Annals of Systems Biology
A Rapid Fluorescent in vitro Assay Suitable for Studying the Kinetics of O6-Alkylguanine Lesion Progression to DNA Inter-Strand Cross-Links and the Kinetics of the Primary Lesion’s Repair
Philip G Penketh*
Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06520, USA
Cite this as
Penketh PG. A Rapid Fluorescent in vitro Assay Suitable for Studying the Kinetics of O6-Alkylguanine Lesion Progression to DNA Inter-Strand Cross-Links and the Kinetics of the Primary Lesion’s Repair. Ann Syst Biol. 2024;7(1): 051-053. Available from: 10.17352/asb.000023Copyright License
© 2024 Penketh PG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.An assay is described that permits the study of the kinetics of DNA cross-link precursor formation, the kinetics of cross-formation, and the kinetics of the repair of these DNA cross-link precursor lesions, at physiological pH values. Due to the relatively rapid nature of these processes existing assays are not suited to the study of these processes. Data obtained using this assay can be used to optimize the design of certain classes of anticancer drugs.
In the author’s opinion, by far the best assay for O6-Methylguanine-DNA-methyltransferase (MGMT) activity in cells is the assay developed by Ishiguro, et al. [1-3]. This assay is based on the transfer of radioactive benzyl residues from [benzene-3H]O6-benzylguanine to MGMT. However, this assay is unsuitable for studying the kinetics of the progression of the primary O6-alkylguanine lesion to DNA-DNA inter-strand cross-links, and the kinetics of the primary lesion’s repair by MGMT, because the rate constants involved in the Ishiguro assay means it takes several hours to determine the MGMT activity, whereas many of the dynamic processes we are interested in occur on the time frames of minutes or even seconds. Thus they could not be readily resolved by the [Benzene-3H]-O6-benzylguanine method as the reaction kinetics of [Benzene-3H]-O6-benzylguanine with MGMT would be highly rate limiting in many cases.
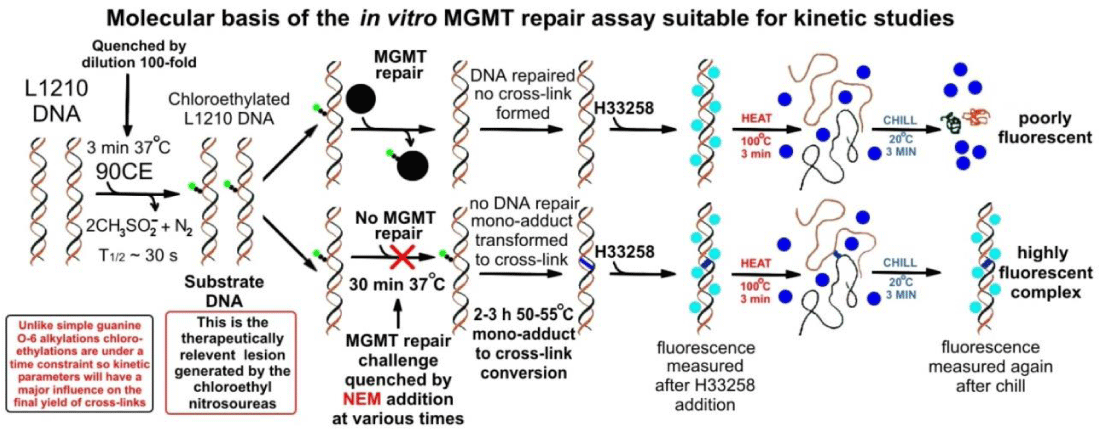
The rapid fluorescent in vitro assay, to study the kinetics of lesion formation and repair, was developed from our DNA cross-linking assay [4]. This assay allows for the determination of the fraction of DNA molecules containing one or more covalent inter-strand cross-links. These cross-linked DNA molecules rapidly renature upon snap cooling following thermal denaturation, because the cross-links hold the complementary DNA strands in close proximity and in register. Since H33258 forms a highly fluorescent complex with double-stranded but not mispaired/denatured DNA, DNA molecules containing cross-links, will yield highly fluorescent complexes following a heat/chill cycle with H33258 dye; whereas DNA molecules devoid of such cross-links do not. Since all assay steps are conducted at neutral pH values, potential problems caused by base-catalyzed lesion hydrolysis are avoided [4].
The fluorescent in vitro assay outlined in this opinion piece allows for the in vitro study of the relative rates of the primary lesions’ progression to cross-links and competing repair processes. This rapid assay technique also permits the investigation of the initial DNA alkylation event which is 1st order with respect to the concentration of the bis-sulfonyl hydrazine (BSH) and has t1/2 values of <30s [5]. This initial reaction can thus be effectively quenched at any point by a 100-fold rapid dilution (see Figure 1) of the reaction mixture. The proportion of DNA molecules that already contained a guanine O6-alkylguanine lesion prior to the dilution step can then be determined by measuring the fraction of the DNA molecules that are cross-linked as described in [5]. The number of cross-linking moieties produced in the test DNA relates to the cross-linked fraction as follows: The average number of cross-links per DNA molecule (A), assuming a Poisson distribution, equals −ln(1-X), where X = the cross-linked fraction (the value obtained from the assay). Thus for a sample of test DNA with, for example, a cross-linked fraction (X) of 0.4, A calculates to be 0.51. The probability that a given DNA molecule has N cross-links equals e−A × AN/N!; where N! equals factorial N. In this example, 60% of the DNA molecules would have 0 cross-links, 30.6% would have 1 cross-link, 7.8% would have 2, 1.3% would have 3, etc., to give a total of 40% of the DNA molecules containing one or more cross-links, with the average number of cross-links per DNA molecule being 0.51. Thus at low levels of cross-linking (<10%) X ≈ A, as very few molecules contain more than one cross-link.
Our research team (now disbanded), designed modular drugs which were composed of domains with specific functions [6,7]. A targeting domain (e.g., a trigger for hypoxic region activation [6], or solid tumor targeting via the EPR effect [7]; a linker domain which could also have a fuse function (time delay to allow drug accumulation [7]), and a BSH domain which functions as the warhead [6].
To efficiently design the modular nano-drug/particle, it is best to optimize each domain separately. This would require the BSH domain to be optimized to maximize its differential toxicity between MGMT-containing cells and those lacking MGMT activity. To do this one would have to maximize the relative repair window (i.e., slow the rate of the transition from the MGMT repairable cross-link precursor to the highly lethal and poorly tolerated DNA-DNA inter-strand cross-link [5]). Using our rapid fluorescent in vitro assay with NEM (1 µL of a 1M solution in DMSO per 20µL of reaction mixture) quenching (Figure 1), we were able to show that the cross-link precursor lesions generated by 90CE (1,2-bis(sulfonyl)-1-(2-chloroethyl)hydrazine) were essentially completely removed in ~20 seconds by a small molar excess of MGMT. This is an extreme rate for two reactants (MGMT and lesion) both at miniscule concentrations. In fact, it is greater than the maximum diffusion-controlled reaction rate of (109 to 1010 M-1 s-1) at 37 ºC and thus would be impossible for reactants in a free solution where the molecules are free to diffuse in 3 dimensions. However, this reaction occurs on a linear DNA molecule with the lesion in a fixed position like a sitting duck, and the MGMT molecule is free to walk/diffuse in a single dimension along the DNA molecule. To avoid lethality the cell needs to have sufficient MGMT molecules to remove all the cross-link precursors before a small (<10/cell) but lethal number has progressed to cross-links [5]. If 90CE analogs were synthesized as potential warheads, e.g., by replacement of the chlorine moiety with pseudohalogens, or of the chloroethyl hydrogens by deuteriums, or replacement of the chloroethyl moiety with a chloroisopropyl moiety as described in [8], they would likely change both the rate of the transition of the cross-link precursor to a G-C ethane cross-link (1-(N3-cytosinyl),-2-(N1-guaninyl)ethane) and its repair rate by MGMT. Thus, the kinetics of these processes would need to be ascertained to ensure these modifications increased the magnitude of the repair window and increased the differential toxicity between MGMT-expressing and non-expressing cells to the BSH warhead.
An association between MGMT promoter methylation and tumorigenesis features in patients with ovarian cancer has been reported [9]. Since MGMT promoter methylation results in the silencing of the MGMT gene [3,6,10] it is likely that such tumors would show dramatic responses when treated with the described modular BSH nano-drugs and existing mildly targeted BSHs such as 101 m, (1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-[(methylamino)carbonyl]hydrazine) [11,12].
This assay had been previously used to determine the inhibitory activities of 2-nitro-6-benzyloxypurine, an analog of O6-benzylguanine, in which the essential 2-amino group is replaced by a nitro moiety and its reduction products. 2-Nitro-6-benzyloxypurine was found to be non-inhibitory (>2000-fold weaker than O6-benzylguanine), whereas its hydroxylamino and amino reduction products were potent inhibitors [13].
Conclusion
This high-speed fluorescence-based assay is ideally suited to study the interactions of BSHs, haloethyl nitrosoureas, and other classes of alkylating drugs whose therapeutic activity is dependent on the attack of the O-6 position of DNA guanine and leads to the formation of DNA-DNA inter-strand cross-links. Moreover, this assay can be performed on low-cost desk top Hoefer minifluorometers (excitation wavelength 365 nm, emission wavelength 460 nm), that are readily available in most laboratories.
- Ishiguro K, Shyam K, Penketh PG, Sartorelli AC. Development of an O(6)-alkylguanine-DNA alkyltransferase assay based on covalent transfer of the benzyl moiety from [benzene-3H]O(6)-benzylguanine to the protein. Anal Biochem. 2008;383(1):44-51. Available from: https://doi.org/10.1016/j.ab.2008.08.009
- Ishiguro K, Zhu YL, Shyam K, Penketh PG, Baumann RP, Sartorelli AC. Quantitative relationship between guanine O(6)-alkyl lesions produced by Onrigin™ and tumor resistance by O(6)-alkylguanine-DNA alkyltransferase. Biochem Pharmacol. 2010;80(9):1317-1325. Available from: https://doi.org/10.1016/j.bcp.2010.07.022
- Ishiguro K, Shyam K, Penketh PG, Baumann RP, Sartorelli AC, Rutherford TJ, Ratner ES. Expression of O6-Methylguanine-DNA Methyltransferase Examined by Alkyl-Transfer Assays, Methylation-Specific PCR and Western Blots in Tumors and Matched Normal Tissue. J Cancer Ther. 2013;4(4):919-931. Available from: https://doi.org/10.4236/jct.2013.44103
- Penketh PG, Shyam K, Sartorelli AC. Fluorometric assay for the determination of DNA-DNA cross-links utilizing Hoechst 33258 at neutral pH values. Anal Biochem. 1997;252(1):210-213. Available from: https://doi.org/10.1006/abio.1997.9996
- Penketh PG, Baumann RP, Ishiguro K, Shyam K, Seow HA, Sartorelli AC. Lethality to leukemia cell lines of DNA interstrand cross-links generated by Cloretazine derived alkylating species. Leuk Res. 2008;32(10):1546-1553. Available from: https://doi.org/10.1016/j.leukres.2008.03.005
- Shyam K, Penketh PG, Baumann RP, Finch RA, Zhu R, Zhu YL, et al. Antitumor sulfonylhydrazines: design, structure-activity relationships, resistance mechanisms, and strategies for improving therapeutic utility. J Med Chem. 2015;58(9):3639-3671. Available from: https://doi.org/10.1021/jm501459c
- Penketh PG, Williamson H, Baumann RP, Shyam K. Design Strategy for the EPR Tumor-Targeting of 1,2-Bis(sulfonyl)-1-alkylhydrazines. Molecules. 2021; 26(2):259-275. Available from: http://doi:10.3390/molecules26020259
- Penketh PG, Shyam K. Design strategy and approach to increase the selectivity of 1,2-bis(sulfonyl)-1-alkylhydrazine warheads in tumor-targeted applications. BJSTR. 2021; 34(3):26781-26790. Available from: https://ideas.repec.org/a/abf/journl/v34y2021i3p26781-26790.html
- Qiao B, Zhang Z, Li Y. Association of MGMT promoter methylation with tumorigenesis features in patients with ovarian cancer: A systematic meta-analysis. Mol Genet Genomic Med. 2018;6(1):69-76. Available from: https://doi.org/10.1002/mgg3.349
- Roh HJ, Suh DS, Choi KU, Yoo HJ, Joo WD, Yoon MS. Inactivation of O6-methyguanine-DNA methyltransferase by promoter hypermethylation: Association of epithelial ovarian carcinogenesis in specific histological types. The Journal of Obstetrics and Gynaecology Research. 2011;37:851–860. Available from: https://doi.org/10.1111/j.1447-0756.2010.01452.x
- Penketh PG, Finch RA, Sauro R, Baumann RP, Ratner ES, Shyam K. pH-dependent general base catalyzed activation rather than isocyanate liberation may explain the superior anticancer efficacy of laromustine compared to related 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazine prodrugs. Chem Biol Drug Des. 2018;91(1):62-74. Available from: https://doi.org/10.1111/cbdd.13057
- Finch RA, Shyam K, Penketh PG, Sartorelli AC. 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-(methylamino)carbonylhydrazine (101M): A Novel Sulfonylhydrazine Prodrug with Broad-Spectrum Antineoplastic Activity. Cancer Res. 2001;61:3033-3038. Available from: https://pubmed.ncbi.nlm.nih.gov/11306484/
- Penketh PG, Shyam K, Baumann RP, Ishiguro K, Patridge EV, Zhu R, Sartorelli AC. A strategy for selective O6-alkylguanine-DNA alkyltransferase depletion under hypoxic conditions. Chem Biol Drug Des. 2012;80(2):279-290. https://doi.org/10.1111/j.1747-0285.2012.01401.x
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
