Global Journal of Biotechnology and Biomaterial Science
Red blood cell alterations by in Vitro action of Trichinella spiralis newborn larvae
Patricia Ponce de León1*, Martin Toderi2, Horacio Castellini3 and Bibiana Riquelme4*
2Degree in Biotechnology, Facultad Cs, Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Argentina
3Doctor in Physics, Facultad Cs, Exactas Ingeniería y Agrimensura, Universidad Nacional de Rosario, Argentina
4Doctor in Physics, Principal Researcher of the CIUNR, Facultad Cs, Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Argentina
Cite this as
Ponce de Leon P, Toderi M, Castellini H, Riquelme B (2020) Red blood cell alterations by in Vitro action of Trichinella spiralis newborn Larvae. Glob J Biotechnol Biomater Sci 6(1): 007-012. DOI: 10.17352/gjbbs.000012Background: T. spiralis establishes an intimate contact with the host erythrocytes during the newborn larvae migration through the bloodstream to their encystment in the muscle.
Objective: In the present work we study the alterations in the mechanical and aggregation properties of red blood cells produced in Vitro by newborn larvae at low concentrations (100, 250, 500, and 1000 larvae/mL).
Methods: The study was performed by incubating human erythrocytes with an equal volume of different concentrations of newborn larvae for 30 minutes, with controlled agitation. To evaluate the erythrocyte alterations by the action of the larvae, the Erythrocyte Rheometer, the Optical Chip Aggregometer, and the Digital Image Analysis were used.
Results: In the treated erythrocyte samples, a decrease in isolated cell numbers and an increase in the aggregates were observed respect to the control at the higher larval concentration (1000 larvae/mL). The deformability index, the elastic modulus and surface viscosity showed no significant differences with the control; however, the storage modulus of the erythrocyte membrane decreased significantly with the increase in larval concentration. Erythrocyte aggregation parameters showed that the aggregation index increased with the larvae concentration present in the incubation, highlighting the presence of larger aggregates and clusters. The time to attain half of the maximum aggregation diminished, which implies faster aggregation kinetics.
Conclusion: The results observed in Vitro describe the red blood cell alterations that could be produced during in vivo infection in the host.
Introduction
Trichinellosis is a major zoonosis in Latin America and represents a problem both to human and animal health [1]. Despite the high overall prevalence of intestinal worms in humans, and the economic impact they cause in the production of livestock, knowledge of the host-parasite relationship is still incomplete [2]. The biological cycle of Trichinella spiralis includes the migration of the new-born larvae (NBL) through the bloodstream, until its encystment in the muscle cell [3].
A key to understanding parasitic infections is the study of the interactions established between the two organisms involved: the host and the parasite.
The existence of more than 50 sialic acid structures is recognized, which are located at the end of the molecules of which they are part. Sialic acid present important biological functions, for it was deduced that some functions of glycoconjugates are determined, at least partially, by the participation of sialic acids in their composition. Their study has allowed us to consider that they will have an important role in the host-parasite relationship since the cellular mechanisms of many infectious processes would involve the surface glycoconjugates of both [4].
Kaneko and Anosa [5], communicated that a decrease in erythrocyte sialic acid alters the erythrocyte surface, modifying it antigenically and biochemically. These alterations could lead to increased phagocytosis of liver cells, contributing to the anemia status reported in this infection [6]. Moreover, erythrocyte desialylation can expose cryptic antigenic determinants of the membrane, among which is the T antigen. It has been reported that T activation may occur in the stage of larvae circulating through the bloodstream (NBL), with consequent hemolysis, thrombocytopenia and thrombosis [7]. It is also important to study the possible hemorheological alterations produced in the host during infection to understand the parasite-host relationship that is established.
Red blood cells (RBC, erythrocytes) are the most abundant cells in the bloodstream and contain hemoglobin, the compound that carries oxygen through the body [8]. Due to their abundance and mechanical properties, RBCs are mainly responsible for blood rheological behavior influencing the blood viscosity, which depends on erythrocyte deformation and aggregation [9-11]. Sialic acid is responsible for the negative charge of the membrane of red blood cells. A decrease in surface electric charge promotes the erythrocyte aggregation leading to an increase in blood viscosity, which in turn induces an increase in flow resistance and could produce obstructions of microcapillaries, altering microcirculation [12-15].
Previous experiences reported that the contact of erythrocytes with T. spiralis larvae produces alterations in the erythrocyte aggregation [16-19].
In previous work, alterations in the viscoelastic and aggregation properties of human red blood cells by in Vitro action of different concentrations of T. spiralis muscle larvae (ML) were studied [20]. NBLs have direct contact with the erythrocytes of the host during the migration, which could lead to hemorheological alterations affecting the microcirculation. Also, a study with high concentrations of newborn larvae (3000 NBL/mL) was carried out with 60 and 120 minute incubations, showing alterations in erythrocyte aggregation. However, at such larval concentrations, it was not possible to determine the erythrocyte viscoelastic parameters due to the formation of large erythrocyte aggregates that introduced noise in the signal [21]. Therefore, the aim of the present work was to study the alterations in the mechanical and aggregation properties of erythrocytes produced in Vitro by lower concentrations of NBL after a 30 min incubation to avoid the interference of aggregates in the signal. The study was performed using techniques developed in our laboratory.
Materials and methods
Newborn larvae T. spiralis
Newborn larvae (NBL) were obtained from CBi mice infected with T. spiralis, which were provided by the animal research facility of the Experimental Genetics Institute (Faculty of Medical Sciences, National University of Rosario). CBi is an inbred mouse strain derived from an outbred population generated by crossing BALB/c, Rockland, NIH and Swiss mice. It was generated to be used as a base population of a broad genetic basis and as the control line of an experiment of artificial body conformation selection, which has given rise, among others, to CBi–and CBi/L mice lines [22,23].
Between 6 and 13 post-infection days, gravid females were obtained by surgery from the small intestine of mice. Females were incubated in a 100 µL of RPMI-1640 medium (Sigma- Aldrich) supplemented with Gibco™ Fetal Bovine Serum and antibiotics for 18 h at 37ºC in an atmosphere of 5% CO2. NBLs were subsequently separated from adult females and collected in saline solution. Larvae concentrates were prepared at 24 h, resulting in 100, 250, 500 and 1000 larvae/mL [24].
Red blood cells
Fresh group O blood samples were collected from healthy donors (n=3) by venipuncture in sterile vials containing EDTA as anticoagulant, stored at 4 ºC, and analyzed within 24 hours. The blood was centrifuged at 1,100 g (25 ºC, 5 min). After removing plasma and buffy-coat, RBCs were washed three times with saline solution (SS: 0.90% w/v of NaCl, 308 mOsm/L). After the signature of the informed consent from participants, the collection and processing of samples were performed within 24 h from extraction time as recommended for hemorheological laboratory techniques [25]. This study has the approval of the Bioethical Committee of the Facultad de Cs. Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario.
Erythrocyte treatment
Treatment involves incubating 100 µL of globular sediment with an equal volume of NBL concentrate (Treated RBCs). Control RBCs were incubated with saline solution (SS) in the same way. To evaluate the effect of NBL on erythrocyte aggregation, Treated and Control RBCs were incubated for 30 minutes at 37 ºC. Then, RBCs were washed in SS and re-suspended in autologous plasma at 0.3% of hematocrit to obtain aggregation parameters by digital image analysis and at 40% of hematocrit to be measured in the optical chip. The erythrocyte treatment and hemorheological tests were carried out within 4 h after extraction.
Erythrocyte aggregation by digital image analysis
RBCs were suspended in autologous plasma (to induce rouleaux formation) at 0.3% hematocrit and poured into an excavated slide, which was placed on the stage of an Optical Inverted Microscope (Union Optical, Japan). After 5 minutes, aggregation of cells was attained and microscopic images of RBCs aggregate populations were registered by triplicate using a 40x objective and a digital camera (Mikova DCM500 USB2.0). Each image was instantaneously stored in a computer file. Then, ImageJ software was used to study alterations in erythrocyte aggregation by analyzing the distribution of aggregate size and by determining the Individual Cell Coefficient (CCA) [15,26]. The CCA parameter was defined as:
where CAinitial is the percentage of the individual cell number before larva treatment (Control) and CAfinal is the individual cell percentage after larva treatment. This coefficient varies between 0 (no differences in aggregation before and after treatment) and 1 (complete aggregation after treatment).
A study of aggregate size distribution was also carried out. To do this, erythrocyte aggregates were counted and classified into four categories [27]:
• individual cells;
• aggregates of two and three cells;
• aggregates of four, five, and six cells;
• aggregates of 7 or more cells.
Viscoelastic parameters of RBCs
Data were obtained with the Erythrocyte Rheometer, which is an instrument developed in our laboratory based on laser diffractometry technique [28-30]. The following erythrocyte stationary and dynamic viscoelastic parameter corresponding to the oscillating shear stress at a cardiac frequency of 60 cycles per minutes (1 Hz) were determined [9,10]:
DI: erythrocyte deformability index
μ: elasticity of erythrocyte membrane
η: surface viscosity of erythrocyte membrane
G”: loss modulus
In order to carry out these measurements, 100 µL of each RBC sample in autologous plasma (40% hematocrit) was poured in 4.5 mL of a solution of polyvinylpyrrolidone (Sigma PVP360) at 5% (w/v) in PBS (viscosity of 22.0 cp, pH 7.40 and 295 mOsmol/kg).
Optical-chip aggregometer
Erythrocyte aggregation kinetics was studied by an optical chip based on the analysis of light transmission through a blood sample recorded in real-time [31,32]. RBCs were suspended in autologous plasma at 40 % hematocrit and 15 µL of this suspension was used to assess erythrocyte aggregation within 400 s. The optical chip is the physical support for the blood sample and consisted of a 5 mm diameter circular chamber of 15 µL of volume. The measurement device sets the sample on a horizontal plane, and after disaggregation by mechanical agitation, a LED beam (λ~ 670 nm) goes vertically through the sample and a photo-multiplier records variation in the incident light intensity. Graphics of light intensity as a function of time were obtained, and the aggregation parameters were used to calculate the following parameters:
AI: indicates the normalized amount of accumulated aggregation by calculating the integral of the registered curves.
t1/2: the time required to reach half of the total transmitted light intensity at 400 s, indicating a characteristic time constant for the average level of aggregation.
Statistical analysis
The mean and confidence interval were calculated in each blood sample measured in quintuplicate (control and treated with the different concentrations of T. spiralis newborn larvae). Then, p values based on two-tailed statistical tests were calculated in each blood sample considering that in cases where p > 0.05, the difference is not significant [33].
Results
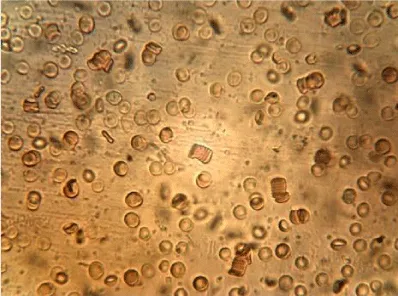
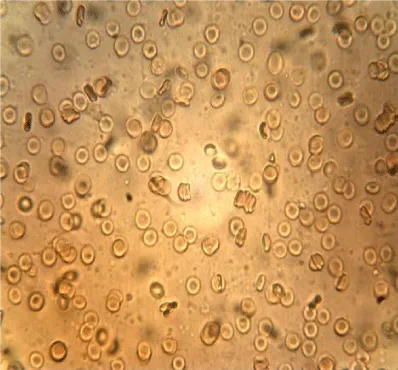
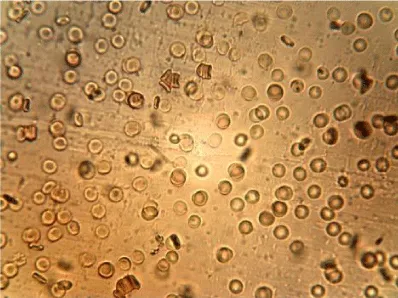
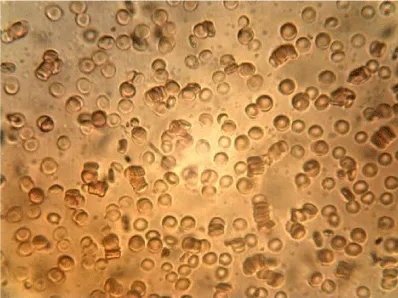
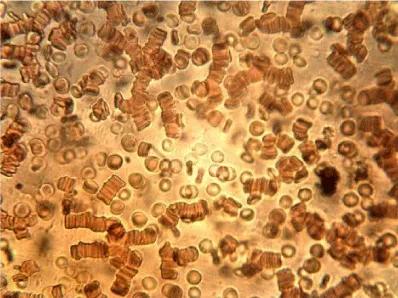
Examples of images of red blood cells untreated and treated with different concentrations of NBL are presented in Figure 1. A decrease in the number of isolated cells and an increase of the aggregates in the RBC treated were observed compared to the control, the most notable effect is when the concentration of NBL used in the treatment was higher. RBC untreated showed a large number of isolated cells and some small aggregates, whereas large networks of erythrocytes were formed in the RBCs incubated with 1000 larvae/mL without morphological cell alterations.
All treated samples show significant alterations in CCA and aggregate size distribution respect to the control (p < 0.001) as shown in Table 1, where CCA values were higher with the increase in NBL concentration. The incubation with 100 larvae/mL showed a CCA = 0.31, indicating that the aggregation was moderate, while in the treatment with 1000 larvae/mL the aggregation was very high (CCA = 0.83).
The mean values of the erythrocyte viscoelastic parameters from RBC untreated and treated with NBL are shown in Table 2. Unlike the results obtained in previous work with ML, in the case of NBL the deformability index and the elastic modulus showed no significant alterations (p > 0.05). As with ML treatment, an increase in the values of the surface viscosity of the membrane is observed in some measurements, but the averages did not show significant differences with the Control. Also, very high noise in the signal was observed at the higher concentration of larvae (1000 larvae/mL), probably due to the presence of micro-aggregates (as can be seen in the images of Figure 1), which interfere with the diffractometric readings of the Erythrocyte Rheometer. The storage modulus of the erythrocyte membrane (G’) decreased significantly with the larvae concentration in the treated samples (p < 0.001).
Erythrocyte aggregation parameters presented in Table 3 show that the AI increased with the number of larvae per mL present in the incubation process. This result evidences the presence of larger and more globular aggregates, while t1/2 diminished, which implies faster aggregation kinetics. A higher concentration of larvae in the incubation presents kinetic curves with greater slopes at the beginning of the phenomenon, which gives shorter t1/2. This could be attributed to a decrease in the surface charge of the cells, favoring the formation of aggregates due to decreased electrostatic repulsion among erythrocytes. Also, it contributes to the formation of globular aggregates, which allows greater transmission of light through the blood sample.
Discussion
Previous work with ML [20], showed a change in the viscoelastic and aggregation properties of the erythrocyte membrane, suggesting that the greater erythrocyte alteration by the action of the larvae occurs in the cytoskeleton and glycocalyx. Besides, in other parasitoses such as Trypanosoma cruzi [34] and Ascaris lumbricoides [15], the authors suggested that these alterations could be associated with the capture of erythrocyte sialic acid by parasite.
In the present work, the results from aggregation parameters of treated samples with new-born larvae showed significant alterations respect to the control. This result is similar to what was observed in the incubation with muscle larvae [20], but a little less pronounced. Also, results showed that the erythrocyte aggregation is higher when the larval concentration used increased, which translates into a decrease in the number of individual cells and the formation of larger erythrocyte aggregates. The incubation with 1000 larvae/ml showed a CCA value of 0.83, indicating the formation of larger erythrocyte aggregates as compared to the control but smaller than those obtained with muscle larvae. This observed increase in aggregation suggests a decrease in the erythrocyte surface electric charge, similar to reported in other work by P. Ponce de León, et al. [16,17]. According to experiments using the Polybrene immune-hematological test, these alterations in the electric charge could be due to a decrease in sialic acid on the membrane [18,35].
The deformability index and the elastic modulus showed no significant alterations, but storage modulus (G’) decreased significantly with the larvae concentration in the treated samples (p<0.05). This relevant result would indicate that the cytoskeleton of erythrocyte may become softer due to the interaction with the NBL.
Results show that t1/2 diminished, indicating faster aggregation kinetics probably related to an alteration in the surface electric charge produced by the action of new-born larvae, as was shown in previous work [21].
Previous work demonstrates that newborn larvae produce alterations in the erythrocyte aggregation [21]. Now, results confirm that the new-born larvae also induce alterations in the viscoelastic properties of the erythrocyte membrane. These results complete the hemorheological evaluation of action de new-born larvae.
Although studies have been carried out on muscle larvae of Trichinella spiralis, the importance of this study with newborn larvae lies in the fact that they circulate through the blood so that the erythrocyte alterations that they produce are of biomedical relevance.
Conclusion
Our investigation group has studied the trichinellosis applying immune-hematological [7,18,32], hemorheological [16,19-21], and physical-mathematical [36-38] techniques. The importance of this type of researches is that the interdisciplinary study enriches the knowledge of the parasite-host relationship and the clinical implications of this parasitosis.
The results observed in Vitro with new-born larvae Trichinella spiralis suggest that hemorheological alterations in the host could be produced during in vivo infection.
Declarations
Funding
This study was carried out with the financial support of Universidad Nacional de Rosario.
Ethics approval (include appropriate approvals or waivers)
This study is part of the research project entitled “Effect of Trichinella spiralis on erythrocyte desialization. Part II” was approved (Resolution CD 826/2018) by the Ethics Committee of the Faculty of Biochemical and Pharmaceutical Sciences of the National University of Rosario, which has elaborated the “Ethics in the treatment of scientific information data” document (Resolution CD 337/2015;
(https://www.fbioyf.unr.edu.ar/evirtual/pluginfile.php/174802/mod_resource/content/1/DOCUMENTO%20DE%20CEUNR%20Pag1-3.pdf) and CICUAL Rules of Procedure (Resolution CD 283/2016;
https://www.fbioyf.unr.edu.ar/evirtual/pluginfile.php/174796/mod_resource/content/1/16CD283.RES.pdf).
Consent to participate (include appropriate statements)
All participants must sign the informed consent in accordance with the regulations of the Facultad de Ciencias Bioquímcias y Farmacéuticas (UNR).
The authors thank the Universidad Nacional de Rosario for financial support. We would also like to extend our gratitude to Doctor Maria Delia Vasconi and Bioq. Mariana Bellini for their significant collaboration in the sample preparation and experiments. We would like to thank the staff from the English Department of the Facultad de Ciencias Bioquímicas y Farmacéuticas (UNR) for the language correction of the manuscript.
- Nweze A (1984) Executive Stress and its Management. The Nigerian Manager: Challenges and Opportunities, Longman Nigeria.
- Isichei A (2000) Posttraumatic stress disorder. N Engl J Med 346: 108-114. Link: http://bit.ly/35TEjZg
- Colman AM (2003) Oxford Dictionary of psychology. Oxford University press Inc New York.
- Sarafino E (2002) Health Psychology: biopsychosocial interactions (4th Ed). New York , NY USA John Wiley and Sons Inc. Link: http://bit.ly/391Gr30
- Baum A (1990) Stress, instrusive imagery, and chronic distress. Health Psychol 9: 653-675. Link: http://bit.ly/35OeZ6V
- Lazarus R, Cohen J (1977) Environmental stress. Hum Behav Environ 89-127. Link: http://bit.ly/2MoIEMk
- Cox T (1978) Stress. Macmillian, London 24: 322. Link: http://bit.ly/2Qe3XRU
- Lazarus W, Folkman M (1984) Conquer your stress. London.
- Lazarus R (1999) Stress and emotion: Anew synthesis. New York Springer Publishing Co. Link: http://bit.ly/2Qj8ra1
- Carroll D (1992) Health psychology: stress, social support, and the buffering hypothesis. Psychological bulletin, 98310-357
- Selye H (1956) Stress of life. MMcGrew-Hill, New York. Link: http://bit.ly/372mVSe
- Rahe RH, Mahan JL, Arthur RJ (1970) Prediction of near future health change from a subjects preceding life change. J Psychosom Res 14: 401-406. Link: http://bit.ly/2ZmO6Ex
- Friedman M, Rosenman R (1974) Type A behaviour and your heart. New York: Knopf. Link: https://amzn.to/2QgsnKA
- Deary MG I, Whiteman MC (2009) Personality Traits (3rd Ed) Cambridge University Press. Link: http://bit.ly/397Lr6n
- Kavanagh T, Shephard RJ (1973) The Immediate Antecedents of Myocardial Infarction in active men. Can Med Assoc J 109: 19-22. Link: http://bit.ly/2MkkylV
- Selye H (1946) The General Adaptation Syndrome and the disease of Adaptation. J Clin Endocrinol Metab 6: 117. Link: http://bit.ly/2tIBOut
- Segal J (2010) The Language of Emotional Intelligence. Link: http://bit.ly/2tLciEZ
- Miller A (1991) Personality Types: A Modern Synthesis, Alta University of Calgary Press. Link: http://bit.ly/2Qeb6Se
- Baum A, Revenson TA, Singer JE (1997) Handbook of Health Psychology. Lawrence Erlbaum Associates, New Jersey
- Fiske D (1949) Consistency of the factorial structure of personality ratings from different sources. J Abnorm Psychol 44: 329-344. Link: http://bit.ly/2Smg0zb
- Goldberg LR (1981) Language and individual differences: The search foruniversal in personality lexicons. In L Wheeler (Ed). Review of personality and social psychology Hillsdale, NJ Eribaum 2: 141-165. Link: http://bit.ly/35TMAwi
- McCrae R, Costa P (1987) Validation of the five-factor model of personality across instruments and observers. J Pers Soc Psychol 52: 81-90. Link: http://bit.ly/2Qf4e6X
- Eysenck H, Eysenck M (1985) Personality and individual differences. New York: Plenum. Link: http://bit.ly/2EQNoWY
- Jenkins CD, Zyzanski S, Rosenman RH (1979) Manual for the Jenkins Activity Survey. New York: Psychological Corp. Link: http://bit.ly/2SobekW
- Friedman M, Roseman S (1974) Affects on performance resulting from variations in stress levels occurring during evaluations. Journal of the South western society of Economists 17: 73-77.
- Spector PE, O’Connell B (1994) The contribution of personality traits, negative affectivity, locus of control and Type A to the subsequent reports of job stressors and job strains. Journal of Occupational Psychology 67: 1-11. Link: http://bit.ly/2QfofdJ
- Caplan R, Jones K (1975) Effects of work load, role ambiguity, and Type A personality on anxiety, depression, and heart rate. J Appl Psychol 60: 713-719. Link: http://bit.ly/2MoGDzO
- Burke RJ, Deszca E (1982) Preferred organizational climates of Type A Individuals. Journal of Vocational Behaviour 21: 50-59. Link: http://bit.ly/34TJiIb
- (2008) Mosby's Dental Dictionary. 2nd edition. Elsevier, Inc.
- David J (2005) Psychology of Stereotyping. Guildford Press, New York. Link: http://bit.ly/2PUmZO0
- Marsland AL, Cohen S, Rabin BS, Manuck SB (2001) Associations between stress, traits, negative affects, acute immune reactivity, and antibody response to hepatitis B inject in healthy ypong adults. Health Psychol 20: 4-11. Link: http://bit.ly/2PRbMhq
- Turner J (1986) The significance of the social identity concept for social psychology. Br J Soc Psycho 25: 231-252. Link: http://bit.ly/37bk61r
- Roy M, Kirschbaum C, Steptoe A (2001) Psychological, cariovscular, and metabolic correlates of individual differences in cortisol stress recovery in young men. Psychoneuroendecrinilogy 26: 375-391. Link: http://bit.ly/2Mq1brL
- Erinoso O (1996) The Sources of Stress among Nigerian retirees. Unpublished B.Sc Thesis, Department of Psychology, University of Lagos.
- Onighaiye M (1996) The impact of length of time on the University on ego identity. self-esteem and stress manifestation in students. Unpublished B.Sc Thesis. Psychology Department University of Lagos.
- Derogatis L, Lipman R, Cori L (1977) SCL-90R: administration, scoring and procedures manual. Baltimore: John Hopkins University Schol of Medicine, Clinical Psychometric Research Unit.
- Barnard (2007) Type A behaviour pattern: A new insight to gender challenges in higher education. dimensions of health and sickness, Tavistock Publications Ltd; Great Britain.
- Umeh C (2009) Interpersonal Dependency: A Predictor of Psychological Distress.
- Bakal DA (1979) Psychology and Medicine: Psychobiological.
- Lundberg B (1996) The Psychology of optimal Experience. Harper Collins, New York.
- McCrae RR, Costa PT (1989) The structure of interpersonal traits. Wiggin’s circumplex and the five-factor model. J Pers Soc Psychol 56: 586-595. Link: http://bit.ly/2ELhXNL
- Norman w (1967) 2,88 personality traits descriptive: Normative operating characteristics for a university population. Ann Arbor: University of Michigan, Department o psychology.Smith BB (1967) Contemporary
- psychology Experiments: Adaptations for Laboratory. Link: http://bit.ly/34RLsI7

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
