Outlook on Microplastic Environmental Impact Assessment, Challenges, and Opportunities. Mol Biotechnol. 2023 Sep 27. doi: 10.1007/s12033-023-00886-w. Epub ahead of print. PMID: 37758971.
- Ahmad S, Munir S, Zeb N, Ullah A, Khan B, Ali J, Bilal M, Omer M, Alamzeb M, Salman SM, Ali S. Green nanotechnology: a review on green synthesis of silver nanoparticles - an ecofriendly approach. Int J Nanomedicine. 2019 Jul 10; 14:5087-5107. doi: 10.2147/IJN.S200254. PMID: 31371949; PMCID: PMC6636611.
- Siddiqi KS, Husen A, Rao RAK. A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnology. 2018 Feb 16;16(1):14. doi: 10.1186/s12951-018-0334-5. PMID: 29452593; PMCID: PMC5815253.
- Vanlalveni C, Lallianrawna S, Biswas A, Selvaraj M, Changmai B, Rokhum SL. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv. 2021 Jan 13;11(5):2804-2837. doi: 10.1039/d0ra09941d. Erratum in: RSC Adv. 2022 May 31;12(25):16093. PMID: 35424248; PMCID: PMC8694026.
- Ijaz M, Zafar M, Iqbal T. Green synthesis of silver nanoparticles by using various extracts: a review. Inorganic and Nano-Metal Chemistry. 2020; 51(5):744-755.
- Patil MP, Singh RD, Koli PB, Patil KT, Jagdale BS, Tipare AR, Kim GD. Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Microb Pathog. 2018 Aug; 121:184-189. doi: 10.1016/j.micpath.2018.05.040. Epub 2018 May 25. PMID: 29807133.
- Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019 Jan 21;9(5):2673-2702. doi: 10.1039/c8ra08982e. PMID: 35520490; PMCID: PMC9059941.
- Jangra SS, Madan VK. Phytochemical and Nutritional Composition of Different Parts of Garden Cress (Lepidium sativum L.). Int. J. Curr. Microbiol. App. Sci. 2018; 7(11):1136-1145.
- Yassin MT, Mostafa AAF, Al-Askar AA, Al-Otibi FO. Synergistic antifungal efficiency of biogenic silver nanoparticles with itraconazole against multidrug-resistant candidal strains. Crystals. 2022;12(6):816.
- Saradha S, Debbie JV, Meera M, Ruckmani A, Arunkumar R. Anti-cancer activity of Manilkara zapota seed and skin extracts on HeLa cell lines. J Posit Sch Psychol. 2022:10422-10427.
- Lee SH, Jun BH. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int J Mol Sci. 2019 Feb 17;20(4):865. doi: 10.3390/ijms20040865. PMID: 30781560; PMCID: PMC6412188.
- Dilbar S, Sher H, Ali H, Ullah R, Ali A, Ullah Z. Antibacterial Efficacy of Green Synthesized Silver Nanoparticles Using Salvia nubicola Extract against Ralstonia solanacearum, the Causal Agent of Vascular Wilt of Tomato. ACS Omega. 2023 Aug 17;8(34):31155-31167. doi: 10.1021/acsomega.3c03164. PMID: 37663485; PMCID: PMC10468922.
- Saradha S, Debbie JV, Meera M, Ruckmani A, Arunkumar R. Anti-cancer activity of Manilkara zapota seed and skin extracts on hela cell lines. Journal of Positive School Psychology. 2022; 10422-10427.
- Sharifi-Rad M, Pohl P, Epifano F. Phytofabrication of Silver Nanoparticles (AgNPs) with Pharmaceutical Capabilities Using Otostegia persica (Burm.) Boiss. Leaf Extract. Nanomaterials (Basel). 2021 Apr 19;11(4):1045. doi: 10.3390/nano11041045. PMID: 33921810; PMCID: PMC8074182.
- Ramadan MF, Oraby HF. Lepidium sativum seeds: therapeutic significance and health-promoting potential. In: Academic Press, editor. Nuts and Seeds in Health and Disease Prevention. 2020. p. 273-289.
- Painuli S, Quispe C, Herrera-Bravo J, Semwal P, Martorell M, Almarhoon ZM, Seilkhan A, Ydyrys A, Rad JS, Alshehri MM, Daştan SD, Taheri Y, Calina D, Cho WC. Nutraceutical Profiling, Bioactive Composition, and Biological Applications of Lepidium sativum L. Oxid Med Cell Longev. 2022 Jan 19; 2022:2910411. doi: 10.1155/2022/2910411. PMID: 35096265; PMCID: PMC8791756.
- Soni V, Raizada P, Singh P, Cuong HN, S R, Saini A, Saini RV, Le QV, Nadda AK, Le TT, Nguyen VH. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environ Res. 2021 Nov; 202:111622. doi: 10.1016/j.envres.2021.111622. Epub 2021 Jul 7. PMID: 34245729.
- Pochapski DJ, Carvalho Dos Santos C, Leite GW, Pulcinelli SH, Santilli CV. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir. 2021 Nov 16;37(45):13379-13389. doi: 10.1021/acs.langmuir.1c02056. Epub 2021 Oct 12. PMID: 34637312.
- de Carvalho Bernardo WL, Boriollo MFG, Tonon CC, da Silva JJ, Cruz FM, Martins AL, Höfling JF, Spolidorio DMP. Antimicrobial effects of silver nanoparticles and extracts of Syzygium cumini flowers and seeds: Periodontal, cariogenic and opportunistic pathogens. Arch Oral Biol. 2021 May; 125:105101. doi: 10.1016/j.archoralbio.2021.105101. Epub 2021 Mar 1. PMID: 33676363.
- Dadar M, Tiwari R, Karthik K, Chakraborty S, Shahali Y, Dhama K. Candida albicans - Biology, molecular characterization, pathogenicity, and advances in diagnosis and control - An update. Microb Pathog. 2018 Apr; 117:128-138. doi: 10.1016/j.micpath.2018.02.028. Epub 2018 Feb 16. PMID: 29454824.
- Rozhin A, Batasheva S, Kruychkova M, Cherednichenko Y, Rozhina E, Fakhrullin R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines (Basel). 2021 Nov 29;12(12):1480. doi: 10.3390/mi12121480. PMID: 34945330; PMCID: PMC8708042.
- Rosenberg M, Ilić K, Juganson K, Ivask A, Ahonen M, Vinković Vrček I, Kahru A. Potential ecotoxicological effects of antimicrobial surface coatings: a literature survey backed up by analysis of market reports. PeerJ. 2019 Feb 11; 7:e6315. doi: 10.7717/peerj.6315. PMID: 30775167; PMCID: PMC6375256.
- Hasanin M, Elbahnasawy MA, Shehabeldine AM, Hashem AH. Ecofriendly preparation of silver nanoparticles-based nanocomposite stabilized by polysaccharides with antibacterial, antifungal and antiviral activities. Biometals. 2021 Dec;34(6):1313-1328. doi: 10.1007/s10534-021-00344-7. Epub 2021 Sep 25. PMID: 34564808; PMCID: PMC8475443.
- Ram VP, Yasur J, Abishad P, Ramesh C, Gourkhede DP, Arya PR, et al. Enhanced therapeutic efficacy of biogenic nanosilver-conjugated thymol: in vitro and in vivo evaluation against emerging multi-drug resistant microbes. J Drug Deliv Sci Technol. 2023; 86:104741.
- Mikhailova EO. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J Funct Biomater. 2020 Nov 26;11(4):84. doi: 10.3390/jfb11040084. PMID: 33255874; PMCID: PMC7711612.
- Radhakrishnan VS, Reddy Mudiam MK, Kumar M, Dwivedi SP, Singh SP, Prasad T. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int J Nanomedicine. 2018 May 3; 13:2647-2663. doi: 10.2147/IJN.S150648. PMID: 29760548; PMCID: PMC5937493.
- Mani M, Okla MK, Selvaraj S, Ram Kumar A, Kumaresan S, Muthukumaran A, Kaviyarasu K, El-Tayeb MA, Elbadawi YB, Almaary KS, Ahmed Almunqedhi BM, Elshikh MS. A novel biogenic Allium cepa leaf mediated silver nanoparticles for antimicrobial, antioxidant, and anticancer effects on MCF-7 cell line. Environ Res. 2021 Jul; 198:111199. doi: 10.1016/j.envres.2021.111199. Epub 2021 Apr 29. PMID: 33932479.
- Mani M, Okla MK, Selvaraj S, Ram Kumar A, Kumaresan S, Muthukumaran A, Kaviyarasu K, El-Tayeb MA, Elbadawi YB, Almaary KS, Ahmed Almunqedhi BM, Elshikh MS. A novel biogenic Allium cepa leaf mediated silver nanoparticles for antimicrobial, antioxidant, and anticancer effects on MCF-7 cell line. Environ Res. 2021 Jul; 198:111199. doi: 10.1016/j.envres.2021.111199. Epub 2021 Apr 29. PMID: 33932479.
- El-Haggar M, El-Hosseiny L, Ghazy NM, El-Fiky FK, El-Hawiet A. Phytochemical investigation, antimicrobial and cytotoxic activities of suspension cultures of Lepidium sativum L. South African Journal of Botany. 2021; 138:500-505. https://doi.org/10.1016/j.sajb.2020.12.024.
- Haj Bloukh S, Edis Z, Abu Sara H, Alhamaidah MA. Antimicrobial Properties of Lepidium sativum L. Facilitated Silver Nanoparticles. Pharmaceutics. 2021 Aug 27;13(9):1352. doi: 10.3390/pharmaceutics13091352. PMID: 34575428; PMCID: PMC8466285.
- Ali T, Hussain F, Naeem M, Khan A, Al-Harrasi A. Nanotechnology Approach for Exploring the Enhanced Bioactivities and Biochemical Characterization of Freshly Prepared Nigella sativa L. Nanosuspensions and Their Phytochemical Profile. Front Bioeng Biotechnol. 2022 May 17; 10:888177. doi: 10.3389/fbioe.2022.888177. PMID: 35656198; PMCID: PMC9152536.
- Antunes Filho S, Dos Santos MS, Dos Santos OAL, Backx BP, Soran ML, Opriş O, Lung I, Stegarescu A, Bououdina M. Biosynthesis of Nanoparticles Using Plant Extracts and Essential Oils. Molecules. 2023 Mar 29;28(7):3060. doi: 10.3390/molecules28073060. PMID: 37049821; PMCID: PMC10095647.
- Li L, Pan H, Deng L, Qian G, Wang Z, Li W, Zhong C. The antifungal activity and mechanism of silver nanoparticles against four pathogens causing kiwifruit post-harvest rot. Front Microbiol. 2022 Aug 31; 13:988633. doi: 10.3389/fmicb.2022.988633. PMID: 36118196; PMCID: PMC9471003.
- Nikzamir M, Akbarzadeh A, Panahi Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J Drug Deliv Sci Technol. 2021; 61:102316.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
