Global Journal of Biotechnology and Biomaterial Science
Biomedical evaluation of green tea herbal liquid with bama miniature pigs
Yufan Li1, Xinggang Zhao2,3, Jun Liu2,3, Mingyue Lin1, Chenxin Wang1, Rui Zhang1, Li Chen4* and Qin Zou1*
2Shenzhen Weiduo Technology Co., Ltd. Shenzhen 518000, P.R. China
3Guangxi Weiduo Technology Co., Ltd. Fangchenggang 538000, P.R. China
4Analytical & Testing Center, Sichuan University, Chengdu 610064, P.R. China
Qin Zou, Research Center for Nano Biomaterials, Analytical & Testing Center, Sichuan University, Chengdu 610064, P.R. China, Tel: +8615982237939; E-mail: [email protected]
Cite this as
Li Y, Zhao X, Liu J, Lin M, Wang C, et al. (2023) Biomedical evaluation of green tea herbal liquid with bama miniature pigs. Glob J Biotechnol Biomater Sci 9(1): 009-016. DOI: 10.17352/gjbbs.000019Copyright Licence
© 2023 Li Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Traditional Chinese Medicine (TCM) is widely used in health preservation and non-radical treatment because of its relatively gentle therapeutic effect, which is reflected in many products. Especially in atomization therapy, TCM shows its strong potential. This experiment focused on analyzing the extract of green tea (tea polyphenols) in a nebulizer, which is very similar to e-cigarettes. After intravenous injection of relevant drugs into small experimental pigs, the results of blood tests and the HE staining of organ sections showed no abnormalities in the experimental group. Since the dose of intravenous injection is much larger than that of nebulized inhalation, the safety of the product was verified by the experiment. The relevant analysis results obtained in this experiment will be used for comparison with subsequent, longer-term (more than 3 months) injection experiments.
Introduction
Atomizing inhalation therapy is a popular treatment method currently [1], which is usually used to treat respiratory diseases [2]. It uses a medical atomizer to atomize the medicine liquid into tiny particles. When patients breathe in the aerosol solution through the nose or mouth, the medicine solution will residue in the trachea, lungs, and other respiratory organs, so as to achieve painless, rapid, and effective treatment. The working principle of the herbal nebulizer is similar to that of a medical atomizer, which atomizes the liquid medicine into tiny particles and makes it reach the respiratory tract and lungs by breathing into complete drug delivery [3]. Similar products include e-cigarettes, which vaporize a concentrated liquid to achieve a similar effect to traditional cigarettes [4]. Most e-cigarette concentrates are said to be free of many of the harmful ingredients found in traditional cigarettes, such as tar and carbon monoxide. Nicotine, however, is still included [5]. The potential dangers of nicotine, including addiction, should not be ignored. Compared with e-cigarettes, the biggest advantage of herbal nebulizer is that it does not contain nicotine. On the contrary, it is replaced by various herbal medicines, which greatly reduces the potential harm.
Traditional Chinese Medicine (TCM) has been widely used nowadays, and its therapeutic effects on many diseases have impressed people deeply. At present, a large number of herbal ingredients, prescriptions, related targets, drugs, and diseases have been recorded, facilitating the modernization of TCM in the world. Existing studies have shown that tea and its biologically active ingredients have various physiological and medicinal effects such as reducing fat and losing weight, lowering blood sugar, protecting the liver, and protecting the liver [6]. Numerous studies have established that green tea contains chemical elements that are directly related to human health. Green tea polysaccharides, caffeine, theanine, and tea polyphenols have pharmacological properties that include anticancer [7], antioxidant [8], neuroprotective [9], and reducing blood sugar [10]. According to the result of Xiong’s group, both raw tea and cooked tea had a strong inhibitory effect on the weight gain of obese rats, and the weight, wet fat weight, and fat coefficient of the rats were decreased [11]. In this experiment, the regent is Pu’er tea, which has a similar main component to green tea extract. Although tea has a variety of pharmacological effects and has a long history of application, its pharmacological effects have not been used in People’s Daily life, and a certain effect has not been developed [12]. To realize the transformation of tea from a daily drink to a medicine that can treat diseases, there are still many places worth studying and discussing, which need further exploration and development by the majority of researchers [13,14]. Therefore, the research on Green Tea and relative production is valuable for the development of pharmacy.
The nebulizer supplied by Shenzhen Vido Technology has been tested by bacterial experiment and rabbits’ intravenous injection [15] but has not been tested on large animals to further demonstrate its safety. In order to further study the effects of this product on the human body, in this experiment, the nebulization solution contained in the product was injected into small experimental pigs, and biological evaluation was conducted regularly. At the same time, since the pigs could not use the nebulizer, the drug was directly delivered through intravenous injection (IV), indicating that a larger than usual dose will be injected into the pigs. If this dose does not cause damage to the organs of the pigs, it means that the dose of the solution in the nebulizer that people normally use is perfectly safe.
Materials and methods
All surgical procedures were approved by the Ethics Committee of West China Animal Experiment Center of Sichuan University. The experimental protocol was approved by the Sichuan Provincial Laboratory Animal Management Committee with approval number 20230512002, and the experimental procedure followed the International Association of Veterinary Medical Editors’ “Consensus of Authors’ Guide on Animal Ethics and Welfare” and local and national regulations.
Pig intravenous injection
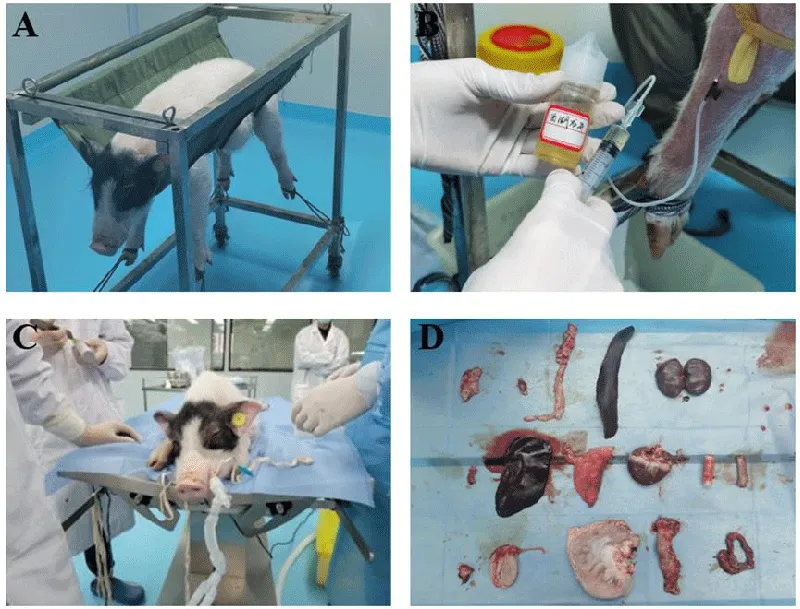
The green tea nebulization solution (378 mg/L) was provided by Shenzhen Vido Technology Co., Ltd. Shenzhen, Guangdong. Diluting the stock solution with normal saline from 378 mg/L to 37.8 mg/L in the clean bench, and 30 mL Green tea diluted solution were prepared [16]. The diluted solution then was injected through the hindlimb vein. Before each injection, the pigs were placed on the restraint device, weighed, and then record their weight. The injected volume was determined by the following formula [17,18]:
In the experiment, two miniature pigs with similar physical conditions are prepared. One of the pigs was taken as the control group. The other pig was taken as the experimental group, marked with the green tea group. The control group was fed for 7 days without injection and then dissected. For the Green tea group, the green tea solution was injected every three days over a period of two weeks and then dissected (Figure 1B).
Histological staining analysis
After the pigs were dissected, their organs (trachea, lung, heart, liver, spleen, kidney, small intestine, large intestine, and duodenum) were removed, sampled, and fixed with a fixative solution (Figure 1D). Samples were dehydrated in gradients (50%, 75%, 83%, 95%, 100%) of ethanol and then embedded in paraffin. The embedded tissues were then sliced into 5 μm ~ 10 μm tissue sections along the transverse or sagittal plane. Hematoxylin-eosin (HE) staining kits were used to stain the tissue sections [19]. Photographs were observed under the bright field of an optical microscope (Ti-U, Nikon, Japan).
Toxicological analysis of the nebulization solution
The blood was sampled from each group of pigs before they were dissected and the serum biochemical tests and blood routine examination were performed [20].
The serum biochemical tests contain 16 items, which are AST (Aspartate Aminotransferase), ALT (Alanine Aminotransferase), ALP (Alkaline Phosphatase), ALB (Albumin), γ-GT (Gamma-Glutamyl), Glu (Glucose), Ca (Calcium), CK (Creatine Kinase), LDH (Lactate Dehydrogenase), UREA (Urea), CREA(Creatinine), TC (Total Cholesterol), TG (Triglyceride), TP (Total Protein), GLO (Globulin), and P (Phosphorus).
The trace elements in blood tests contain 6 items, which are: K (kalium), Cl (chlorine), Ca (Calcium), Mg (Magnesium), Fe (Iron), and Na (sodium).
The blood routine examination contains 12 items, which are WBC (White Blood Cell), HGB (Hemoglobin), RBC (Red Cell Count), HCT (Hematocrit), MCV (Mean Red Cell Volume), MCH (Mean Red Cell Hemoglobin), MCHC (Mean Red Cell Hemoglobin Content), RDWCV (Coefficient of variation of RBC distribution width), PLT(Platelet), MPV (Mean Platelet Volume), and PDW (Platelet Distribution Width).
Results
HE staining of the green tea group
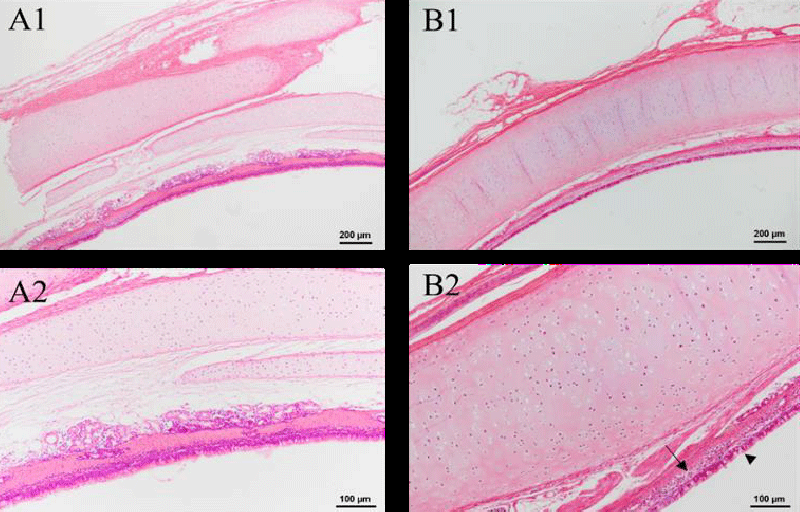
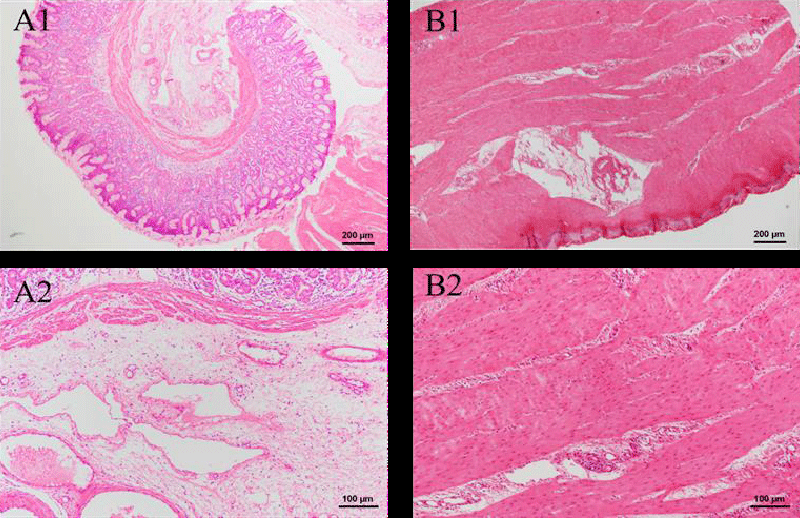
The histopathological examinations of the trachea are shown in Figure 2. After intravenous injections for two weeks, there is no change in the green tea group. Mucosa, submucosa, and adventitia parts are clear [22] in the green tea group. Additionally, inflammatory factors were not observed, either. Thus, there is no influence on the trachea during two weeks of injection, which indicates the biosafety of the green tea nebulization solution.
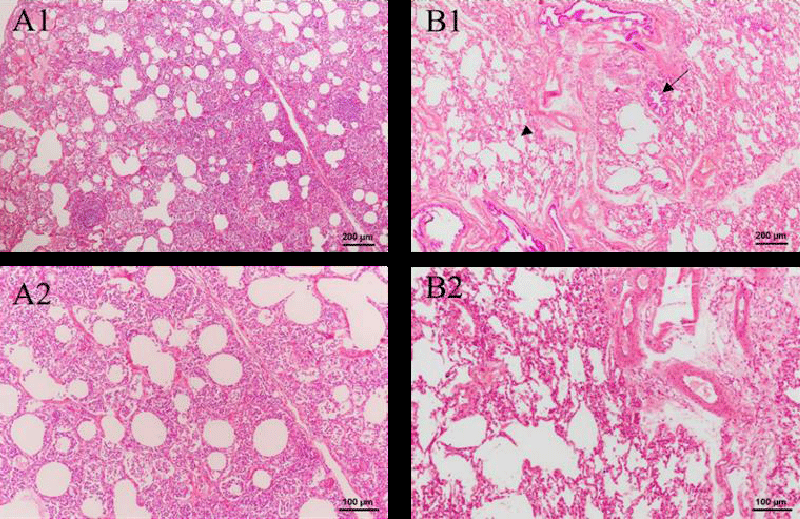
Figure 3 shows the HE-stained images of the control and green tea groups. The structure of lung tissue is clear and complete. Uniform pulmonary alveolar was observed, as well as the smooth alveolar wall. Besides, no edema and inflammatory cells are in the surrounding tissue. The findings lead to the conclusion that a two-week injection of green tea nebulization solution is safe for the pig’s lung.
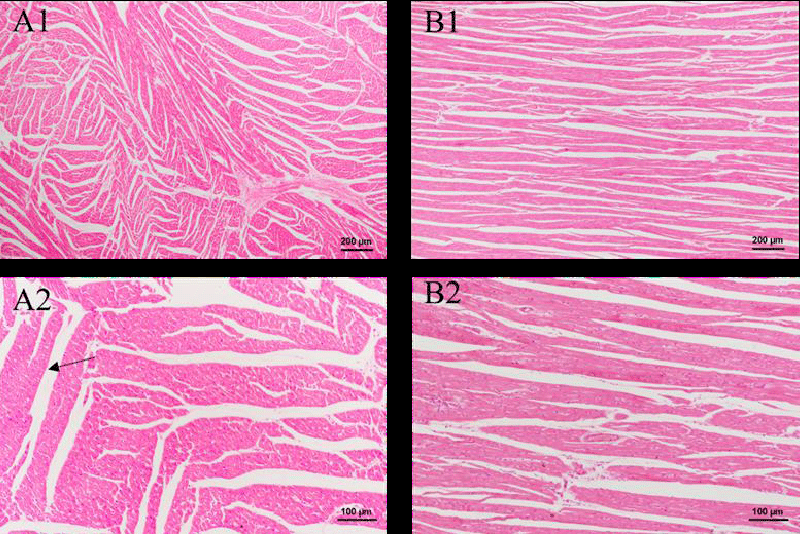
In Figure 4, the structures of the stained section of the heart are shown. The images of both the control group and the green tea group have similar appearances. Red-stained myocardial cells and blood vessels are embodied in the section. Considerable inflammatory cells are not observed, which indicates that the green tea nebulization solution makes no damage to the pig’s heart.
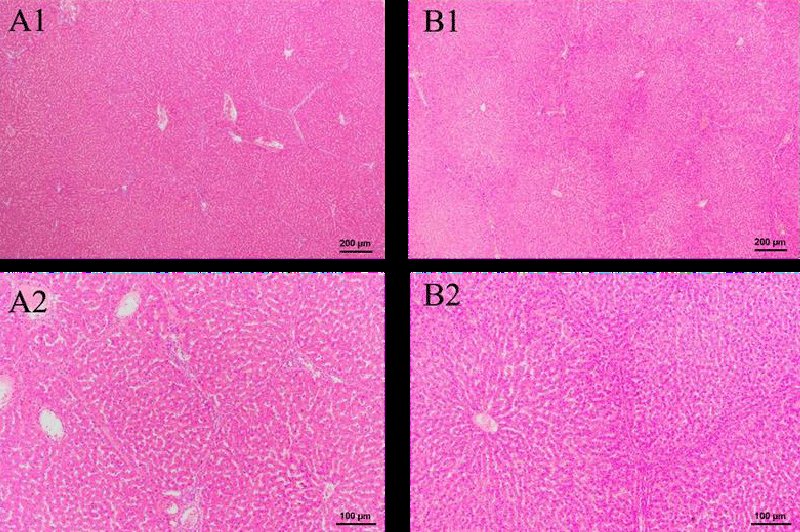
In Figure 5, both the control and green tea groups have complete hepatic lobules with clear boundaries. The contour and color of the liver in the HE-stained image are normal. Thus, the results provide evidence of the safety of green tea nebulization solution on the liver.
As shown in Figure 6, both the control group and green tea group have complete red medullar slice region. No obvious cell aggregation is observed, which means no inflammation is found. The result proves that green tea nebulization solution is safe for the spleen.
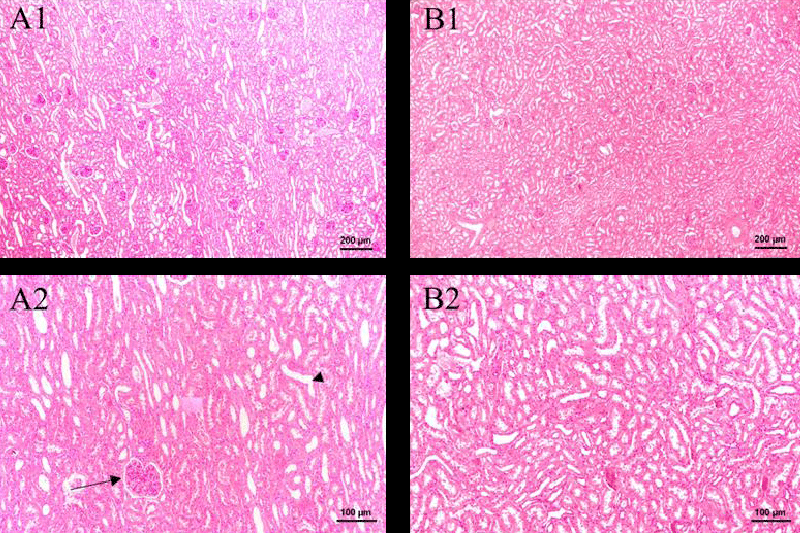
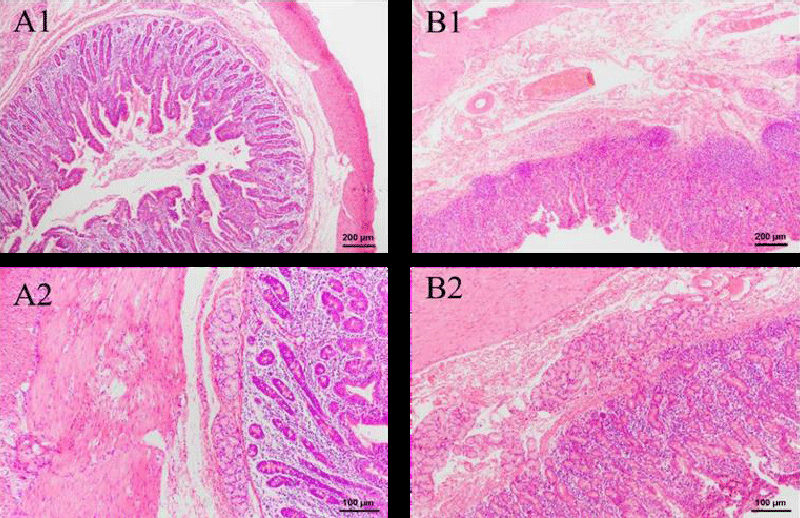
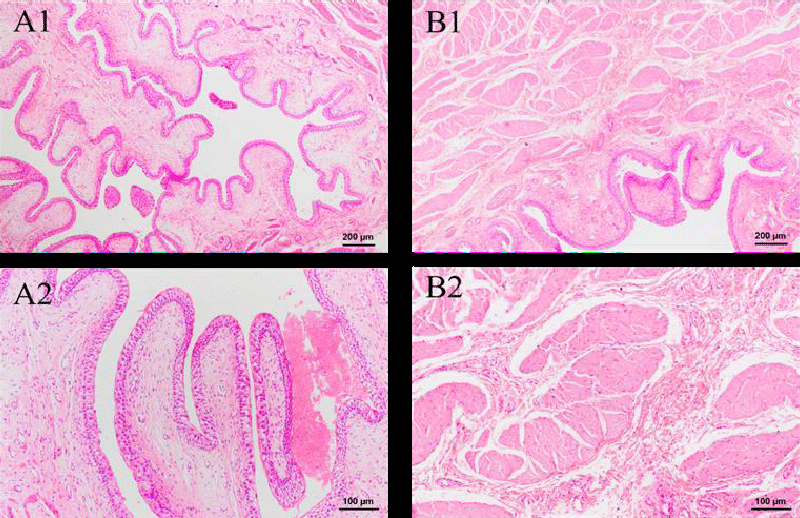
Figure 7 shows the result of histopathological examinations of the kidney in two weeks. In the Green tea group, no significant difference is observed after an intravenous injection of two weeks. The renal corpuscle and renal tubule are still clear and remain normal in Figure 1-B1. In addition, no obvious inflammation is observed. Therefore, the green tea nebulization solution has no harmful effect on the pig’s kidney for two weeks.
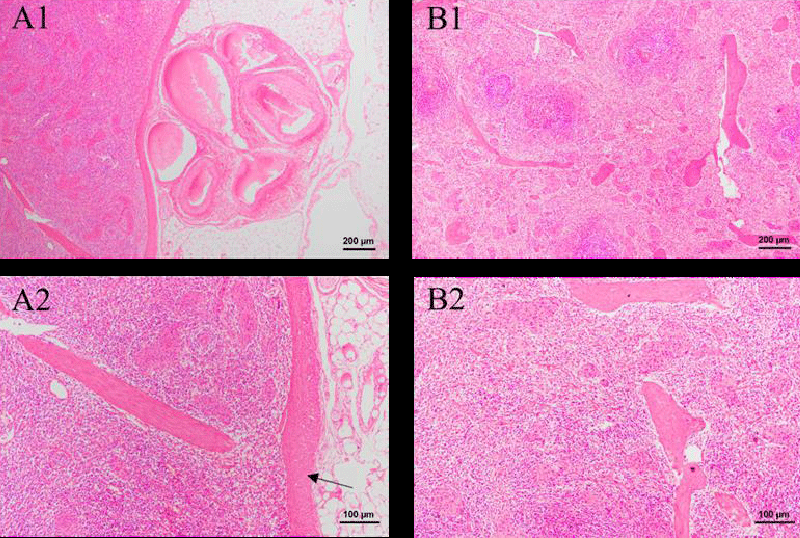
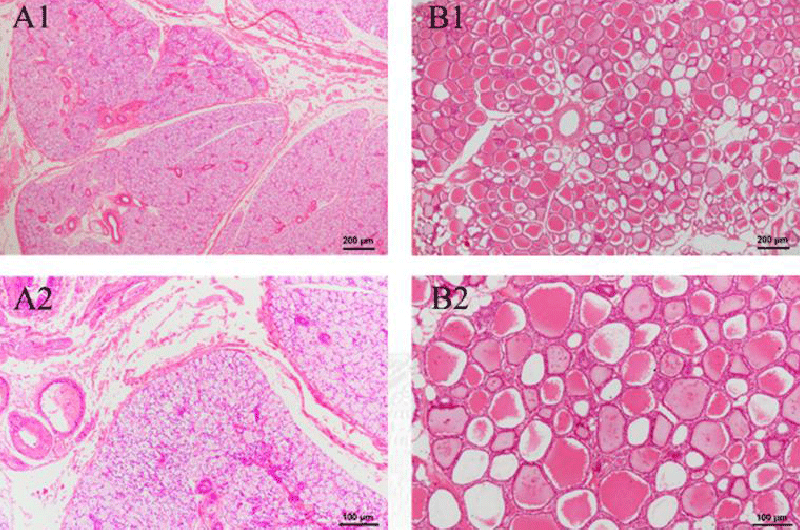
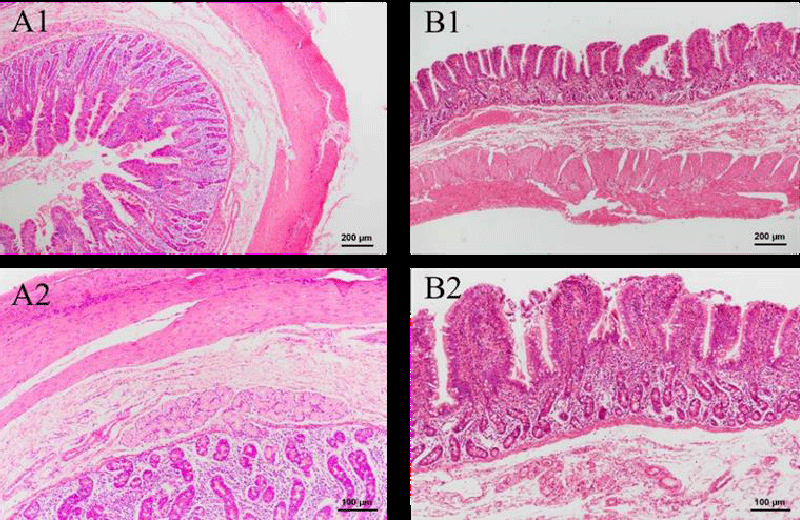
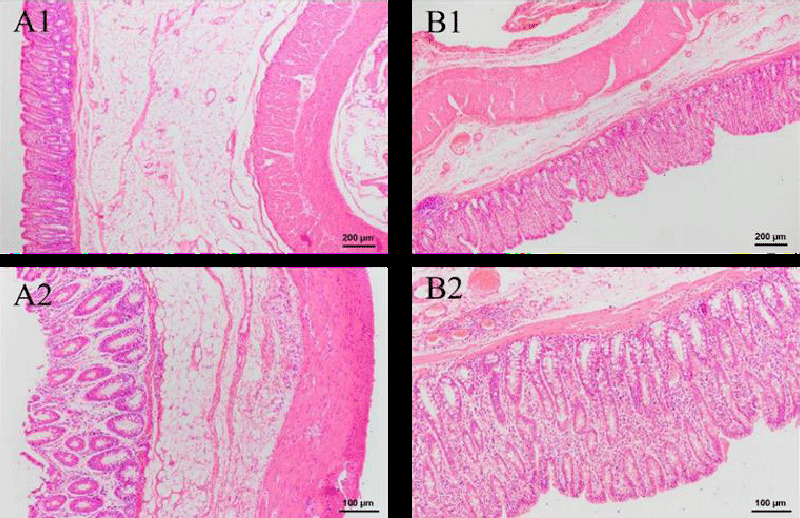
HE-stained sections of other organs were also made, which include the thyroid, stomach, small intestine, large intestine, duodenum, and bladder. As seen in Figure 8B, there was a slight sign of enlargement of the thyroid follicles in the experimental group. Given that no other parts of the organ sections showed obvious inflammatory reactions, it is likely that this is a disturbance of the endocrine system caused by irregular and unstable rest in the experimental animals, thus increasing the burden on the thyroid cells. No goblet cells were discovered in stomach sections, and there were tight connections of mucus cells that had significant protective effects in the gastric fovea. Both groups exhibited entire and transparent mucosa, submucosa, and muscle linings in the small intestine, large intestine, and duodenum. A lot of goblet cells and absorptive cells, as well as a dearth of stem cells and endocrine cells, were present in the densely and regularly distributed small and large intestinal glands. In these sections, no structure change and inflammatory cells are observed. These findings show that intravenous injection of green tea nebulization solution for two weeks does not cause histopathological changes in pigs. The results are shown below (Figure 8-13).
Blood analyses of the green tea group
The blood biochemical, blood electrolyte, and blood routine of the pigs were analyzed. There is no standard blood index for Bama pig up to now, therefore, according to the literature, the reference data come from Chen’s [28] and Zhao’s research [29]. A small difference between the blood test results and reference is acceptable.
Biochemical blood test: The biochemical blood test is frequently used to determine the composition of various ions, lipids, proteins, different enzymes, and other bodily metabolites in the blood. It is a reflection of the roles and conditions of diverse animal tissues and organs [30]. Blood glucose (Glu) and inorganic salt ions are stable and kept in the body in a specific equilibrium range under normal health conditions to maintain the normal metabolism and osmotic pressure of the body [31]. Otherwise, there will be an electrolyte imbalance and a disturbance in glucose metabolism. In Table 1, Glu, CK and TG data range differs greatly from the reference value range. However, these data can be affected before and after feeding. In the experiment, all pigs stayed on empty stomachs for eight hours before the blood was collected, so Glu and other items data can be below reference data and these data will not influence the normal growth and development of pigs. Since the performance of both the control and experimental groups was similar as seen in the other results, we speculate that this may be a change due to fasting. The values of the control group, however, were closer to the reference values. Therefore, further experiments are still needed to justify the speculation.
AST, ALT and ALP are widely distributed in the human liver, bone, intestine, kidney, and placenta that are excreted by the liver to the bile [32]. These enzymes will increase in the serum when the liver cell membrane is harmed or when there is cell necrosis. The degree of liver cell damage can be determined by monitoring the activity of enzymes in serum or plasma. In the control group and experiment group, both of the ALP data were below the reference group, which means pigs may have Hepatitis or liver injury. However, the liver sections of both the control group and the green tea group look similar, and no inflammatory reaction was found. Thus, these data changes may be caused by individual differences in pigs. In addition, the ALP value is significantly affected by the amount of vitamin D intake or the fasting time before the blood draw. So, it can be predicted that during the experiment, pigs get too much vitamin D from their diet and short fasting time resulted in lower ALP values than normal.
After comparing the blood biochemical data of the control and green tea groups, it was found that most of the test items’ data are within a reasonable range, which reveals the biosafety of the Green tea nebulization solution.
Blood electrolyte test Table 2: It can be found that Blood electrolyte test data are relatively within the range of the reference group. For K, Fe, Cl, and Na content, the values in the experiment are slightly smaller than the reference group. As pigs were fasting before blood was drawn, these electrolytes could flow out of the body through sweating, urination, etc. As a result, these items’ content shows lower than the reference. Overall, pigs’ blood electrolyte was stable before and after medical injection, which proves that the green tea nebulization solution has no significant effect on the metabolism of blood electrolyte in pigs.
Complete blood routine: A complete blood routine is an essential fundamental marker for biomedical evaluation, which alter over time and can show a variety of disorders [33]. It can be used to assess the experimental animals’ general physiological condition.
In Table 3, it can be seen that except for the WBC value, Blood routine numbers are all in the range of reference, which indicates the biosafety of the solution. Both the control group and green tea group’s WBC are higher than the reference range. However, as shown by the HE staining sections, inflammation was not present in the major organs of the pigs. Therefore, it can be hypothesized that the repeated injections and blood collection may have caused a localized subcutaneous infection in the pigs, which led to an increase in the leukocyte count, rather than being caused by the green tea nebulization solution.
Discussion
With the rise of new tobacco products, glycerin has become an important part of tobacco raw materials, especially in e-cigarettes, glycerin is often used as an atomizer of e-cigarette liquid, content can be as high as 90% [34]. In the product used in this experiment, the main composition of the nebulization solution is also glycerin and a small amount of TCM herbal ingredients. Previously, nebulized inhalation studies based on mice [35] and rats [36,37] and intravenous injections in rabbits [15] have been performed. The result shows that glycerol makes no considerable influence on experimental animals’ respiratory systems. Therefore, glycerol fogging used as an electronic cigarette agent is free of harm to animals.
Generally speaking, the higher rank of the experiment animal, the more complex its biological structure, and the closer its response is to human beings. Furthermore, the pig has a similar circulatory system to a human being [38]. Thus, compared with rats and rabbits, the pig has more advantages. As a large mammal, a pig has a much similar biological structure to a human being, and the circulatory system, which will ensure the medicine through intravenous injection can simulate the effect in the human body. Although the pig is much more expensive in price, in order to have a better simulation of the human body, the pig is selected as the experimental animal.
Drug injection dose is another part that needs to be decided before the experiment. A low dose makes the result not so believable and a high dose will cause toxicity. For different animals, the injection dose is absolutely different. However, in Nair and Jacob’s research, they point out a dose conversion between animals which is based on the weight [39]. Thus, it is reasonable to decide the injection dose for pigs based on other animals’ injection doses. For the removal of blood, too large an amount of removal could not only cause fluctuation of blood biochemical index or even death of experiment animal and too small an amount will cause insufficiency for blood examination. Research shows that an animal can recover in two weeks if the removal of blood is not beyond 10% of its total amount of blood and hemodynamics will not change if not beyond 20% [40]. Thus, for our experimental cycle, the collection of less than 10% of the total amount is considered not to influence the experiment result.
In this study, most of the results of HE section staining and blood tests showed that the experimental group remained in a similar condition to the control group after intravenous injection. This proves the safety of green tea herbal nebulized products. However, there are still data that may be influenced by diet or emotion such as Glu/CK/TG that differ from the control group. Therefore, it is necessary to focus on testing the changes in these data in further long-term experiments in the follow-up.
Conclusion
This experiment verified the safety of green tea nebulizer through intravenous injection of small experimental pigs. The results of blood tests and HE staining of organ sections together confirm the safety of this herbal nebulizer. Although some of the indicators in the blood tests fluctuated, these parameters were influenced by factors such as eating and were similarly affected in the control group. Combined with the observations of organ sections, no inflammatory reactions were observed in the organs of the pigs. Therefore, the biosafety of the green tea nebulizer can be assured by the results of this experiment, which indicate a promising prospect for the application of TCM in herbal nebulizers. For further studies, longer-term (more than 3 months) injection experiments will be considered to further ensure the safety of the nebulizer so that it can be used in clinical trials and daily life as early as possible.
This work was supported by the China NSFC project [No. 32171338], Key Science and Technology Special Project of Sichuan Province [No.2020ZDZX0008], Innovation and Reform Project of Postgraduate Education of Sichuan University in 2021 and Experimental Technology Research Project of Sichuan University [SCU221099], Biological Evaluation of Atomizer and Herbal Atomizing Liquid [No.21H0588], and Effect of Atomized Liquid of Traditional Chinese Medicine on Metabolism and Ultrastructure of Stem Cells [No.21H0928].
- Li C, Liu S, Luo G, Wang G, Zhang B, Nie Q. Comparison of plasma pharmacokinetics of Tanreqing solution between intratracheal aerosolization and intravenous injection in rats. Biomed Chromatogr. 2018 Mar;32(3). doi: 10.1002/bmc.4116. Epub 2017 Nov 22. PMID: 29027677.
- Zhang G, Li Y, Chen T, Gao Y, Sun J, Yang W, Song L, Su P, Ma M, Zhang Z, Zhang H, Yang Y, Li H, Ye Z, Hou H. Comparative study of the efficacy and pharmacokinetics of reduning injection and atomization inhalation. Biomed Pharmacother. 2019 Oct;118:109226. doi: 10.1016/j.biopha.2019.109226. Epub 2019 Aug 1. PMID: 31377471.
- Wang Z, Cao Y, Lu Y, Zhang F, Su Y, Guo Y. Ultrasonic extraction and nebulization in real-time coupled with carbon fiber ionization mass spectrometry for rapid screening of the synthetic drugs adulterated into herbal products. Anal Chim Acta. 2020 Nov 1;1136:62-71. doi: 10.1016/j.aca.2020.08.045. Epub 2020 Aug 28. PMID: 33081950.
- Blagev DP, Harris D, Dunn AC, Guidry DW, Grissom CK, Lanspa MJ. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet. 2019 Dec 7;394(10214):2073-2083. doi: 10.1016/S0140-6736(19)32679-0. Epub 2019 Nov 8. PMID: 31711629.
- Poschenrieder F, Rotter M, Gschwendtner A, Hamer OW. E-cigarette-induced lung disease: from acute to chronic. Lancet. 2020 Aug 22;396(10250):564. doi: 10.1016/S0140-6736(20)31755-4. PMID: 32828188.
- Du WH, Peng SM, Liu ZH, Shi L, Tan LF, Zou XQ. Hypoglycemic effect of the water extract of Pu-erh tea. J Agric Food Chem. 2012 Oct 10;60(40):10126-32. doi: 10.1021/jf302426w. Epub 2012 Oct 1. PMID: 22957968.
- Zhao T, Li C, Wang S, Song X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules. 2022 Jun 18;27(12):3909. doi: 10.3390/molecules27123909. PMID: 35745040; PMCID: PMC9231383.
- Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010 Sep 1;501(1):65-72. doi: 10.1016/j.abb.2010.06.013. Epub 2010 Jun 15. PMID: 20558130; PMCID: PMC2946098.
- Yoneda Y, Kuramoto N, Kawada K. The role of glutamine in neurogenesis promoted by the green tea amino acid theanine in neural progenitor cells for brain health. Neurochem Int. 2019 Oct;129:104505. doi: 10.1016/j.neuint.2019.104505. Epub 2019 Jul 13. PMID: 31310779.
- Amorim ND, Moreira L, Vaz Ribeiro S, Cesario Grasiele. Effect of green tea extract on bone mass and body composition in individuals with diabetes. Journal of functional foods. 2018.
- Xiong CY, Yang B, Peng YJ, Yan G, Pu-Ming HE. Anti-obesity and Lipid-decreasing Effects of Pu-er Tea. Southwest China Journal of Agricultural Sciences. 2018.
- Xu L, Xia G, Luo Z, Liu S. UHPLC analysis of major functional components in six types of Chinese teas: Constituent profile and origin consideration. LWT-Food Science & Technology. 2019; 102.
- Zhu K, Ouyang J, Huang J, Liu Z. Research progress of black tea thearubigins: a review. Crit Rev Food Sci Nutr. 2021;61(9):1556-1566. doi: 10.1080/10408398.2020.1762161. Epub 2020 May 29. PMID: 32468849.
- Liu L, Wu X, Zhang B, Yang W, Li D, Dong Y, Yin Y, Chen Q. Protective effects of tea polyphenols on exhaustive exercise-induced fatigue, inflammation and tissue damage. Food Nutr Res. 2017 Jun 1;61(1):1333390. doi: 10.1080/16546628.2017.1333390. PMID: 28659745; PMCID: PMC5475289.
- Rui Z, Mingyue L, Chenxin W, Yufan L, Xinggang Z, Jun L. Biological toxicity evaluation of traditional medicine white tea extract liquid. Global Journal of Biotechnology and Biomaterial Science. 2023;9:001-8. doi: 10.17352/gjbbs.000018.
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016 Mar;7(2):27-31. doi: 10.4103/0976-0105.177703. PMID: 27057123; PMCID: PMC4804402.
- Sultana N. A simple practice guide for dose conversion between animals and human. 2021.
- Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C; European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001 Jan-Feb;21(1):15-23. doi: 10.1002/jat.727. PMID: 11180276.
- Kather JN, Krisam J, Charoentong P, Luedde T, Herpel E, Weis CA, Gaiser T, Marx A, Valous NA, Ferber D, Jansen L, Reyes-Aldasoro CC, Zörnig I, Jäger D, Brenner H, Chang-Claude J, Hoffmeister M, Halama N. Predicting survival from colorectal cancer histology slides using deep learning: A retrospective multicenter study. PLoS Med. 2019 Jan 24;16(1):e1002730. doi: 10.1371/journal.pmed.1002730. PMID: 30677016; PMCID: PMC6345440.
- Liu Y, Mao X, Yu B, He J, Zheng P, Yu J. Excessive dietary taurine supplementation reduces growth performance, liver and intestinal health of weaned pigs. Livestock Science. 2014; 168:109-19.
- Kojima K, Vacanti CA. Tissue engineering in the trachea. Anat Rec (Hoboken). 2014 Jan;297(1):44-50. doi: 10.1002/ar.22799. Epub 2013 Dec 2. Erratum in: Anat Rec (Hoboken). 2016 Jun;299(6):703-10. PMID: 24293389.
- Watanabe K. Trachea. Bergman's Comprehensive Encyclopedia of Human Anatomic Variation. 2016; 1212-6.
- Hoang TV, Nardiello C, Surate Solaligue DE, Rodríguez-Castillo JA, Rath P, Mayer K, Vadász I, Herold S, Ahlbrecht K, Seeger W, Morty RE. Stereological analysis of individual lung lobes during normal and aberrant mouse lung alveolarisation. J Anat. 2018 Mar;232(3):472-484. doi: 10.1111/joa.12773. Epub 2018 Jan 9. PMID: 29315540; PMCID: PMC5807959.
- Marshall RA, Luchkanych AMS, Morton JS, Boyes NG, Zhai A, Marciniuk DD, Mei Y, Allison EY, Shoemaker JK, Al-Khazraji BK, Allen MD, Tomczak CR, Olver TD. Cerebral haemodynamics during arrhythmia in health, ischaemic heart disease and heart failure with reduced ejection fraction, and in a preclinical swine model. J Physiol. 2022 May;600(10):2311-2325. doi: 10.1113/JP283112. Epub 2022 May 4. PMID: 35389526.
- Leiskau C, Baumann U. Structure, Function, and Repair of the Liver. Diseases of the Liver and Biliary System in Children 2017; 1-17.
- Lewis SM. The Spleen. Postgraduate Haematology. 2005; 358-369.
- Lin J, Chen Q, Hu J. Urinary Organs. In: Lin J, Chen Q, Hu J, editors. Color Atlas of Zebrafish Histology and Cytology. Singapore: Springer Nature Singapore. 2022; 131-42.
- Ke-Yan C, Wen S, Qian S, Na LU, He Z, Yang W. Adaptability Observation and Detection of the Blood Physiological and Biochemistry Indicators of Bama Minipig in Liaoning Area. China Animal Husbandry & Veterinary Medicine. 2014.
- Yuqiong Z, Shulin Y, Ning Q, Yafen G, Shuguang WU, Lei X, et al. Screening with Some Blood Biochemistry Parameters in GUIZHOU Minipigs and GUANGXI Bama Minipigs. Laboratory Animal Science. 2017.
- Williams LB, Co C, Koenig JB, Tse C, Lindsay E, Koch TG. Response to Intravenous Allogeneic Equine Cord Blood-Derived Mesenchymal Stromal Cells Administered from Chilled or Frozen State in Serum and Protein-Free Media. Front Vet Sci. 2016 Jul 22;3:56. doi: 10.3389/fvets.2016.00056. PMID: 27500136; PMCID: PMC4956649.
- Jeklova E, Leva L, Knotigova P, Faldyna M. Age-related changes in selected haematology parameters in rabbits. Res Vet Sci. 2009 Jun;86(3):525-8. doi: 10.1016/j.rvsc.2008.10.007. Epub 2008 Nov 28. PMID: 19041105.
- Meki MH, Miller JM, Mohamed TMA. Heart Slices to Model Cardiac Physiology. Front Pharmacol. 2021 Feb 4;12:617922. doi: 10.3389/fphar.2021.617922. PMID: 33613292; PMCID: PMC7890402.
- Kallendrusch S, Merz F, Bechmann I, Mayr SG, Zink M. Long-Term Tissue Culture of Adult Brain and Spleen Slices on Nanostructured Scaffolds. Adv Healthc Mater. 2017 May;6(9). doi: 10.1002/adhm.201601336. Epub 2017 Feb 20. PMID: 28218503.
- Ooi BG, Dutta D, Kazipeta K, Chong NS. Influence of the E-Cigarette Emission Profile by the Ratio of Glycerol to Propylene Glycol in E-Liquid Composition. ACS Omega. 2019 Aug 5;4(8):13338-13348. doi: 10.1021/acsomega.9b01504. PMID: 31460462; PMCID: PMC6705204.
- Tao C, Tang Y, Zhang L, Tian Y, Zhang Y. Atomization method for verifying size effects of inhalable particles on lung damage of mice. Sci Total Environ. 2017 Feb 1;579:1476-1484. doi: 10.1016/j.scitotenv.2016.11.150. Epub 2016 Dec 1. PMID: 27914648.
- YAN D, Gao Y, Sheng Y, Liang D, Hu Y, Zhen S. Inhalation toxicity testing on Wistar rats exposed to atomized glycerol in 90 days. Acta Tabacaria Sinica. 2020;26:1-9.
- OECD. Test No. 413: Subchronic Inhalation Toxicity: 90-day Study. Paris: OECD Publishing. 2018.
- Xue-Mei LI, Zhou X, Chi CT, Hou JF, Jia SH, Jin-Ming LI. Animal Experiments and Experimental Animal Selection in Traditional Chinese Medicine. Research and Exploration in Laboratory. 2011.
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016 Mar;7(2):27-31. doi: 10.4103/0976-0105.177703. PMID: 27057123; PMCID: PMC4804402.
- Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C; European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001 Jan-Feb;21(1):15-23. doi: 10.1002/jat.727. PMID: 11180276.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
