Annals of Marine Science
Fluorescence of plankton fish eggs of Black Sea Mullus barbatus ponticus for test-analysis of the cell fertilization and development
Victoria V Roshchina1*, Valerii A Yashin1, Tatyana N Petrova2 and Vladimir I Maltsev2
2T.I. Vyazemsky Karadag Scientific Station, Nature Reserve of RAS, Branch of A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS, Nauki str., 24, Kurortnoye Settlement, Feodosia, 298188, Crimea, Russia
Cite this as
Roshchina VV, Yashin VA, Petrova TN, Maltsev VI (2024) Fluorescence of plankton fish eggs of Black Sea Mullus barbatus ponticus for test-analysis of the cell fertilization and development. Ann Mar Sci 8(1): 002-007. DOI: https://dx.doi.org/10.17352/ams.000042Copyright License
© 2024 Roshchina VV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The autofluorescence of fish eggs from the Black Sea Mullus barbatus ponticus Essipov was studied in order to identify the state of their fertilization and development in the ichthyoplankton. The study differed from the earlier genetic fish investigations with artificial fluorescent dyes. In our experiments, among the eggs were the objects with various stages of development: from unfertilized and just-fertilized cells emitted in blue to well-seen embryos that fluoresced in red. Red-fluorescing embryonal probes were peculiar to living eggs, unlike blue-fluorescing cells with damaged structures. The fluorescent method (luminescence microscopy with or without microspectrofluorimetry) allows us to identify the earlier stages of development of the fish eggs. The autofluorescence of the fish eggs of the species studied was possible to recommend for the testing of fertilized and living eggs at the early stages of development that may be applied to the fish industry in the future.
Introduction
Analysis of planktonic fish eggs has not yet included a study of their autofluorescence in visible spectral regions. Meanwhile, the position of the maxima of the fluorescent components and the intensity of egg emission in the visible spectral range potentially could be useful for testing its condition, fertilization, and development in modern environmental practice. In the beginning, it had perspectives for ecological monitoring of the fish population near the Nature Reserve of RAS, but the data could be useful for the future of the fish industry as a whole.
For this purpose, it may be convenient to apply fluorescence techniques such as luminescence microscopy without and with microspectrofluorimetry [1-5]. However, there is still no data on the eggfish fluorescence in the visible region of the spectrum from 400 nm to 700 nm. Here, we set the task of obtaining images and fluorescence spectra of eggs from Black Sea fish from plankton in order to find out the possibilities of applying different spectral techniques in practical testing of the state and stage of cellular development that may be of large commercial importance. In this first paper, we reflected on the process of determining maturity and fertilization on the examples of the Black Sea red mullet roe Mullus barbatus ponticus Essipov.
Materials and methods
The objects of research were samples from plankton - fish eggs of Mullus barbatus ponticus Esipov, 1927 (trivial name the Black Sea red mullet) belongs to the family Mullidae [6], in the Black Sea basing near Karadag Biological Station of Feodosia, Russia. It was collected for experiments as described in special publications [7,8]. Eggs’ samples were fixed with 4% or 10% formalin for best storing before spectral experiments. The first fixation has advantages for fast visualization during one week, while the second one permits storage for a longer time (up to 1 month).
Measurement of fluorescence. Autofluorescence of samples in 4% and 10% formalin was observed and photographed directly on slides [4,5] using a Leica DM 6000 B luminescent microscope (Germany) and the MSF-15 microspectrophotometer/fluorimeter (LOMO, Russia) [8] with the Levenhuk M300 Base camera (USA). The fluorescence spectra were recorded using the MSF-15 microspectrofluorimeter (LOMO, Russia).
Results and discussions
The base principle of the animal egg structures – from a formation of oocytes (oocyte is a developing female structure that cannot yet bind sperm or be fertilized) has been described by Gilbert or Gilbert and Barresi to mature embryo developing after fertilization [9,10]. This mature egg consists of a nuclear part in the cytoplasm (pronucleus) covered with yolk, and this all is surrounded by the yolk membrane. Outside the membrane, there is a shell with several layers. Observing with the usual microscope, the red mullet fish egg cell from Black Sea species studied as a whole structure of the generative female cell could be seen covered with a dense thick yolk membrane and containing a large amount of yolk with one or more yolk fatty drops and their structure has the image, similar to described for many invertebrates [11]. Eggshell consists of several layers: 1. cellular membrane, which regulates the flow of specific ions during fertilization and must be capable of fusing with the sperm cell membrane. 2. Outside this egg cell membrane is an extracellular matrix that forms a fibrous mat around the egg and is often involved in sperm-egg recognition. In invertebrates (likely it is similar for fish too), this structure is usually called the vitelline envelope containing several different glycoproteins [9-11]. Many types of eggs also may have a layer of egg jelly outside the vitelline envelope. This glycoprotein meshwork can have numerous functions, but most commonly it is used either to attract or to activate sperm. Within the enormous volume of egg cytoplasm resides a large nucleus is present, usually not seen under transmitted light of a microscope because all interior is covered by yolk. Yolk formed in the oocyte serves as the nutrient material for the future embryo. Mainly, we see yolk, which can occupy up to 95% of the total volume of the cell. Often yolk and yolk fatty drops contain pigments carotenoids, lipids, and proteins. The meiotic divisions lead the oocyte to either synthesize or absorb proteins, and yolk and yolk fatty drops act as food reservoirs for the developing embryo. Besides, here are protective chemicals that are needed as a defense against predators or for a safer environment. Many animal eggs may contain ultraviolet filters and DNA repair enzymes that protect them from sunlight.
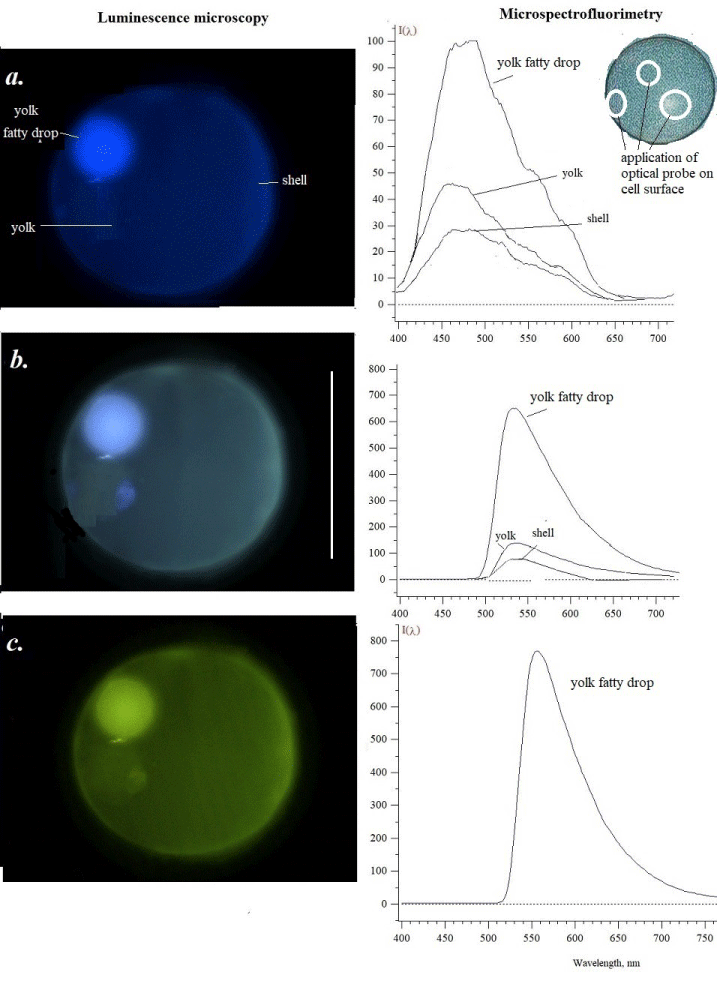
In our study, the fluorescence of red mullet fish eggs as the main object was analyzed because its cellular probes were more successful in the analysis. It was possible to see a different state of eggs given from the sample. We have tried to select unfertilized and developing eggs from red mullet plankton and have seen the changes that occurred based on the fluorescence of fixed material. In the photo in Figure 1, we show an unfertilized egg when excited by light of various wavelengths. Photographs taken under a luminescent microscope show the natural color of fluorescence. Using an optical probe of microspectrofluorimeter, sizes from 0.5 mm up to 2 µm [1,3] we saw the emission of some parts of egg – yolk, yolk fatty drop, and shell. More intensive was a fluorescence of yolk fatty drop under various used light excitation – ultraviolet 360-380 nm (a), blue 430-450 nm (b) and blue-green 480-500 light (c). It should be noted that in oocytes of Vertebrata, the spectral region in blue is associated with NAD+/NADH and in green – with flavins (riboflavin) of mitochondria [12]. When the yolk, yolk fatty drop, and shell were excited by green-yellow light 515-550 nm, there was no visible fluorescence in the orange-red region. The main background is respectively blue, slightly blue, and greenish (Figure 1 a-c).
Under ultraviolet light (a) the yolk fatty drop emits brighter with a maximum of 480-500 nm, then all structures in blue, blue-greenish, and green. Yolk emitted with a maximum of 460 nm while shell picked 470 nm in their spectra. The possible fluorophores in the spectral region may be some phenolic compounds and NAD+/NADH. The excitation by violet light (b) gave the emission with maxima 530 nm, but different heights as well as under green light excitation which induced the fluorescence with a maximum of 560 nm. (c) A similar fluorescence maximum is characteristic for carotenoids [2,12]. Some of them are found in the fish eggs of various species.
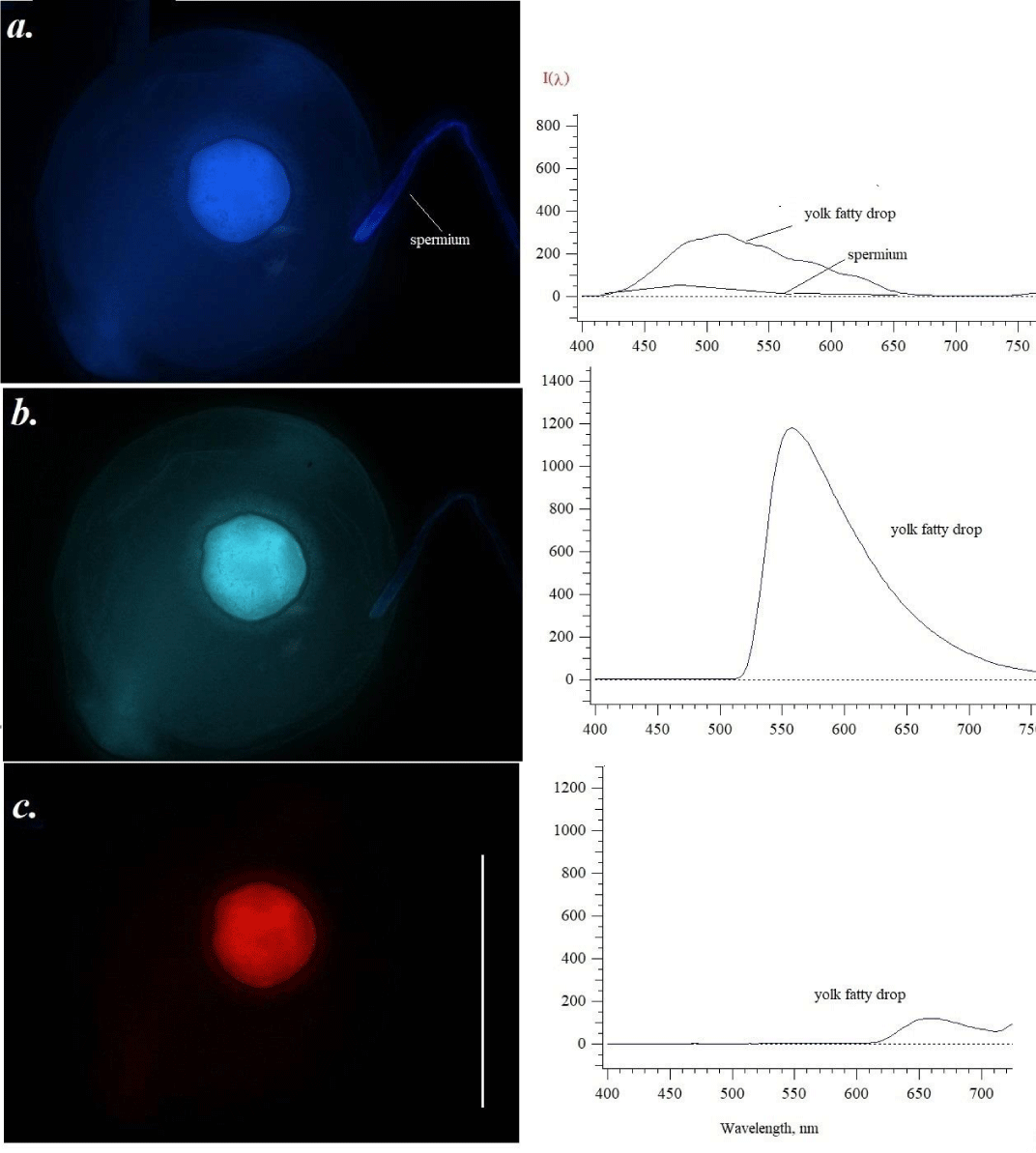
Unlike unfertilized eggs, Figure 2 shows the first changes in fluorescence just or after the contact with spermium (spermatozoid). According to Gilbert [9], fertilization involves the contact and fusion of the mature sex cells, the sperm and the egg named gametes. The fusion of the male gamete cells (sperm) stimulates the egg to begin development (the developing egg is called the oocyte before it reaches the stage of meiosis at which it is fertilized) and initiates a new individual. The contents of the egg also vary greatly from species to species. In any case, it accumulates nuclear components and a remarkable cytoplasmic storehouse during its maturation. This cytoplasmic trove includes nutritive proteins to supply energy and amino acids. In many species, this is accomplished by accumulating yolk proteins in the egg.
The egg picture changes somewhat when a spermatozoid/spermium (Figure 2a) fuses into the egg. The nucleus constitutes the sperm head. An individual sperm is able to travel by whipping its flagellum. It is clearly visible as a glowing blue filament, when excited by ultraviolet light (a), with the tip brighter than the rest of the filament. The yolk area of the eggs inside shows brighter structures as compared to unfertilized eggs without a spermium (spermatozoon). The boundaries and contents of the egg are already clearly marked. After excitation with violet (b) and green-yellow (c) light, the spermatozoon is no longer visible against the general background. The greatest changes are observed when excitation with light 515-550 nm occurs (c): the yolk fatty drop fluoresces in the red region of the spectrum (c). We could not see red emission in the photo of Figure 1. If to compare with unfertilized and undeveloped eggs (Figure 1a), we saw twice an increase in yolk fatty drop emission in blue, and – in green for 30 % of the fluorescence intensity.
The clearest picture is well seen for the big yolk fatty drop. At excitation by ultraviolet light, we saw blue fluorescence of both the yolk fatty drop and the head of spermium. In the first case, the main maximum shifted to a longer wavelength – 510 nm - 520 nm. A weak 480-500 nm pick was recorded for the spermium. Formalin as a fixator, interacting with monoamines, can induce additional blue luminescence [2,3]. However, we did not see a specially marked difference in the character of the emission, although for a short-time storage of the samples, 4% fixation may be better. Under violet light, the fluorescence image of the yolk fatty drop contains some structures, that were absent or not seen earlier, but the main maximum of 560 nm is not changed in the comparison with Figure 1b. It should be noted that there was the appearance of red fluorescence of yolk fatty drop (Figure 2c). A clear maximum of 655-660 nm was seen. According to the monograph of Mikulin [12], fish yolk may include pigments, not only lipophilic compounds such as carotenoids-antioxidants of lipid peroxidation, but also - proteins (porphyrin-containing structures such as cytochrome b560 and other hemе-proteins). Fluorescence of carotenoids is possible at 530-540 nm. Some pterins are also emitted in blue under ultraviolet light [12,13]. The most intense emission with a maximum of 540-550 nm in the green region was noted for the cell after excitation by violet light. We only hypothesized about the inclusion in the visible red fluorescence of some carotenoids and iron-containing heme-proteins. There is the information that yolk of fish eggs contains heme-proteins [14]. Some porphyrins excited by ultraviolet 400 nm, emitted with maxima in regions 600-750 nm - 630, 660, and 690 nm [15,16]. Hematoporphyrin from blood erythrocytes (hemoglobin) treated with 0.5-1.5 N HCL or sulfur acid fluoresces with maxima 580-588, 600, 625, 660, 625 nm [3], According to some authors (17-19] acetone extracts of blood elements have maxima 585 and 630 nm in the fluorescence spectra at the excitation 400 nm.
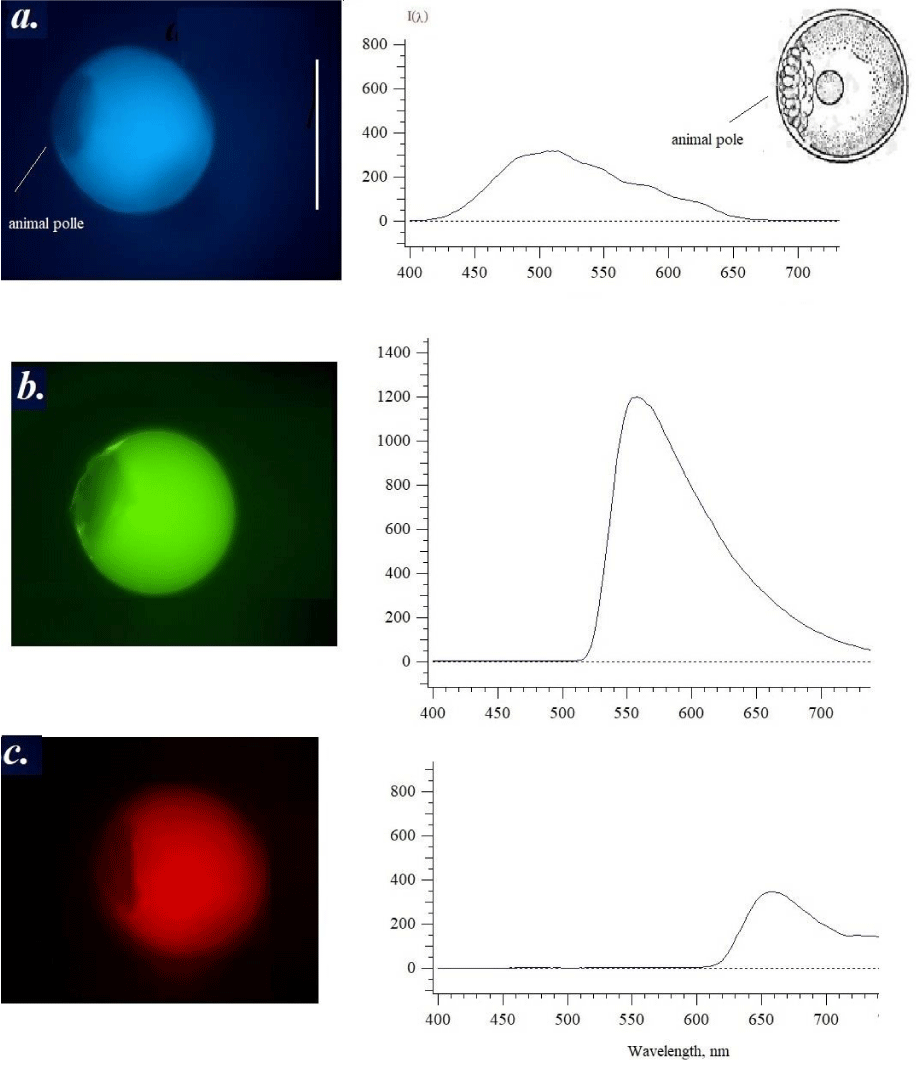
There is the sample of the following development phase (Figure 3) where is the animal pole formation [9]. The formation of the animal pole, on which the formation of germ cells occurs as a result of the splitting of the zygote of the structure after the fusion of the nuclei of the egg cell and sperm. This stage demonstrated the formation of a non-fluorescent animal pole contacted with the main mass of yolk. When excited by ultraviolet light one can see a non-fluorescent area (dark spot) formed on the side of the egg, and the yolk fatty drop is no longer visible since the fluorescence is homogeneous. Separation of part of the contents from the yolk, and the yellow spot in other cells may merge with the yolk part (see upper cell on the left). Excitation by ultraviolet or violet light somewhat shifts the yolk emission maximum to the long-wavelength region, although the nature and appearance of the spectrum resemble the same pattern as previously in Figure 2. However, for the first time (Figure 2c) red fluorescence appears and a maximum of 650-660 nm in the emission spectrum increases three-fold (Figure 3c). It turns out that it is the red fluorescence (c) that indicates the beginning of the development of the embryo after fertilization. When excited by violet light (b), the same samples had maxima in the green-yellow region of the spectrum of 540-550 nm, characteristic of carotenoids [12].
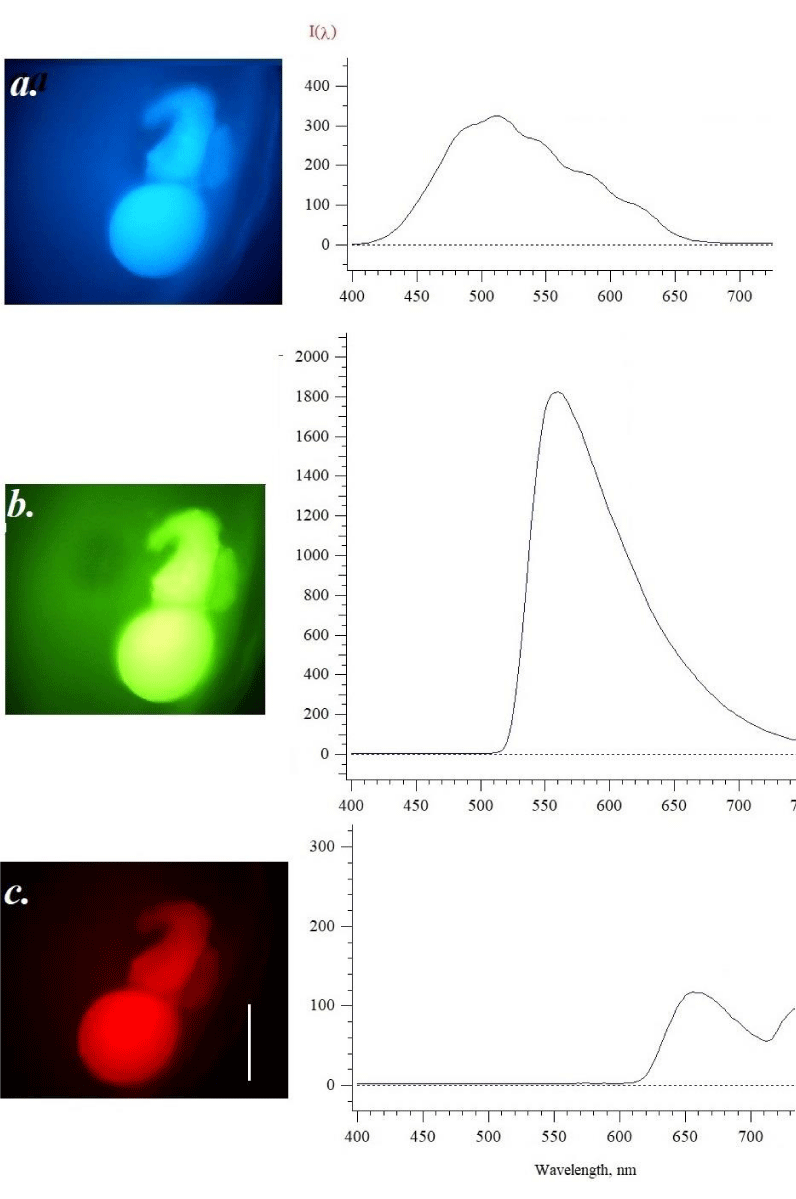
The emission spectra were recorded at the initial stages of development (Figures 1-3) and the following sample (Figure 4) is related to the stage when the embryo has appeared. Figure 4. Shows the photo and the fluorescence spectra when excited by light of different wavelengths. The photo clearly demonstrates the presence of an embryo that is seen as a blue structure under ultraviolet excitation, green in violet light, and red under green excitation. Partly as a dark spot the observer sees possible yolk fatty drop, and lack of fluorophore. The embryo emission in blue (Figure 4a) has no marked changes in intensity, while in green (b) increases up to 30 % in the comparison as shown in Figure 3. As for red fluorescence, the intensity in maximum 650-660 nm decreased. The fact that images on Figures 2-4 have a red color emission may indicate the possibility of the appearance of hemoglobin, which has maxima in the region of 630-640 nm [14,15-19]. Autofluorescence of mammalian blood has been explored as a label-free approach for the detection of cell types, as well as for diagnosis and detection of infection, cancer, and other diseases [18-19]. Porphyrins in the plasma of blood at excitation 400-450 nm show emission in the range 630-700 nm, flavins, for example, riboflavin at excitation 400 nm has an emission maximum 510-530 nm, while at excitation 444 nm - peak 558 nm. Visible fluorophores with a maximum of 630-640 nm are more real to porphyrins as precursors of blood.
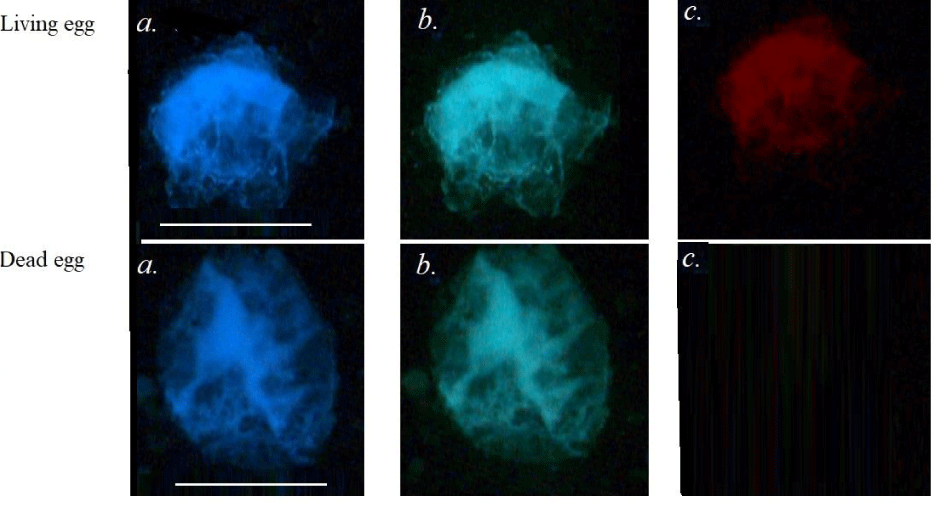
In order to confirm this supposing, we compare fluorescent images of living and dead (with damaged structures) eggs from red mullet (Figure 5). As we can see, in the dead sample the red fluorescence is absent, therefore this emission is linked with living and developing egg.
We understand that the first step in the use of fluorescent methods of staining is without artificial dyes. There are only a few publications dealing with genetics, where fish eggs were studied with fluorescent dye binding with nuclei such as Ethidium bromide or Hoechst dyes [20,21]. Autofluorescence has perspective in the fast analysis if there is a luminescence microscope, and for more deep research the recording apparatuses such as microspectrofluorimetry may be recommended. The main limitation of the method consists of the procedures of the samples’ preservation. Water-sensitive gel structures need either fixation or maintaining aquarium conditions for cellular fluorescent analysis. In the future, the new special technique should be exploited. For sea practical use in ecological monitoring of the cell fertility and damages or in fisheries, we hope, a new portative fluorescent technique should be applied, side by side with our laboratory apparatus.
In planktonic eggs, it is possible to detect the earlier stages of development. Thus, we present the first data on the observation and measurement of the fluorescence of planktonic eggs on the example of red mullet. Following studies of the autofluorescence application in fishery practice may be important for the quantitative determination of fertilized and unfertilized cells or living and dead cells. Moreover, visual pictures of the light emission under a luminescence microscope may be useful in education, especially in embryology, histology without damaging the cell structures, and cell biology as a whole.
Conclusion
The fluorescent method allows us to identify the earlier stages of development of the fish eggs. It was possible to test fertilized eggs and primary stages of development. Observers may see the internal structure of the egg cell under the excitation of ultraviolet, violet, and green the living and dead cells and the difference between living and dead cells. This approach should be recommended for practice in both fundamental cell biology and industrial fishery.
We are grateful to Optical Microscopy and Spectrophotometry core facilities, ICB RAS, Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences for the use of Leica microscopes.
Absolute author contribution
Common idea, all experiments and the paper writing (Roshchina VV), experiments and discussion (Yashin VA), receiving of fish eggs’ plankton and identification as well as discussion (Petrova TN and Maltsev VI).
- Karnauykhov VN, Yashin VA, Kulakov VI. Apparatus for investigation of fluorescence characteristics of microscopic objects. US patent 4354114. US. 1982.
- Karnaukhov VN. Carotenoids: recent progress, problems and prospects. Comp Biochem Physiol B. 1990;95(1):1-20. doi: 10.1016/0305-0491(90)90241-k. PMID: 2184985.
- Karnaukhov VN. Luminescent spectral analysis of cell. Moscow: Nauka. 1978; 204.
- Roshchina VV, Mel'nikova EV, Iashin VA, Karnaukhov VN. Avtofluorestsentsiia intaktnykh spor khvoshcha Equisetum arvense L. v protsesse razvitiia [Autofluorescence of intact Equisetum arvense L. spores during their development]. Biofizika. 2002 Mar-Apr;47(2):318-24. Russian. PMID: 11969171.
- Roshchina VV. Fluorescence of Living Plant Cells for Phytomedicine Preparations. Boca Raton: CRC Press. 2020.
- Dekhnik TV. Ichthyoplankton of Black Sea Kiev: Naukova Dumka. 1973; 237.
- Maltsev VI. Coastal ichthyocomplex of specially protected waters of the south-eastern Crimea. Marine Technologies: Problems and Solutions – 2021: a collection of articles by participants of the National Scientific and Practical Forum. Conf. Kerch, April 19-30, 2021), Ed. E. P. Masyutkina.– Kerch : KSMTU. 2021; 277–280.
- Maltsev VI, Vasilets VE, Shaganov VV, Petrova N. Revision of the species composition of fish of the coastal ichthyocomplex of the water area of the Karadag Nature Reserve. Bulletin of the Kerch State Marine Technological University. 2021; 2: 50-65.
- Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates. 2000; 290.
- Gilbert SF, Barresi MJF. Developmental Biology. 12th ed., 2020 Sunderland, Massachusetts USA: Sinauer Associates. 2020; 450.
- Tan TCY, Dunning KR. Non-invasive assessment of oocyte developmental competence. Reprod Fertil Dev. 2022 Dec;35(2):39-50. doi: 10.1071/RD22217. PMID: 36592982.
- Mikulin AE. Functional Significance of pigments and pigmentation in fish ontogenesis. Moscow: VNIRO Publishing, 2000; 231.
- Thomas AH, Lorente C, Capparelli AL, Pokhrel MR, Braun AM, Oliveros E. Fluorescence of pterin, 6-formylpterin, 6-carboxypterin and folic acid in aqueous solution: pH effects. Photochem Photobiol Sci. 2002 Jun;1(6):421-6. doi: 10.1039/b202114e. PMID: 12856711.
- Gouterman M, Rentzepis PM, Straub KD. Porphyrins. Excited States and Dynamics. Washington: Amer Chem Soc. 1986; 321.
- Masilamania V, Al-Zhrania K, Al-Salhia M, Al-Diabb A, Al-Ageilyc M. Cancer diagnosis by autofluorescence of blood components Journal of Luminescence. 2004; 109:143–154.
- Courrol LC, de Oliveira Silva FR, Coutinho EL, Piccoli MF, Mansano RD, Vieira Júnior ND, Schor N, Bellini MH. Study of blood porphyrin spectral profile for diagnosis of tumor progression. J Fluoresc. 2007 May;17(3):289-92. doi: 10.1007/s10895-007-0171-7. Epub 2007 Mar 29. PMID: 17393286.
- Courrol LC, de Oliveira Silva FR, Coutinho EL, Piccoli MF, Mansano RD, Vieira Júnior ND, Schor N, Bellini MH. Study of blood porphyrin spectral profile for diagnosis of tumor progression. J Fluoresc. 2007 May;17(3):289-92. doi: 10.1007/s10895-007-0171-7. Epub 2007 Mar 29. PMID: 17393286.
- Al-Salhi M, Masilamani V, Vijmasi T, Al-Nachawati H, VijayaRaghavan AP. Lung cancer detection by native fluorescence spectra of body fluids--a preliminary study. J Fluoresc. 2011 Mar;21(2):637-45. doi: 10.1007/s10895-010-0751-9. Epub 2010 Oct 19. PMID: 20957416.
- Shrirao AB, Schloss RS, Fritz Z, Shrirao MV, Rosen R, Yarmush ML. Autofluorescence of blood and its application in biomedical and clinical research. Biotechnol Bioeng. 2021 Dec;118(12):4550-4576. doi: 10.1002/bit.27933. Epub 2021 Sep 14. PMID: 34487351.
- Sarvel AK, Kusel JR, Araújo N, Coelho P, Katz N. Comparison between morphological and staining characteristics of live and dead eggs of Schistosoma mansoni. Mem Inst Oswaldo Cruz. 2006 Sep;101 Suppl 1:289-92. doi: 10.1590/s0074-02762006000900045. PMID: 17308784.
- Tonelli FMPT, Lacerda S, Procopio MS, Resede RRR. Gene delivery to Nile tilapia cells for transgenesis and the role of PI3K-c2α in angiogenesis. Scientific Reports. 2017; 7(1):44317.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
