Global Journal of Biotechnology and Biomaterial Science
Treatment of diabetic muscular hyperplasia with natural and nutritional supplements
Safir Ullah Khan1* and Munir Ullah Khan2
2MOE Key Laboratory of Macromolecular Synthesis and Functionalization, International Research Center for X Polymers, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027, China
Cite this as
Khan SU, Khan MU (2022) Treatment of diabetic muscular hyperplasia with natural and nutritional supplements. Glob J Biotechnol Biomater Sci 8(1): 001-008. DOI: 10.17352/gjbbs.000016Copyright Licence
© Khan SU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Skeletal muscle is an important part of the human body. Most glucose metabolism is accomplished by skeletal muscle through insulin mediation. Skeletal muscle metabolism disorder can affect glucose metabolic homeostasis and insulin sensitivity of the body, and diabetic muscular Hypoplasia is a secondary lesion of muscle tissue caused by diabetes. In recent years, it has been found that in addition to mainstream western medicine and traditional Chinese and Western medicine combined treatment programs, natural products, and nutritional supplements also play an important role in the prevention and treatment of diabetic muscular Hypoplasia. Therefore, this paper will discuss the definition and pathogenesis of diabetic muscular Hypoplasia, as well as the prevention and treatment mechanism of some natural products and nutritional supplements, to provide more theoretical reference for non-drug targeted therapy of diabetic muscular Hypoplasia.
Introduction
It is estimated that by 2045, the number of patients with type 2 diabetes worldwide will reach 693 million [1] and the accompanying diabetic muscular Hypoplasia has gradually become one of its complications that cannot be ignored. In 1995, Professor GALRAND [2] formally named the muscle Hypoplasia caused by diabetes diabetic muscular Hypoplasia. Diabetic muscular Hypoplasia occurs in peripheral neuropathy caused by diabetes. Peripheral neuropathy can lead to increased loss of motor units in patients, resulting in insufficient innerve of muscle fibers and compensatory nerve redistribution, further inducing muscle fiber Hypoplasia, and ultimately resulting in loss of skeletal muscle mass, strength, and endurance [3]. As an important metabolic tissue of the human body, skeletal muscle wilting will affect glucose absorption and further aggravate insulin resistance, which further aggravates skeletal muscle Hypoplasia, thus forming a vicious cycle that has a serious negative impact on the life of type 2 diabetes patients. Therefore, glycosuria muscle Hypoplasia has been paid more attention to. In recent years, studies have found that in addition to the mainstream western medicine and Chinese and western medicine combined treatment program, diet therapy means can play a role in the pathogenesis of diabetes to relieve and prevent muscular Hypoplasia. Among them, natural products such as berberine (BBR), tea polysaccharides (TPS), and salidroside (SALidroside, Sal can inhibit inflammation, reduce the activity of reactive oxygen species (ROS) and reduce the degree of peripheral nerve injury. However, nutritional supplements such as Leucine (Leu), creatine (Cr) and omega-3 fatty acids (fatty acids) can promote protein synthesis in skeletal muscle and relieve the symptoms of skeletal muscle Hypoplasia caused by diabetes. Therefore, this paper mainly discusses the alleviating or prevention of diabetes-induced muscular Hypoplasia from the perspectives of supplementing natural products or regulating protein metabolic balance, and explores the role and targeted molecular mechanism of nutritional intervention in diabetic muscular Hypoplasia, so as to provide theoretical reference for the diversified selection of prevention or intervention strategies.
Inducible factors and mechanism of diabetic muscular hypoplasia
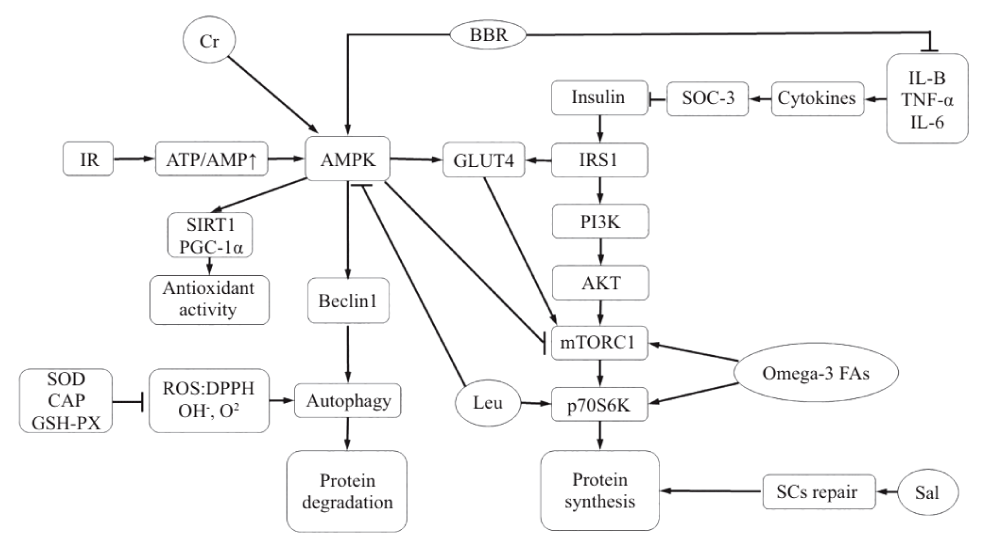
Diabetes muscle Hypoplasia, as a kind of multiple factors of chronic disease, its pathogenesis is mainly of skeletal muscle protein synthesis and degradation of signal tong road damage [4], peripheral neuropathy, oxidative stress, mitochondrial dysfunction, cell dysfunction autophagy, and apoptosis, etc., in its essence is the protein metabolism disorder, low synthesis, and decomposition, This results in a negative balance of skeletal muscle metabolism, resulting in skeletal muscle Hypoplasia and mass loss [5]. Among them, protein decomposition is mainly regulated by the ubiquitin protein system (UPS) and autophagolysosome system. Meanwhile, UPS is mainly composed of ubiquitin activase E1, ubiquitin-binding enzyme E2, and ubiquitin-protein ligase E3 [6]. On the one hand, E3 contains muscle Hypoplasia F-box protein (MUSCLE Hypoplasia F-box, Atrogin-1 (MAFbx/ Atrogin-1), and Muscle Ring Finger protein 1(MuRF1). Protein synthesis, on the other hand, is mediated by phosphatidylinositol 3 kinase (PI3K)/ protein kinase B, AKT)/ mammalian target of Rapamycin complex 1(mTORC1) pathway [7]. However, insulin resistance will break the balance of protein synthesis above, leading to intensified protein decomposition [8].
Insulin receptor substrate 1/2(IRS1/2), In addition, downstream PI3K, Phosphoinositide dependent kinase 1(PDK1), and AKT are activated, and the activated AKT further activates downstream mTORC1, thereby increasing protein synthesis. Among them, mTORC1 not only regulates protein synthesis and acts as a negative regulator in autophagy, but also affects skeletal muscle homeostasis and quality control [9]. At the same time, activated AKT promotes the expression of glucose transporter 4(GLUT4), which speeds up glucose uptake in the blood, thereby lowering blood glucose levels.
Insulin resistance refers to the decline in the efficiency of insulin in promoting glucose uptake and utilization due to various reasons. The body compensates for excessive secretion of insulin to produce hyperinsulinemia to maintain the stability of blood glucose [10]. Among them, IRS1 gene mutation and GLUT4 expression reduction will lead to abnormal glucose uptake in tissues, thus accelerating the occurrence and development of insulin resistance [11]. Lack of energy intake leads to hunger, which increases AMP/ATP value and activates AMPactivated protein kinase (AMPK) to promote Beclin1 expression, which can induce autophagy and protein degradation. At the same time, the downstream histone deacetylase (sirtuin1, SIRT1)/ peroxisome proliferator-activated receptor-1 α(peroxisome proliferator-activated receptor-γ coactivator-1 α) pathway, Enhance mitochondrial function and antioxidant capacity [12].
As a common chronic metabolic inflammation in diabetes, obesity also affects the occurrence of diabetic muscular Hypoplasia. Among them, IκB inhibitor Kappa B kinase β(IKK1β)/ Nuclear factor kappa-B (NF-κB) is a common pathway of insulin resistance and inflammation. It can affect the progression of diabetic muscular Hypoplasia [13]. The expression of IKK1β and C-Jun N-terminal kinase (JNK) was activated by lipopolysaccharide (LPS). Activation of NF-κB and downstream activator protein-1 (AP-1) can increase inflammatory factors such as tumor necrosis factor-α (TNF-α) and interleukin-6 (INTERleukin-6). Il-6) and IL-1β promote the binding of cell signaling molecule SOC-3 with IRS and inhibit the activation of AKT, thereby aggravating insulin resistance [14]. At the same time, insulin is also a kind of angiotensin, which can slow down blood flow and skeletal muscle metabolism when resistance occurs, which accelerates protein decomposition of skeletal muscle [15]. Lipase can induce vascular angiogenesis, endothelin-1 (END1) secretion, an increase in nitric oxide levels, and induce vascular systolic and diastolic dysfunction [16]. In addition, peripheral neuropathy complicated by diabetes, such as peripheral nerve axonal degeneration and demyelination, can affect the function and structure of peripheral nerves, reduce the survival rate of Schwann cells and the regeneration capacity of peripheral nerves, and thus affect the microcirculation of bone iliac muscle [17].
In summary, due to the blocked IRS function in diabetes, the sensitivity of pancreatic insulin is reduced, which then leads to the accumulation of oxidative stress, resulting in autophagy dysfunction, weakened microcirculation, impaired vascular endothelial function, and peripheral neuropathy, which further accelerates the decomposition of protein and leads to diabetic muscle Hypoplasia.
The mechanism of natural products on diabetic muscular hypoplasia
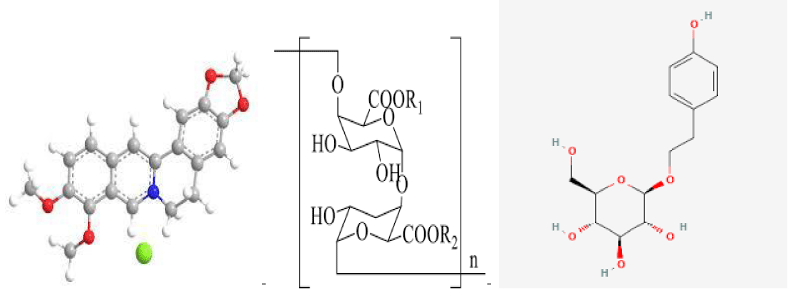
Berberine: Berberine, also known as berberine, berberine, the chemical formula for the C2OH18NO4 Chinese name (S)-5,8,13,13 A-tetrahydro-6H-dibenzo [A,G] quinazine; It belongs to isoquinoline quaternary ammonium alkaloid and is the main part of the efficacy of traditional Chinese medicine, such as Coptis Chinensis, Sanzicao and Phellodendron chinense [18]. The medicinal part is mainly extracted from the bark, root, and stem of berberis [19]. Preocular studies have found that BBR has certain efficacy in anti-inflammatory, antioxidant, hypoglycemic, improvement of insulin resistance, and lipid metabolism disorders. As a common anti-inflammatory drug in clinical practice, BBR can play an anti-inflammatory role by inhibiting inflammation [20]. In a randomized controlled trial of 182 diabetic patients, TNF-α, IL-6, and C-reactive protein (C-REACTIVE protein) in 3T3-L1 adipocytes were found in the control group after taking compound berberine tablets and berberine. CRP, IL-1β, and other inflammatory factors were significantly reduced [21]. Inhibition of the above inflammatory factors can reduce the activity of NF- κB, and AP-1 and thus alleviate the inhibition of IRS, promote the activation of the PI3K/AKT pathway to accelerate the translocation of GLUT4 membrane and increase the uptake of glucose by muscle cells, thereby reducing insulin resistance and lowering blood glucose [14]. In addition, BBR can increase AMP/ATP value and activate the AMPK/ PGC-1α pathway to enhance mitochondrial function in skeletal muscle and achieve the antioxidant effect. In addition, activation of AMPK may increase the transcription-promoting hypoglycemic effect of GLUT4 [22]. At the same time, Berberis (Berberis) fruit also has a similar effect, such as distribution in Iran, the whole Berberis, with anti-inflammatory, lipid-lowering effect; However, domestic Barberis Barberis, Barberis Barberis, berberis Barberis, and berberis Barberis are edible and have the effect of relieving hypertension and dispelling wind and fire [19]. In summary, BBR can inhibit the expression of inflammatory factors, weaken the inhibitory effect on IRS, increase insulin sensitivity, and promote protein synthesis in diabetic muscular Hypoplasia (Figure 1).
Chemical structures of Berberine, Tea polysaccharide, and Salidroside.
Tea polysaccharide
Tea is a traditional Chinese drink, which is rich in tea polysaccharides and tea polyphenols, etc. TPS is a heteropoly sugar combined with protein and has extensive biological activities in anti-inflammatory, antioxidant, anti-tumor, immune modification, and other aspects [23]. Polysaccharides are widely distributed in plants and are polymers of high molecular weight linked by at least 10 monosaccharides [24]. TPS is mainly extracted from leaves, flowers, and bark [25]. It has been found that it has certain biological activities in antioxidant and INSULIN resistance [26]. Experimental studies have shown that TPS can reduce blood glucose and increase body weight in diabetic mice by activating the PI3K/AKT/GLUT4 pathway. In addition, studies have found that oral TPS can enhance superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GCE) in mice. Gsh-px has the ability to remove free radicals such as 1,1-diphenyl-2-picrylhydrazyl (DPPH), OH−, and O2−, thus showing a good antioxidant effect [27]. Due to the different antioxidant effects of TPS with different quality, TPS with medium molecular weight was found to have the strongest antioxidant activity in damaged cells simulated by HK-2 cells, and has antioxidant activity and repair effect on mitochondria, lysosome, and intracellular DNA [28], and also has therapeutic effect on ROS induced vascular endothelial injury [23]. TPS can significantly reduce the expression of TNF-α and other pro-inflammatory factors, and also increase the levels of immunoglobulin A(IgA), IL-4, IL-2, IgG, IgM, IL-10, and other anti-inflammatory factors [29]. At the same time, TPS is widely distributed in green tea, black tea, and other tea drinks. Moderate consumption of tea drinks, especially coarse tea and aged tea, can increase the intake of TPS, thus removing ROS in the body and reducing cell tissue damage.
Salidroside
Salidroside also known as salidroside; Rhodiola is a phenylpropanoside extracted from plants. Named by Soviet scholars after the main component of Rhodiola, salidroside is found in plants such as ligustrine, bilberry, and salix, and has functions of nerve repair and regeneration, anti-oxidation, and anti-muscle Hypoplasia [30]. One of the causes of diabetic muscular Hypoplasia is peripheral nerve injury caused by poor peripheral microcirculation, hypoxia of nerve cells, insufficient nutrient supply, ROS accumulation, etc., thus aggravating skeletal muscular Hypoplasia [31]. Sal plays a significant protective role against ROS and hypoxic nerves [32]. In hypoxia-induced models, Sal can activate the SIRT1/FoxO3α pathway to improve hypoxia-induced vascular smooth muscle injury and nerve injury to reduce apoptosis. Moreover, intervention on SIRT1-related pathways can effectively improve type 2 diabetes [33]. In terms of neural repair, Sal regulates the proliferation and growth of RSC96 Schwann cells in vitro and regulates neurotrophic factors such as brain-derived neurotrophic factor, BDNF, Glial cell line-derived neurotrophic factor, GDNF and cerebral dopamine neurotrophic factor (CDNF) were also up-regulated [34]. Immunohistochemistry and HE staining showed that bone marrow mesenchymal stem cells pretreated with Sal had a significant inhibitory effect on LPS-induced neuroinflammation [35], indicating that Sal can delay nerve injury and neurodegenerative diseases caused by inflammation [36]. At the same time, relevant reviews on the effects of Sal on nerves show that Sal also has positive effects on neurotransmission and regeneration, choline system, anti-apoptosis, anti-oxidative stress, and improvement of AD, PD, and epilepsy [37]. Thus, Sal can directly and interconnect to ameliorate peripheral nerve injury, increase nerve action on the muscle, and achieve the purpose of slowing down the occurrence of skeletal muscle Hypoplasia (Figure 1).
Skeletal muscle protein synthesis related nutritional supplements.
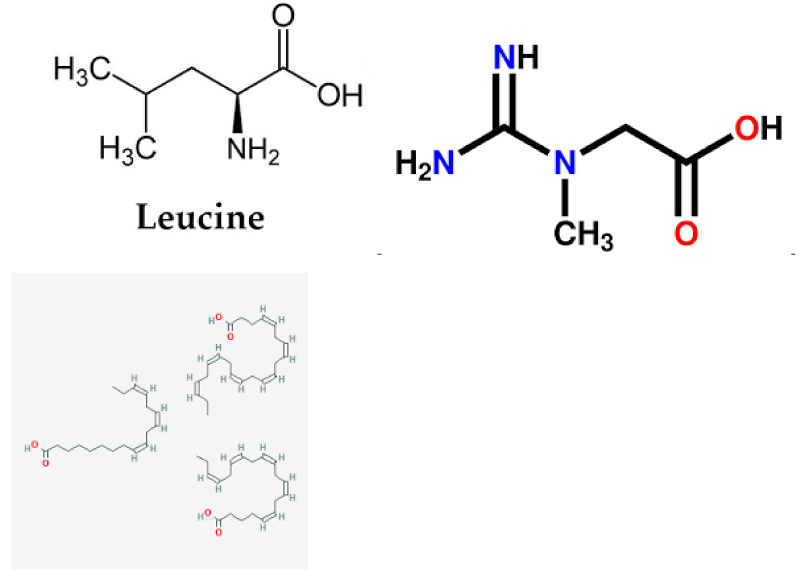
Leucine
Leu is a kind of essential branched-chain amino acid of the human body, which can produce ATP similar to the carbon skeleton of other amino acids. Leu can regulate some cellular processes, such as tissue regeneration, metabolism, and protein synthesis [38]. It has the most significant effect on promoting protein synthesis among all branched-chain amino acids [39]. It can activate the mTOR signaling pathway, an important regulator of mammalian protein synthesis, and reduce the inhibition of mTOR by phosphorylation of AMPK, thereby increasing protein synthesis [40]. However, there was no significant difference in promoting protein synthesis between oral and injection in terms of uptake [41]. Experiments showed that the protein synthesis rate approached the maximum value when Leu reached 0.14 g/kg in rats [42]. Oral essential Leu supplements need at least 15~20 g per day in young and old people to cause an increase in muscle protein synthesis [43], and an increase in the proportion of Leu intake can restore inactivated muscle protein synthesis and increase insulin synthesis and metabolism [44]. Studies have found that in the 2-week Leu supplement experiment, on the assumption that catabolism did not change, venous blood and muscle biopsy found that muscle egg white synthesis increased by about 4% after Leu intake [45]. Therefore, supplementation on the day after exercise, which is the peak of protein synthesis, is more likely to increase muscle protein synthesis [46], possibly through the activation of the AMPK/mTORC1 pathway by Leu. This caused an increase in the downstream P70 ribosomal protein S6 kinase (p70S6K) [47]. Therefore, appropriate supplementation of Leu can effectively reverse muscle protein loss in the treatment and prevention of diabetic muscular Hypoplasia.
Creatine
Cr is a nitrogen-containing organic acid composed of glycine, arginine, and methionine, which can promote protein synthesis and increase muscle strength in skeletal muscle [48]. Cr is a natural non-protein amino acid complex with a high content in red meat and seafood [49]. Cr supplementation prevents movement injury, promotes post-exercise recovery, and regulates body temperature; In addition, for neurodegenerative diseases, diabetes, fibromyalgia, aging, and other aspects, the daily supplementation of 3 g has significant health benefits [50]. Half of Cr in the human body is absorbed through diet, and the rest is synthesized by the liver and kidney [51]. Studies have shown that Cr supplementation can increase the storage of intramuscular phosphocreatine, promote the faster recovery of ATP level after exercise, reduce the expression level of pro-inflammatory factors, enhance the stimulation of satellite cell proliferation, and promote the up-regulation of protein synthesis and cell repair genes, to improve exercise time and intensity [52]. One mechanism may be activation of AMPK, alteration of glucose metabolism and oxidation, reduction of Lactoacid production, and mitochondrial ROS production [53]. On the other hand, it may be that Cr supplementation can activate metabolic pathways such as AMPK/IGF-1/mTOR, regulate GLUT4 and increase protein synthesis of muscle fibers [54]. In addition, in the 16-week Cr combined resistance exercise experiment, it was found that the number of muscle satellites and muscle nuclei induced by training increased, the response of muscle fibers to force training was enhanced, and the performance of sub-maximum strength functional tasks was improved, thereby enhancing the maximum muscle strength [55]. Therefore, Cr combined with exercise training can effectively promote and improve skeletal muscle mass and motor function.
Omega-3 fatty acids
Omega-3 fatty acids, also known as N-3 fatty acids, are a kind of polyunsaturated fatty acids essential to the human body, composed of eicosapentaenoic acid and docosahexaenoic acid. Fish, fish oil, and some vegetable oils are their rich sources and play an increasingly important role in disease prevention and health. Lack of intake of essential fatty acids can lead to a series of diseases such as diabetes, hypertension, infant development, cancer, and so on [56]. In addition, omega-3 fatty acids can also reduce skeletal muscle Hypoplasia, anti-inflammatory and improve blood lipids, etc. [57]. Studies on obese mice induced by a high-fat diet have found that 20-week conjugated linoleic acid /ω-3 combined exercise intervention can inhibit protein degradation, improve muscle strength and mass, and enhance muscle protein synthesis rate [58-59]. It was found that ω-3 fatty acids can increase the expression of muscle protein through the mTOR/p70S6K signaling pathway, increase the value of modulator muscle satellite cells, and improve the protein synthesis of skeletal muscle [60-63]. Similarly, increasing fish intake in the diet has been shown to promote protein synthesis by activating the mTORC1/p70S6 K-related pathway [64]. At the same time, increased omega-3 intake in adolescents improved glucose tolerance and insulin sensitivity [65-68]. In addition, muscle strength and neuromuscular function were significantly improved during 90 days of progressive resistance exercise combined with 2 g of omega-3 fatty acids per day. It is suggested that resistance exercise combined with omega-3 fatty acids has a significant additive effect on muscle gain [69-72]. At the same time, it has also been found that ω-3 fatty acids can not only increase protein synthesis in muscle but also regulate blood lipids and reduce glucose uptake, thus reducing the formation of obesity [73-75].
Chemical structures of Leucine, Creatine, and Omega-3 fatty acids
Conclusion
With the rapid increase of the diabetic population, diabetic muscular Hypoplasia has gradually become a factor affecting the health and quality of life of diabetic patients, so the control of diabetic complications and inducing factors attracts special attention. In this paper, the mechanism of tea polysaccharides, creatine, ω-3 fatty acids, and other natural products and nutritional supplements were discussed. Among them, the natural product BBR reduces protein degradation and increases protein synthesis by inhibiting inflammatory factors, increasing insulin secretion, and activating AMPK, TPS reduces ROS in vivo by enhancing antioxidant factors, and Sal reduces peripheral nerve injury by promoting the repair of Schwann cells. Leu, Cr, and ω-3 fatty acids in nutritional supplements can increase protein synthesis by acting on the mTOR/p70S6K pathway, and can also promote glucose uptake by activating the AMPK/GLUT4 pathway to regulate protein homeostasis. Both natural products and nutritional supplements have potential therapeutic value in dealing with the sub mechanism of diabetic muscular Hypoplasia. Therefore, food or drink containing a high amount of effective natural products can be purposefully selected in the food regimen, or the combination of natural products and nutritional supplements can be considered in dealing with diabetes-induced muscular Hypoplasia. In addition, the effect of nutritional supplements combined with exercise intervention may be better, which can be used as a further research point for the prevention and intervention strategies of diabetic muscular Hypoplasia.
- Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules. 2020 Apr 23;25(8):1987. doi: 10.3390/molecules25081987. PMID: 32340373; PMCID: PMC7221535.
- O'Hare JA, Abuaisha F, Geoghegan M. Prevalence and forms of neuropathic morbidity in 800 diabetics. Ir J Med Sci. 1994 Mar;163(3):132-5. doi: 10.1007/BF02965972. PMID: 8200777.
- Kong XX, Kong YR, Wang XF. The relationship between distal symmetric polyneuropathy and muscle mass loss in type 2 diabetes mellitus .Chinese Journal of Diabetes). 2020; 12(9):716-720.
- Jaiswal, Kumar K, Banerjee I, Mayookha VP. Recent trends in the development and diversification of sericulture natural products for innovative and sustainable applications. Bioresource Technology Reports 13(2021):100614.
- Khan SU, Khan MU. The mechanism of mammalian mitochondrial quality control system. Journal of Chemistry and Nutritional Biochemistry. 2021;59-69.
- O'Neill BT, Bhardwaj G, Penniman CM, Krumpoch MT, Suarez Beltran PA, Klaus K, Poro K, Li M, Pan H, Dreyfuss JM, Nair KS, Kahn CR. FoxO Transcription Factors Are Critical Regulators of Diabetes-Related Muscle Atrophy. Diabetes. 2019 Mar;68(3):556-570. doi: 10.2337/db18-0416. Epub 2018 Dec 6. PMID: 30523026; PMCID: PMC6385751.
- Meex RCR, Blaak EE, van Loon LJC. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes Rev. 2019 Sep;20(9):1205-1217. doi: 10.1111/obr.12862. Epub 2019 Jun 26. PMID: 31240819; PMCID: PMC6852205.
- Guo H, Ma Y, Chang XT. Relationship between UPS-E3 ubiquitin ligase ITCH and insulin resistance. Chinese Journal of Pathophysiology). 2020; 36(5):930-935.
- Zhou YZ, Chen PJ, Zheng LF. Mechanism and treatment of disuse muscular Hypoplasia.Chinese Journal of Rehabilitation Medicine). 2017; 32(11):1307-1313.
- Yoon MS. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients. 2017 Oct 27;9(11):1176. doi: 10.3390/nu9111176. PMID: 29077002; PMCID: PMC5707648.
- Fu YP, Jia J, Zhu RX. The regulatory mechanism of autophagy pathway in hypoxia exposureInduced skeletal muscle Hypoplasia .Chinese Journal of Cell Biology). 2021; 43(6):1221-1230.
- Dong G, Chen PJ, Xiao W. Roles of long non-Coding RNAs on hepatic insulin resistance .Chinese Journal of Cell Biology). 2020; 42(4): 698-704.
- Zheng LF, Chen PJ, Xiao WH. [Roles and mechanism of microRNAs in the regulation of skeletal muscle insulin resistance]. Sheng Li Xue Bao. 2019 Jun 25;71(3):497-504. Chinese. PMID: 31218342.
- He Y, Yang LX, Guo XY. Research progress on mechanism of berberine in treating type 2 diabetes mellitus.Traditional Chinese Research Medcine). 2020; 33(12):69-73.
- Miao H, Ou J, Zhang X, Chen Y, Xue B, Shi H, Gan L, Yu L, Liang H. Macrophage CGI-58 deficiency promotes IL-1β transcription by activating the SOCS3-FOXO1 pathway. Clin Sci (Lond). 2015 Apr;128(8):493-506. doi: 10.1042/CS20140414. PMID: 25431838.
- Wu YL, Chang XT. Cross-talk among insulin signals, inflammatory signals and ubiquitin-proteasome system.Chinese Journal of Biochemistry and Molecular Biology). 2016; 32(11):1177-1184.
- Konda PY, Poondla V, Jaiswal KK, Dasari S, Uyyala R, Surtineni VP, Natesan V. Pathophysiology of high fat diet induced obesity: impact of probiotic banana juice on obesity associated complications and hepatosteatosis. Scientific Reports. 2020. 10(1):1-17.
- Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016 Jan;59(1):44-55. doi: 10.1007/s00125-015-3751-0. Epub 2015 Sep 24. PMID: 26404065.
- Wang Y, Zhang J, Wier WG, Chen L, Blaustein MP. NO-induced vasodilation correlates directly with BP in smooth muscle-Na/Ca exchanger-1-engineered mice: elevated BP does not attenuate endothelial function. Am J Physiol Heart Circ Physiol. 2021 Jan 1;320(1):H221-H237. doi: 10.1152/ajpheart.00487.2020. Epub 2020 Oct 30. PMID: 33124883; PMCID: PMC7847073.
- Zou DW, Gao YB, Zhu ZY, Zhou H, Zhang TJ, Li BM, Wang JY, Li MZ, Ma MF, Zhang N. Traditional chinese medicine tang-luo-ning ameliorates sciatic nerve injuries in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2013;2013:989670. doi: 10.1155/2013/989670. Epub 2013 Oct 28. PMID: 24288572; PMCID: PMC3830865.
- Cheng J, Zhou XJ. Research progress in the antitumor mechanism of berberine .Central South Pharmacy. 2021; 19(5):921-925.
- Khan SU, Khan MU, Kalsoom F, Khan MI, Gao S, Unar A, Zubair M, Bilal M. Mechanisms of gene regulation by histone degradation in adaptation of yeast: an overview of recent advances. Arch Microbiol. 2022 Apr 28;204(5):287. doi: 10.1007/s00203-022-02897-8. PMID: 35482104.
- Yao X, Xiao W, Meng YF. Perspective on resource utilizaton of the berberis fruits .Modern Chinese Medicine). 2016; 18(11):1506-1512.
- He XY. Research progress of berberine in the regulation of inflammation .Medical Journal of West China). 2018; 30(11):1714-1716.
- Sun SP. Effect of Berberine on the serum levels of IL-10, IL-6 and CRP in patients with type 2 diabetes mellitus. Journal of Changchun University of Chi-nese Medicine), 2017, 33(3): 431-3.
- Khan S, Khan M. Molecular developments in cell models of fatty liver disease. DYSONALife Science. 2022; 1:16-29.
- Xiao Y, Xu M, Alimujiang M, Bao Y, Wei L, Yin J. Bidirectional regulation of adenosine 5'-monophosphate-activated protein kinase activity by berberine and metformin in response to changes in ambient glucose concentration. J Cell Biochem. 2018 Dec;119(12):9910-9920. doi: 10.1002/jcb.27312. Epub 2018 Aug 21. PMID: 30129983; PMCID: PMC6282525.
- Du LL, Fu QY, Xiang LP, Zheng XQ, Lu JL, Ye JH, Li QS, Polito CA, Liang YR. Tea Polysaccharides and Their Bioactivities. Molecules. 2016 Oct 30;21(11):1449. doi: 10.3390/molecules21111449. PMID: 27809221; PMCID: PMC6274327.
- Prabhusaran N. Konda PY, Yadav Egi J, Dasari S, Katepogu R, Jaiswal KK.
- Wang J, Hu S, Nie S, Yu Q, Xie M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid Med Cell Longev. 2016;2016:5692852. doi: 10.1155/2016/5692852. Epub 2015 Nov 22. PMID: 26682009; PMCID: PMC4670676.
- Le B, Anh PT, Yang SH. Polysaccharide Derived from Nelumbo nucifera Lotus Plumule Shows Potential Prebiotic Activity and Ameliorates Insulin Resistance in HepG2 Cells. Polymers (Basel). 2021 May 28;13(11):1780. doi: 10.3390/polym13111780. PMID: 34071638; PMCID: PMC8199337.
- Ren D, Hu Y, Luo Y, Yang X. Selenium-containing polysaccharides from Ziyang green tea ameliorate high-fructose diet induced insulin resistance and hepatic oxidative stress in mice. Food Funct. 2015 Oct;6(10):3342-50. doi: 10.1039/c5fo00557d. PMID: 26267675.
- Khan US, Khan MU. The mechanism of mammalian mitochondrial quality control system. Journal of Chemistry and Nutritional Biochemistry. 2021; 2(2):59-69.
- Li S, Chen H, Wang J, Wang X, Hu B, Lv F. Involvement of the PI3K/Akt signal pathway in the hypoglycemic effects of tea polysaccharides on diabetic mice. Int J Biol Macromol. 2015 Nov;81:967-74. doi: 10.1016/j.ijbiomac.2015.09.037. Epub 2015 Sep 26. PMID: 26410811.
- Konda PY, Chennupati V, Dasari S, Sharma N, Muthulingam M, Ramakrishnan R, Sade A, Jagadheeshkumar V, Natesan V, Jaiswal KK. Ethno-pharmacological insulin signaling induction of aqueous extract of Syzygium paniculatum fruits in a high-fat diet induced hepatic insulin resistance. J Ethnopharmacol. 2021 Mar 25;268:113576. doi: 10.1016/j.jep.2020.113576. Epub 2020 Nov 7. PMID: 33171270.
- Sun XY, Wang JM, Ouyang JM, Kuang L. Antioxidant Activities and Repair Effects on Oxidatively Damaged HK-2 Cells of Tea Polysaccharides with Different Molecular Weights. Oxid Med Cell Longev. 2018 Nov 21;2018:5297539. doi: 10.1155/2018/5297539. PMID: 30584463; PMCID: PMC6280578.
- Li X, Chen S, Li JE, Wang N, Liu X, An Q, Ye XM, Zhao ZT, Zhao M, Han Y, Ouyang KH, Wang WJ. Chemical Composition and Antioxidant Activities of Polysaccharides from Yingshan Cloud Mist Tea. Oxid Med Cell Longev. 2019 Aug 21;2019:1915967. doi: 10.1155/2019/1915967. PMID: 31531180; PMCID: PMC6721110.
- Khan SU. Extra Chromosomal Circular DNA: Recent Advances in Research. 2022.
- Sun MF, Shen YQ. Research progress on neuroprotective effect and mechanism of salidroside .Drug Evaluation Research). 2017; 40(7):1019-1028.
- Lang F, Aravamudhan S, Nolte H, Türk C, Hölper S, Müller S, Günther S, Blaauw B, Braun T, Krüger M. Dynamic changes in the mouse skeletal muscle proteome during denervation-induced atrophy. Dis Model Mech. 2017 Jul 1;10(7):881-896. doi: 10.1242/dmm.028910. Epub 2017 May 25. PMID: 28546288; PMCID: PMC5536905.
- Gu C, Li L, Huang Y, Qian D, Liu W, Zhang C, Luo Y, Zhou Z, Kong F, Zhao X, Liu H, Gao P, Chen J, Yin G. Salidroside Ameliorates Mitochondria-Dependent Neuronal Apoptosis after Spinal Cord Ischemia-Reperfusion Injury Partially through Inhibiting Oxidative Stress and Promoting Mitophagy. Oxid Med Cell Longev. 2020 Jul 23;2020:3549704. doi: 10.1155/2020/3549704. PMID: 32774670; PMCID: PMC7396093.
- Xu L, Jia L, Wang Q, Hou J, Li S, Teng J. Salidroside attenuates hypoxia/reoxygenation-induced human brain vascular smooth muscle cell injury by activating the SIRT1/FOXO3α pathway. Exp Ther Med. 2018 Jan;15(1):822-830. doi: 10.3892/etm.2017.5446. Epub 2017 Nov 6. PMID: 29434685; PMCID: PMC5772920.
- Khan SU. Therapeutic application of genetically engineered ribosome-inactivating toxin proteins for cancer. J Biomed Res Environ Sci. 2021;2(12):1216-1228.
- Liu H, Lv P, Wu H, Zhang K, Xu F, Zheng L, Zhao J. The Proliferation Enhancing Effects of Salidroside on Schwann Cells In Vitro. Evid Based Complement Alternat Med. 2017;2017:4673289. doi: 10.1155/2017/4673289. Epub 2017 Jun 7. PMID: 28680451; PMCID: PMC5478829.
- Maadawi ZME. Conditioned Medium Derived from Salidroside-Pretreated Mesenchymal Stem Cell Culture Ameliorates Mouse Lipopolysaccharide-Induced Cerebral Neuroinflammation- Histological and Immunohistochemical Study. Int J Stem Cells. 2017 May 30;10(1):60-68. doi: 10.15283/ijsc16055. PMID: 28446004; PMCID: PMC5488777.
- Khan SU, Khan MU. Review on gene regulation: DNA-protein and protein-protein interactions and their regulatory elements. Journal of Chemistry and Nutritional Biochemistry. 2021; 2(2):35-45.
- Pu WL, Zhang MY, Bai RY, Sun LK, Li WH, Yu YL, Zhang Y, Song L, Wang ZX, Peng YF, Shi H, Zhou K, Li TX. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed Pharmacother. 2020 Jan;121:109552. doi: 10.1016/j.biopha.2019.109552. Epub 2019 Nov 9. PMID: 31715370.
- Zhong Z, Han J, Zhang J, Xiao Q, Hu J, Chen L. Pharmacological activities, mechanisms of action, and safety of salidroside in the central nervous system. Drug Des Devel Ther. 2018 May 24;12:1479-1489. doi: 10.2147/DDDT.S160776. PMID: 29872270; PMCID: PMC5973445.
- Pedroso JA, Zampieri TT, Donato J Jr. Reviewing the Effects of L-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis. Nutrients. 2015 May 22;7(5):3914-37. doi: 10.3390/nu7053914. PMID: 26007339; PMCID: PMC4446786.
- Xu D, Shimkus KL, Lacko HA, Kutzler L, Jefferson LS, Kimball SR. Evidence for a role for Sestrin1 in mediating leucine-induced activation of mTORC1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2019 May 1;316(5):E817-E828. doi: 10.1152/ajpendo.00522.2018. Epub 2019 Mar 5. PMID: 30835510; PMCID: PMC6580170.
- Wang XJ, Yang X, Wang RX, Jiao HC, Zhao JP, Song ZG, Lin H. Leucine alleviates dexamethasone-induced suppression of muscle protein synthesis via synergy involvement of mTOR and AMPK pathways. Biosci Rep. 2016 Jun 17;36(3):e00346. doi: 10.1042/BSR20160096. PMID: 27129299; PMCID: PMC5293580.
- Khan SU, Khan MU. Recent Developments and Applications of Single-Cell RNA Sequencing Technology in Cell Classifi cation. J Biomed Res Environ Sci. 2021 Dec 29; 2(12):1283-1290.
- Kobayashi H. [Amino Acid Nutrition in the Prevention and Treatment of Sarcopenia]. Yakugaku Zasshi. 2018;138(10):1277-1283. Japanese. doi: 10.1248/yakushi.18-00091-4. PMID: 30270272.
- Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005 Mar;135(3):376-82. doi: 10.1093/jn/135.3.376. PMID: 15735066.
- Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJ, van Loon LJ. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006 Sep;84(3):623-32. doi: 10.1093/ajcn/84.3.623. PMID: 16960178.
- Lim CH, Gil JH, Quan H, Viet DH, Kim CK. Effect of 8-week leucine supplementation and resistance exercise training on muscle hypertrophy and satellite cell activation in rats. Physiol Rep. 2018 Jun;6(12):e13725. doi: 10.14814/phy2.13725. PMID: 29952091; PMCID: PMC6021278.
- Khan SU, Khan MU. The role of amino acid metabolic reprogramming in tumor development and immunotherapy. Biochemistry and Molecular Biology. 2022;7(1):6-12.
- Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr. 2012 Aug;31(4):512-9. doi: 10.1016/j.clnu.2012.01.005. Epub 2012 Feb 20. PMID: 22357161; PMCID: PMC3640444.
- Waskiw-Ford M, Hannaian S, Duncan J, Kato H, Abou Sawan S, Locke M, Kumbhare D, Moore D. Leucine-Enriched Essential Amino Acids Improve Recovery from Post-Exercise Muscle Damage Independent of Increases in Integrated Myofibrillar Protein Synthesis in Young Men. Nutrients. 2020 Apr 11;12(4):1061. doi: 10.3390/nu12041061. PMID: 32290521; PMCID: PMC7231404.
- Perry RA Jr, Brown LA, Lee DE, Brown JL, Baum JI, Greene NP, Washington TA. Differential effects of leucine supplementation in young and aged mice at the onset of skeletal muscle regeneration. Mech Ageing Dev. 2016 Jul;157:7-16. doi: 10.1016/j.mad.2016.05.007. Epub 2016 Jun 18. PMID: 27327351; PMCID: PMC5002371.
- Xu L, Li CY, Chen N. Exer-cise and nutrition interventions of sarcopenic obesity and underly-ing mechanisms. Food Science. 2017; 38(21): 279-86.
- Kaviani M, Shaw K, Chilibeck PD. Benefits of Creatine Supplementation for Vegetarians Compared to Omnivorous Athletes: A Systematic Review. Int J Environ Res Public Health. 2020 Apr 27;17(9):3041. doi: 10.3390/ijerph17093041. PMID: 32349356; PMCID: PMC7246861.
- Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, Candow DG, Kleiner SM, Almada AL, Lopez HL. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. 2017 Jun 13;14:18. doi: 10.1186/s12970-017-0173-z. PMID: 28615996; PMCID: PMC5469049.
- Brosnan ME, Brosnan JT. The role of dietary creatine. Amino Acids. 2016 Aug;48(8):1785-91. doi: 10.1007/s00726-016-2188-1. Epub 2016 Feb 13. PMID: 26874700.
- Cella PS, Marinello PC, Borges FH, Ribeiro DF, Chimin P, Testa MTJ, Guirro PB, Duarte JA, Cecchini R, Guarnier FA, Deminice R. Creatine supplementation in Walker-256 tumor-bearing rats prevents skeletal muscle atrophy by attenuating systemic inflammation and protein degradation signaling. Eur J Nutr. 2020 Mar;59(2):661-669. doi: 10.1007/s00394-019-01933-6. Epub 2019 Feb 26. PMID: 30806774.
- Ceddia RB, Sweeney G. Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol. 2004 Mar 1;555(Pt 2):409-21. doi: 10.1113/jphysiol.2003.056291. Epub 2004 Jan 14. PMID: 14724211; PMCID: PMC1664837.
- Gualano B, Rawson ES, Candow DG, Chilibeck PD. Creatine supplementation in the aging population: effects on skeletal muscle, bone and brain. Amino Acids. 2016 Aug;48(8):1793-805. doi: 10.1007/s00726-016-2239-7. Epub 2016 Apr 23. PMID: 27108136.
- Aguiar AF, Januário RS, Junior RP, Gerage AM, Pina FL, do Nascimento MA, Padovani CR, Cyrino ES. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur J Appl Physiol. 2013 Apr;113(4):987-96. doi: 10.1007/s00421-012-2514-6. Epub 2012 Oct 7. PMID: 23053133.
- Kaur N, Chugh V, Gupta AK. Essential fatty acids as functional components of foods- a review. J Food Sci Technol. 2014 Oct;51(10):2289-303. doi: 10.1007/s13197-012-0677-0. Epub 2012 Mar 21. PMID: 25328170; PMCID: PMC4190204.
- Buoite Stella A, Gortan Cappellari G, Barazzoni R, Zanetti M. Update on the Impact of Omega 3 Fatty Acids on Inflammation, Insulin Resistance and Sarcopenia: A Review. Int J Mol Sci. 2018 Jan 11;19(1):218. doi: 10.3390/ijms19010218. PMID: 29324650; PMCID: PMC5796167.
- Oh SL, Lee SR, Kim JS. Effects of conjugated linoleic acid/n-3 and resistance training on muscle quality and expression of atrophy-related ubiquitin ligases in middle-aged mice with high-fat diet-induced obesity. J Exerc Nutrition Biochem. 2017 Sep 30;21(3):11-18. doi: 10.20463/jenb.2017.0028. PMID: 29036761; PMCID: PMC5643205.
- Liang JL, Wang CY, Chen N. Recent progress in regulation of dietary nutrition for sarcopenia Food Science). 2019; 40(1): 303-312.
- Bhullar AS, Putman CT, Mazurak VC. Potential Role of Omega-3 Fatty Acids on the Myogenic Program of Satellite Cells. Nutr Metab Insights. 2016 Feb 3;9:1-10. doi: 10.4137/NMI.S27481. PMID: 26884682; PMCID: PMC4747635.
- Rodacki CL, Rodacki AL, Pereira G, Naliwaiko K, Coelho I, Pequito D, Fernandes LC. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr. 2012 Feb;95(2):428-36. doi: 10.3945/ajcn.111.021915. Epub 2012 Jan 4. PMID: 22218156.
- Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016 Mar 2;8(3):128. doi: 10.3390/nu8030128. PMID: 26950145; PMCID: PMC4808858.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
