Global Journal of Biotechnology and Biomaterial Science
New Aspects of Treatment and Organ Involvement in Pediatric Tuberous Sclerosis
1National Heart Hospital, 1373, Sofia, Bulgaria
2Medical University, 1431, Sofia, Bulgaria
3Department of Pediatric Trakia University, 6014, Stara Zagora, Bulgaria
4Department of Clinical Genetics, “Iv. Mitev” Hospital, 1431, Sofia, Bulgaria
5UMBAL “Pirogov”, 1606, Sofia, Bulgaria
6Medical Faculty, Sofia University “St. Cliement Ohridski”, Sofia, Bulgaria
Author and article information
Cite this as
Marinov R, et al. New Aspects of Treatment and Organ Involvement in Pediatric Tuberous Sclerosis. Glob J Biotechnol Biomater Sci. 2025; 11(1): 001-004. Available from: 10.17352/gjbbs.000023
Copyright License
© 2025 Marinov R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Tuberous sclerosis complex (TSC) is a rare genetic disorder marked by benign tumor formation in multiple organs, frequently involving the heart and central nervous system (CNS). We present a case study of a premature infant with genetically confirmed TSC and coexisting cardiac rhabdomyomas (CRs) and subependymal giant-cell astrocytomas (SEGAs).
The patient was treated with oral sirolimus starting at 3 months of age. Cardiac lesions exhibited complete resolution within 6 months of therapy, as evidenced by echocardiography and normal myocardial strain. CNS imaging at 12 months showed partial SEGA regression. Treatment was well tolerated, with no adverse events reported. This case illustrates the potential of early sirolimus therapy to accelerate tumor regression in both cardiac and CNS lesions, highlighting its value in the conservative management of TSC in infancy.
Tuberous sclerosis complex (TSC) is a rare autosomal-dominant syndrome with variable expression, characterized by benign, non-invasive, tumor-like lesions (hamartomas) in the brain, heart, aorta, skin, kidneys, lungs, and liver.
Reports suggest approximately 60% of children with TSC present with cardiac rhabdomyomas (CRs). These tumors can undergo spontaneous regression, although the underlying mechanisms are not fully understood. Partial regression of CRs has been reported in about 50% of cases, and with complete resolution in approximately 18% [1]. CRs can lead to serious cardiac complications, typically observed during fetal life or the early neonatal period. Such complications may result from obstruction of intracavitary spaces and/or cardiac valves, involvement of the conduction system causing atrioventricular block, or the formation of a substrate for atrial or ventricular tachycardia. These issues can lead to low cardiac output syndrome, congestive heart failure, and even sudden cardiac death. Mortality from cardiac complications is estimated at around 9.5% [1].
In most cases, treatment is not required as the lesions regress spontaneously. However, patients with left ventricular outflow tract obstruction or refractory arrhythmias may require surgical resection. Prognosis depends on the number, size, and location of lesions, as well as the presence of associated anomalies. Overall, despite the potential for regression, cardiac manifestations present a clinical problem in about one-third of cases. New options for conservative therapy are being explored.
CNS changes in TSC include subependymal giant-cell astrocytomas (SEGAs), epilepsy, intellectual disability, and autism spectrum disorder. In certain cases, SEGA may require surgical resection, particularly when causing obstructive hydrocephalus; incomplete excision is associated with a propensity for recurrence.
Recently introduced oral mTOR inhibitors in mammalian models have shown efficacy in reducing SEGA volume and improving seizure control in patients with TSC-related intractable epilepsy. Currently, no randomized clinical trials have been conducted in infants (0–36 months), particularly those younger than 12 months, to assess drug dosing and CNS lesion evolution.
Our study aimed to assess the safety and efficacy of daily sirolimus treatment for CRs and SEGAs in a single case under 12 months of age with genetically confirmed TSC. Unresolved issues—such as the optimal duration of therapy and criteria for treatment discontinuation—are discussed further.
Casepresentation
A multidisciplinary team—including a clinical geneticist, pediatric cardiologist, neonatologist, and pediatric oncologist specializing in solid tumors—managed the case of a 1-month-old premature infant diagnosed with TSC according to international criteria. The infant was born at 35 weeks’ gestation, weighing 2000 g and measuring 44 cm, and was one of four siblings; the mother had experienced three spontaneous abortions.
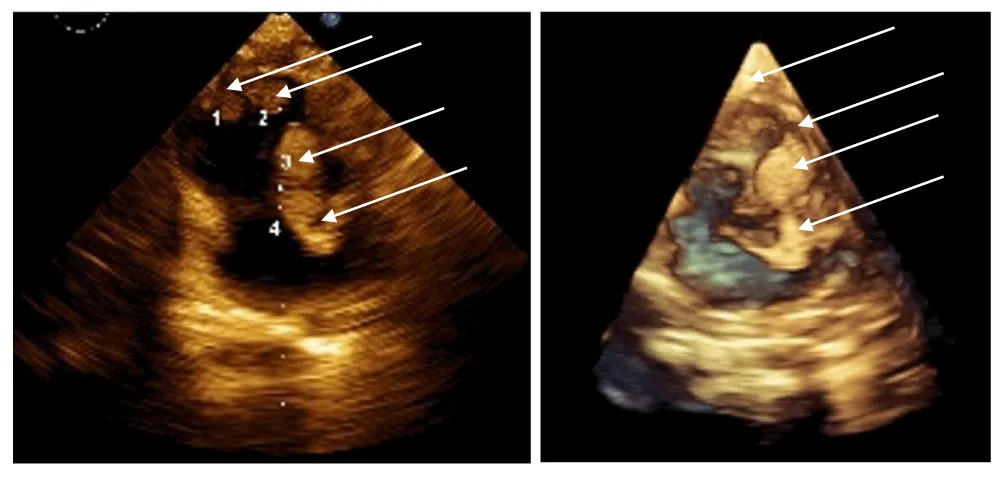
The neonatal period was complicated by infection and respiratory failure. Diagnosis occurred at 1 month due to a detected heart murmur. Echocardiography revealed cardiac rhabdomyomatosis (Figure 1 prompting clinical and genetic evaluation. A TSC2 gene (chromosome 16p13.3) mutation was identified – “c.976-15G>A”, and imaging (Gd-enhanced MRI and transcranial ultrasound) confirmed multiple SEGAs and CRs. Despite the multiple SEGA’s, no seizures have been observed in this patient.
Clinically, the infant thrived, with no heart failure, and demonstrated normal weight gain. Sirolimus therapy (Rapamune) began at 3 months of age. Follow-ups were conducted at 6 and 9 months via echocardiography and at 12 months via CNS MRI.
The initial findings of cardiac rhabdomyomatosis consisted of multiple tumor-like lesions in the septum, the right ventricle, and the apex of the left ventricle, with sizes between 20 and 25 mm for the largest ones (three in number).
Sirolimus, administered as an oral suspension, was started at a weight-adjusted dose based on the standard 1 mg/m² once daily (1 m² ≈ 30 kg). Serum levels were monitored every 8 weeks (therapeutic range: 4–10 ng/mL), and dose adjustments were made accordingly. Ethics committee approval and parental consent were obtained.
After initiation of treatment, regression was already observed by the end of the first month, both in density (echogenicity of the formations) and in size, cutting their sizes to around half (10-12mm). By the end of the third month, the lesions were almost completely resorbed—the intracavitary ones entirely—while data for hyperechogenic areas in the septum persist.
Toxicity was assessed using CTCAE v3.0. SEGA response was evaluated using RECIST v1.1.
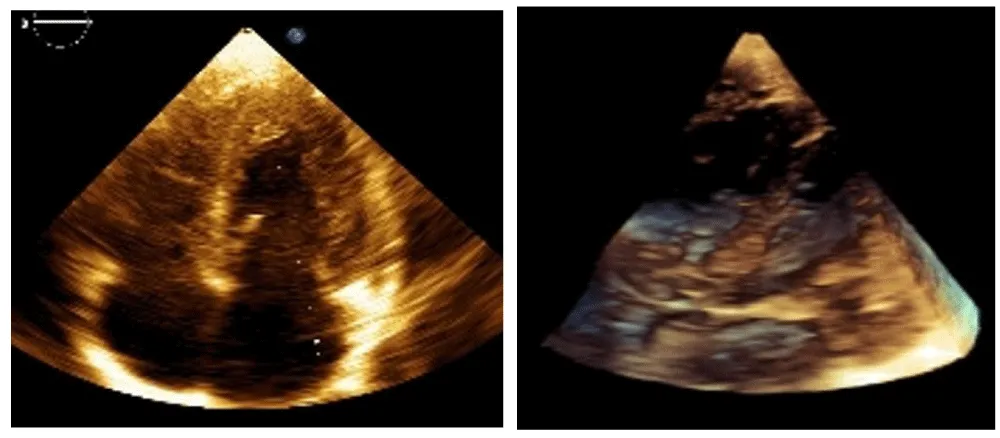
The best cardiac response (BCR) occurred between 4 and 6 months of therapy, with complete resolution of intracardiac tumors on echocardiography (Figure 2) [3].
Following discontinuation of therapy and up to the present moment, no recurrences of the formations have been observed. The child has a normal cardiac and hemodynamic status.
Results
The patient, a male infant (therapy from April 2024 to January 2025), had a birth weight of 1980 g, increasing to 4000 g by therapy initiation. He remained clinically stable, with no cardiac or CNS symptoms, nor abnormalities found by exams.
Serial echocardiography with Philips Epic 7C and GE Vivid E95 (version 206) documented cardiac involvement.
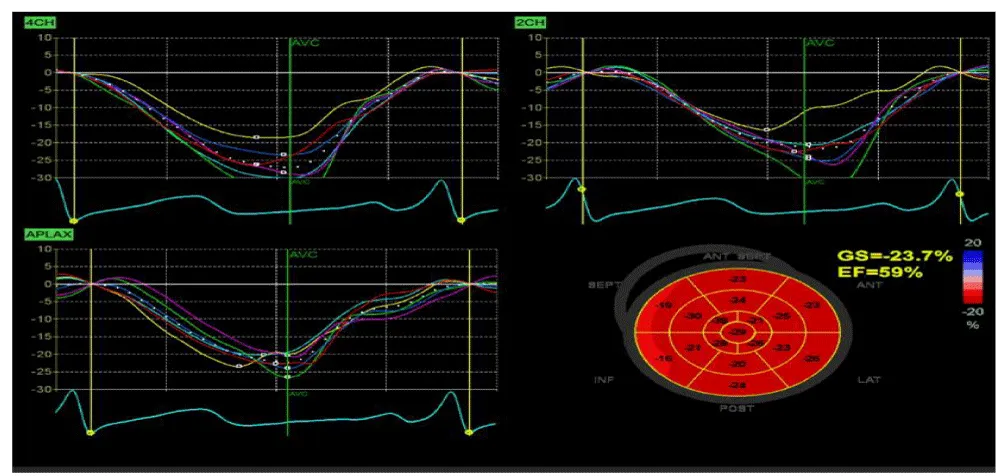
Myocardial strain at 9 months showed normal global longitudinal strain (GLS, −23.7%), supporting therapy discontinuation in light of tumor disappearance (Figure 3).
4CH, 2CH and APLAX respectively refer to “Apical 4-Chamber View”, “Apical 2-Chamber View” and “Apical Long-Axis View (3CH)”. In standard LV (left-ventricle) strain analysis, speckle tracking uses these three apical views (4CH, 2CH, APLAX) to compute the “Global Longitudinal Strain” (GLS): the average of all segmental longitudinal strains across the LV (typically 17 segments). Each of those segments is assigned a different color on the strain graph, The y-axis → % deformation (strain). Negative strain = shortening (normal during systole). Positive strain = lengthening (normal during diastole). The x-axis → time through the cardiac cycle.
Cardiac rhabdomyomas are known to undergo spontaneous regression in infancy, however the time from the beginning of therapy to the onset of a visible cardiac response (echocardiography/EchoCG) in our case was approximately 3–4 months. Regarding the CNS lesions, documented regression was observed after approximately 6 months (transfontanelle ultrasound). These time periods were deemed too short to be attributed to natural spontaneous regression.
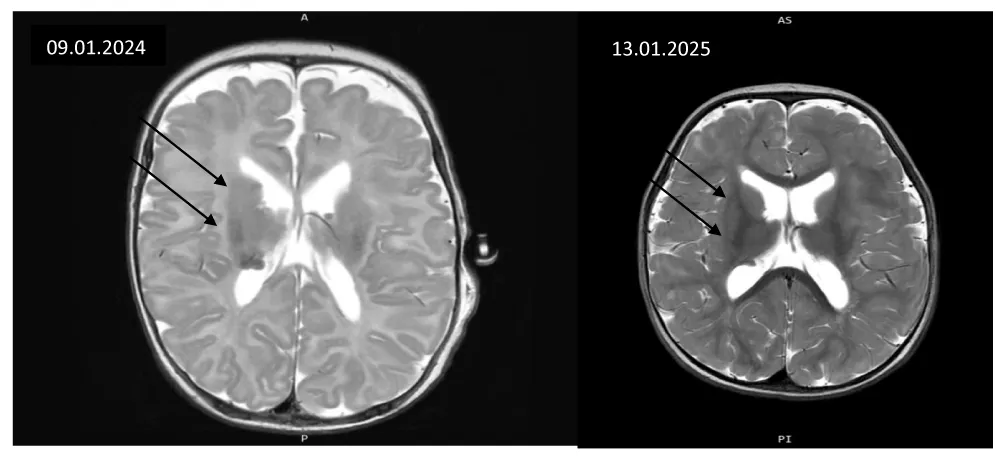
MRI-confirmed SEGA diagnosis pre-therapy and at 12 months showed partial response without significant size progression (Figure 4).
Main CNS lesions were subependymal along both lateral ventricles, with normal ventricular morphology. MRI follow-up showed no lesion progression and slight tumor shrinkage.
With regard to psychomotor development, there is some delay, which cannot be definitively associated with lesions in the central nervous system. Further follow-up and assessment in this regard are planned.
Discussion
Cardiac involvement in TSC often predominates in the fetal and early infant periods. In our case, sirolimus demonstrated significant efficacy in treating multiple, large CRs, both intracavitary and intramural, without rhythm or conduction abnormalities. This activity of the drug may be crucial in newborns or infants with problematic localization and cardiac complications. In fact, the faster reduction in tumor volume improves prognosis by allowing better control of life-threatening arrhythmias or relieving severe intracardiac obstructions, thus avoiding the need for surgical resection. Initial evaluation of cardiac rhabdomyomatosis revealed multiple tumor-like lesions involving the with the three largest lesions measuring approximately 20–25 mm.
Following initiation of therapy, an early treatment response was observed, with reduction in both lesion size and echogenicity evident by the end of the first month. By the third month of treatment, near-complete regression of the lesions was documented, with full resolution of the intracavitary masses and persistence only of limited hyperechogenic areas within the septum. While spontaneous resorption is noted in such cases, the time frame for such resorption to occur is normally far wider.
At the most recent clinical follow-up, after discontinuation of therapy, no recurrence or progression of cardiac lesions has been detected on echocardiographic evaluation. The patient remains clinically stable, with preserved cardiac function and normal hemodynamic status, supporting a sustained therapeutic response and favorable short-term outcome.
A key question during follow-up was the timing of therapy discontinuation, confirmation of intramural lesion resolution, and monitoring of myocardial deformation indices. Treatment resulted in complete tumor resorption—i.e., a full therapeutic response. CNS manifestations stabilized, with partial regression in over 50% of lesions.
The interval from treatment initiation to observable cardiac response was approximately two months; documented CNS regression occurred after six months. This suggests pharmacological enhancement of natural tumor regression. While this patient had not displayed seizure activity, such enchancement would be notably beneficial in cases where seizures are present.
mTOR inhibitors may be crucial in neonates or infants with CRs and cardiac complications, as rapid tumor volume reduction can improve prognosis by alleviating life-threatening arrhythmias or intracardiac obstructions, reducing surgical intervention. Case reports and small series support mTOR inhibitor use in infants and fetuses [4-7].
Everolimus’s benefits in SEGA have been demonstrated in over 100 patients (age >3 years to 34 years), while sirolimus has been used in children >36 months [8].
Sirolimus responses have been classified as: (1) stable disease; (2) partial response with <50% reduction; (3) partial response with >50% reduction; and (4) complete regression. Time to BCR was recorded.
Our case is notable for early therapy initiation at 3 months in a preterm infant.
Treatment adhered to the 2014 JACC consensus on cardiac involvement in TSC: Cardiovascular Manifestations of Tuberous Sclerosis Complex and Summary of the Revised Diagnostic Criteria and Surveillance and Management Recommendations From the International Tuberous Sclerosis Consensus Group.
Unresolved questions
- What happens to fibrous tissue in affected parenchymal organs?
- Why do cardiac hamartomas spontaneously regress while lesions in other organs persist?
- What is the incidence of sudden cardiac death in patients with CRs, and does sirolimus treatment reduce this risk?
- What is the prevalence of latent hypertrophy and dysfunction of the left and right ventricles?
- Should myocardial deformation parameters like GLS be routinely assessed as prognostic markers and indicators of spontaneous regression?
Prospective clinical trials are warranted, focusing on subclinical cardiac function evaluation, including assessments post-therapy cessation.
Author Contributions
Dr. Rumen Marinov - Main oversight of the study, Echocardiology, Neonatology, Writing - Original Draft
Dr. Ivan Chakarov - Hematological and Oncological analysis and consultant
Dr. Daniela Avdzhieva-Tzavella - Genetics consultant and analysis
Dr. Valentin Dimitrov - Pediatrics consultant
Assistant Radoslav Iliev: Writing - Review and Editing, Translation
- Lucchesi M, Chiappa E, Giordano F, Mari F, Genitori L, Sardi I. Sirolimus in infants with multiple cardiac rhabdomyomas associated with tuberous sclerosis complex. Case Rep Oncol. 2018;11(2):425-430. Available from: https://doi.org/10.1159/000490662
- Watson GH. Cardiac rhabdomyomas in tuberous sclerosis. Ann N Y Acad Sci. 1991;615:50-57. Available from: https://doi.org/10.1111/j.1749-6632.1991.tb37747.x
- Jóźwiak S, Kotulska K, Kasprzyk-Obara J, Domańska-Pakieła D, Tomyn-Drabik M, Roberts P, et al. Clinical and genotype studies of cardiac tumors in 154 patients with tuberous sclerosis complex. Pediatrics. 2006 Oct;118(4):e1146-1151. Available from: https://doi.org/10.1542/peds.2006-0504
- Holmes GL, Stafstrom CE; Tuberous Sclerosis Study Group. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48(4):617-630. Available from: https://doi.org/10.1111/j.1528-1167.2007.01035.x
- Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. 2014;15(13):1513-1520. Available from: https://doi.org/10.1016/S1470-2045(14)70489-9
- Breathnach C, Pears J, Franklin O, Webb D, McMahon CJ. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134(4):e1199-1202. Available from: https://doi.org/10.1542/peds.2013-3293
- Chang JS, Chiou PY, Yao SH, Chou IC, Lin CY. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38(7):1478-1484. Available from: https://doi.org/10.1007/s00246-017-1688-4
- Weiland MD, Bonello K, Hill KD. Rapid regression of large cardiac rhabdomyomas in neonates after sirolimus therapy. Cardiol Young. 2018;28(3):485-489. Available from: https://doi.org/10.1017/S104795111700244X
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
