Annals of Marine Science
Aquaculture and its conservation potential of critically endangered Jipe Tilapia (Oreochromis jipe) in Lake Jipe
Paul S Orina1*, Sheban Hinzano2, Domitila Kyule3, Abwao Jacob3, Cecilia M Githukia1, Tonny Orina4 and Mercy Chepkirui1,5
2Kenya Marine and Fisheries Research Institute, Mombasa Research Centre, PO Box 81651-80100, Mombasa, Kenya
3Kenya Marine and Fisheries Research Institute, Sagana Aquaculture Centre, PO Box 451- 10230, Sagana, Kenya
4Faculty of Agriculture, Department of Animal Sciences, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya
5Kisii University, Department of Fisheries and Aquatic Sciences, PO Box 408-40200, Kisii, Kenya
Cite this as
Orina PS, Hinzano S, Kyule D, Jacob A, Githukia CM, et al. (2023) Aquaculture and its conservation potential of critically endangered Jipe Tilapia (Oreochromis jipe) in Lake Jipe. Ann Mar Sci 7(1): 045-050. DOI: 10.17352/ams.000038Copyright License
© 2023 Orina PS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Tilapia jipe (Oreochromis jipe) is listed as a critically endangered Cichlidae endemic to fast-shrinking Lake Jipe. Climate change and anthropogenic activities have significantly impacted the lakes’ ecology and species’ genetic integrity. Four months of Jipe tilapia culture growth performance was conducted in four selected farms within the Chala area, Taveta Sub-County of Taita Taveta County along the slopes of Mt. Kilimanjaro’s Kenya side. The study assessed the growth performance of 3rd generation mixed-sex Oreochromis jipe under aquaculture conditions. Fish sampling was done monthly to assess the growth performance of fingerlings stocked at an average weight of 5 ± 0.02 g and a stocking density of 5/m3. Total weight ranged between 123.72 g, and 74.70 g from the entire growth trial sample population. The highest and the least mean weight gains recorded were 108.87 ± 4.31g and 87.12 ± 4.40g for Farm B and D respectively. Similarly, farm B recorded the highest mean length (18.80 ± 0.27) while farm C recorded the least (17.31 ± 0.25) with no significant difference between the selected farms. Water quality parameters were within the normally acceptable tilapia culture ranges. The study findings indicated a negative allometric growth (b < 3) for all farms except Farm A. All growth trials demonstrated a good Condition factor (K) with a range of 1.82 to 2.19. The present study concluded that O. jipe has the potential for aquaculture and species genetic conservation and restocking due to its ease of propagation. The study recommends further G x E breeding program strategies, nutritional and general management studies, and a policy limiting genetic material transfer from its catchment.
Introduction
Tilapia jipe (Oreochromis jipe) is a freshwater fish belonging to the Cichlidae family which migrated to African countries. Cichlidae is the richest family comprising more than 1600 taxa [1]. Although the majority of freshwater inland fisheries are cichlids, only a few candidates are exploited for protein sources commercially [2]. O. jipe is a slender-bodied tilapia with a small head and mouth, teeth at slender jaws, very fine, and crowded with pharyngeal teeth. They are bottom dwellers inhabiting rivers and lakes hence providing refuge with plenty of microscopic algae and detritus [3]. They are grazers feeding on bottom deposits and phytobenthos. Just like Oreochromis niloticus, they are mouth brooders with high maternal care [4]. O. jipe supports artisanal fisheries in dams and lakes across the Pangani catchment, with the largest fishery at Nyumba ya Mungu and Lake Jipe [5].
Lake Jipe is situated along the transboundary of Kenya and Tanzania [6] along the southern slopes of Mt. Kilimanjaro. The lake is a watershed that contributes significantly towards food security, biodiversity, recreation, transport, and economic empowerment [7]. It is the main source of livelihood for the locals and supports artisanal fisheries across the two countries [8]. Despite the lake benefits gained by the local community, lake Jipe faces a lot of challenges including but not limited to vegetation loss, increased siltation from wildlife pressure and uncontrolled pastoralism [9], climate change, and intensified horticulture activities along the feeder rivers and streams. The lake further experiences swift extension of Typha domingensis, loss of breeding grounds, mining depositions, water abstraction upstream, deforestation, overfishing, and introduction of new species [10]. Dwindling in fisheries production led to the introduction of Tilapia singida (Oreochromis esculentus) and Nile Tilapia (Oreochromis niloticus) to boost fisheries production [11]. However, introduced species have been reported to outcompete the indigenous O. jipe in the lake leading to its disappearance [12,13]. The decline of O. jipe catches from 1970 to 2000 has contributed to its listing in the IUCN Red List of Critically endangered species (IUCN, 2020), a major concern necessitating the need for species conservation through aquaculture.
Globally, aquaculture is the fastest-growing food production sector but has also been recognized as one that has the potential to enjoy growth at the expense of the environment [14]. Aquaculture despite contributing significantly to aquatic resource role in food security poses a danger to the culture of alien species and genotypes introduction to the natural aquatic ecosystems if sustainable practices are not fully implemented [14,15]. Globally, freshwater aquaculture contributes 86% of total fish production [14] and this is mainly by the Tilapiines production both in tropical and subtropical freshwater bodies [16]. They are the most desired cultured fish species due to their highly formulated feed acceptability, ease of captive propagation, fast growth, resistance to diseases, wide agroecological range, and high acceptability by consumers due to the high quality of flesh [17,18]. Further, the decline in native fisheries biodiversity has led to recent development in aquaculture through the introduction of indigenous fish species from the wild [19-23]. The commercially important indigenous species such as Orechromis esculentus, Oreochromis variabilis, Labeo victorianus, and O. jipe are now a rare catch among the fisherfolk in the Kenyan freshwater systems. This therefore provides a great opportunity for aquaculture to ensure sustainable aquaculture by promoting aquaculture of this critically endangered species within their catchment thus limiting alien species introduction and possible genotype interference. Though Lake Jipe is currently suffering the introduction of O. niloticus and O. esculentus, aquaculture efforts have an opportunity to collect wild pure genetic material of O. jipe for conservation and food security, and nutrition. Even though, limited studies have been undertaken on the aquaculture potential of O.jipe. Therefore, this study attempted to assess the cultural potential of O. jipe for possible aquaculture adoption and conservation within the Taita Taveta County water catchment.
Methodology
A 4-month growth trial was conducted in four selected farms located within the Chala agricultural area along the slopes of Mt. Kilimanjaro of Taita Taveta County in Kenya. The sites were preferred due to the cultural facilities’ existence and farmer management knowledge and skills, unlimited quality water supply from Mt. Kilimanjaro, and the presence of County technical support required for the study. Though they enjoy long rains between March and May and short rains between August and October yearly, climate change has significantly altered the rain intensity and patterns limiting agricultural activities with high water demand to Lumi River. The study area is situated in topography that drains into Lake Jipe; a rare biodiversity hotspot in a semi-arid region, leeward south eastern slopes of Mt. Kilimanjaro along the trans-national border of Kenya and Tanzania.
O. jipe broodstock was sourced from four locations of Lake Jipe and three spots along Lumi River to ensure genetic vigor, packed in oxygenated open tanks, and transported as filial brooders to KMFRI Kegati Research Center for domestication and propagation. A 3rd generation of fingerlings measuring 5 ± 0.02 g was conditioned and transported in oxygenated bags to the Chala area in Taveta Sub-County. A total of 1,000 fingerlings were stocked upon re-conditioning in each pond of the four farmers within a 300m proximity. The stocking was at a density of 5 fingerlings/m3. The introduced fingerlings we allowed to acclimatize for 3 days before feeding began using 40% Crude Protein (CP) commercial feed for 1 month, then 32% in the subsequent 2 months before subjecting them to 28% in the final 4th month. Feeding was done thrice in the 1st and 2nd months before dropping to twice daily in the 3rd and 4th months.
Water quality monitoring and fish sampling
In-situ water quality parameters for temperature, Dissolved Oxygen (DO), pH, conductivity, salinity, and Total Dissolved Solutes (TDS) were monitored. Monthly sampling was done to check the growth performance of the fingerlings. Sampling was conducted very early in the morning at 8:00 am EAT to reduce handling stress which would result in mortality. At every sampling, a sample population of 30 fingerlings was taken from each pond by seining using a knotless seine net. They were put into a bucket half full of water and supported with portable aerators before measuring total length and weight. The total length was taken using calibrated measuring board with readability of 0.1cm. This was done by measuring fingerling from the snout to the caudal fin whereas total weight was measured using a weighing balance readability 0.001 g. After taking both total length and body weight measurements, the fingerlings were immediately returned to the ponds. All measurements recorded were used to calculate growth performance parameters as follows;
Where:
- (Log = Natural logarithm, W0 = Initial weight (g), Wt = Final weight (g) and t = Time (days)
Length-weight relationship
The relationship between fish total length and body weight was determined using the Le Cren (1951) equation, W = aLb, following Le Cren (1951).
Where:
W- Body weight of fish (g)
L- Total Length of fish (cm)
a- Intercept
b- slope
Condition factor (K)
The k value of fingerlings was determined from length-weight data. Fish condition factor (K) was determined according to Froesce’s (2006) equation:
Where,
W and L are the mean weight and length respectively.
Data analysis
All data recorded were keyed in an MS Excel sheet on Windows 2013 and reported as mean ± standard error. One -Way ANOVA was carried out to compare means between four selected farms. Tukey HSD analysis was conducted to check specific differences between the four selected farms. Determination of a and b coefficients was performed using non-linear regression. All statistics were done using SPSS software version 2022.S.
Results and discussions
Water quality parameters
Water quality parameters were within the normal acceptable range for the growth of Tilapiines (Table 1). Temperature and DO ranged between 24.12 ± 1.29 to 25.98 ± 0.31 0C and 4.34 ± 0.85 to 5.94 ± 0.21 mg/l respectively. There was no significant difference (p > 0.05) in all the selected farms.
Growth performance
The growth performance of Tilipines is affected by stocking density, nutrition, water quality, and health condition [24]. Four farms coded as A, B, C & D were sampled to determine fish growth performance (Table 2). Farm B recorded the highest mean length 18.80 ± 0.27 cm, while farm C recorded the least 17.31 ± 0.25 cm with no significant difference (p > 0.05) in all selected farms. Total weight ranged between 123.72 g, and 74.70 g from the entire growth trial sample population. Meanwhile, the highest and the least mean weight recorded was 108.87 ± 4.31 g and 87.12 ± 4.40 g for Farm B and D respectively. Further, a significant difference in average weight was recorded between farms A and C (p = 0.03); B and C (p = 0.02) according to Tukey HSD.
The WG was significantly (p < 0.05) higher in Farm B. However, there was no significant difference in all the selected farms throughout the study period. FCR refers to the volume of feeds utilized to achieve one kilogram of fish. Low FCR indicates efficient feeds, low production costs, and sustainable processes [25]. In the present study, low FCR was recorded in Farm B (2.07 ± 0.84) whereas Farm D recorded a high FCR value (3.57 ± 0.26). The high FCR recorded indicates that fingerlings ineffectively convert consumed feeds into flesh. This was attributed to O. jipe’s short aquaculture history and thus short exposure to formulated feeds along the generation line.
Condition factor
The condition factor (K) reflects the biological and physical state (well-being) of the fish [26]. It also reflects the fluctuation in interaction among feeding, parasitic and physiological factors [27]. K value may vary between species depending on the season, length, weight, and habitat [28]. The mean K values ranged between 1.42 and 2.19 with Farm A being the least and Farm D being the highest. Even though, all mean K values recorded were more than 1 implying that the fish were in good condition (Table 3) [29].
Generally, a K value of 1 and above indicates good health of the fish [30]. However, it decreases with an increase in length indicating that the weight of a fish at a certain length is in good health [31,32]. K reported was within values reported by [33,34], indicating a good condition factor for all the fish. In general, the K value of Farm D was higher (2.17) compared to other farms. The significant (p < 0.05) difference in condition factor between farms A and D is attributed to management and sex ratio related to reproductive behavior. Generally, condition factor variation is attributed to maturity stage, sex, feeding behavior, eutrophication, and spawning [35-37].
Length-weight relationship
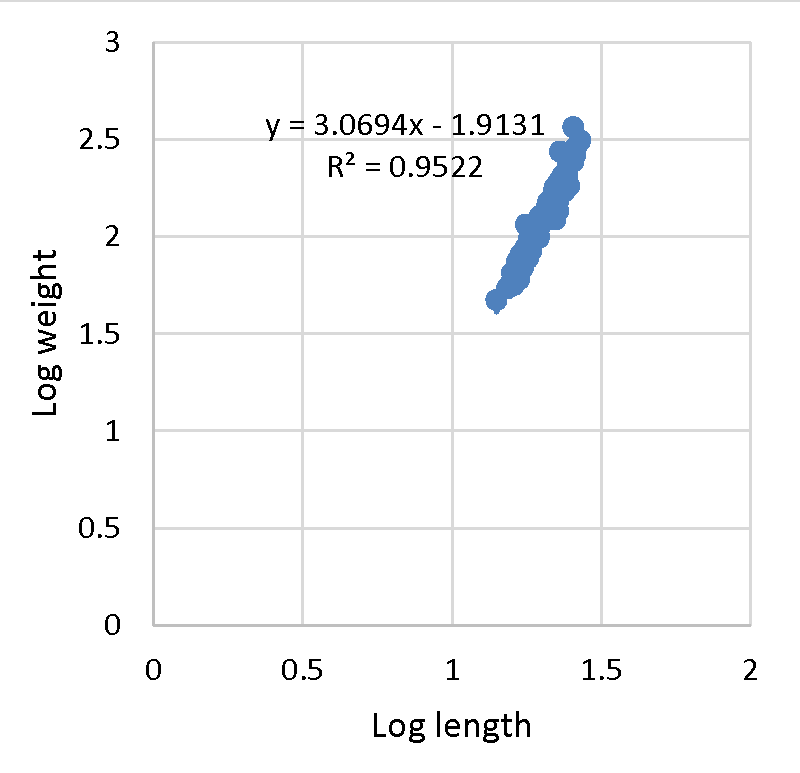
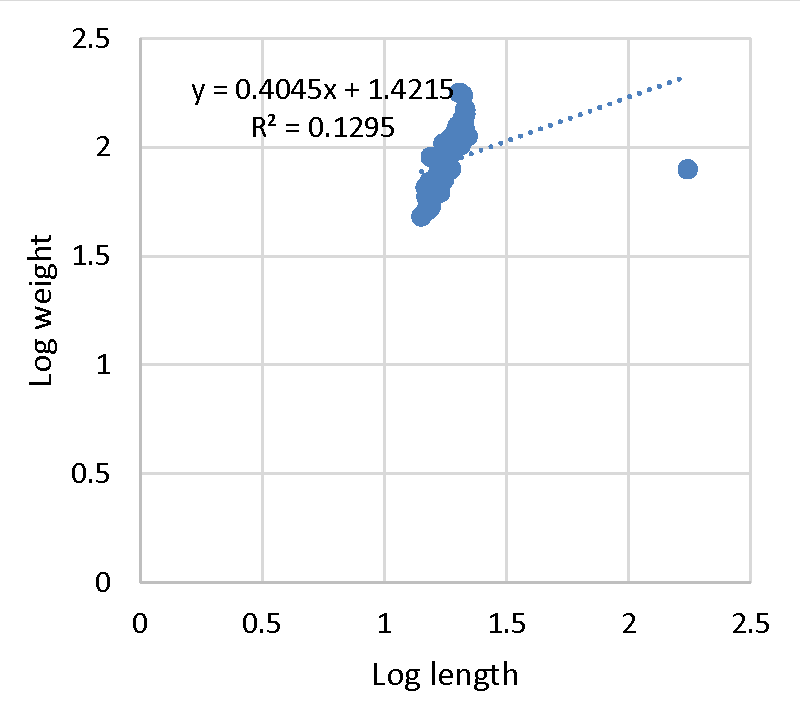
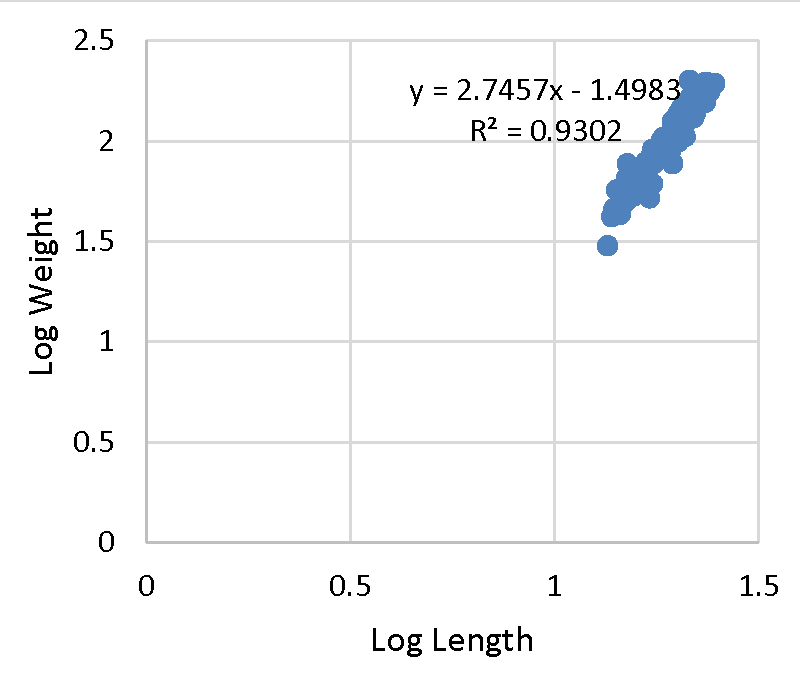
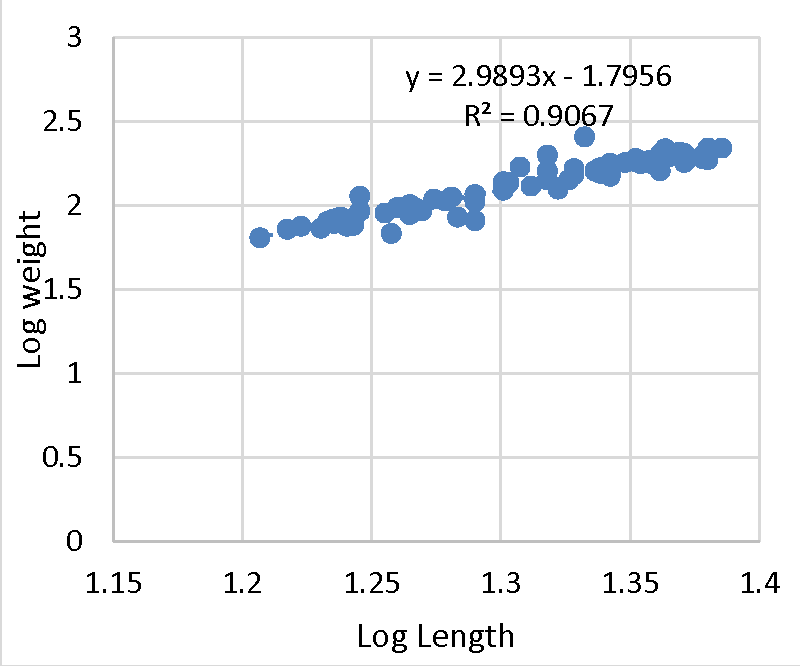
Effective fisheries management is ideal with the understanding of demographic factors such as the length-weight relationship and its relationship to the growth of a population [38-40]. The exponent b from the equation is used to determine the growth status; allometric (b ˂ 3 = negative, b ˃ 3 = positive allometric) or isometric (b = 3). Negative allometric growth is a relative increase in length than body weight compared to positive allometric growth where fish comparatively broaden and increase in flesh with an increase in length. All selected farms with the exception of farm A (b = 3.07), recorded negative allometric growth (b ˂ 3) in mean values of log length-weight exponents (Figure 1). The mean b-exponent values ranged between 3.07 to 0.40 for Farm A and B respectively. The average b-exponent value for all selected farms was 2.30.
The b value in fish may range from 2.5 to 3 [41] while observed may range between 2.5 and 4.0 [42,43]. The ideal isometric growth pattern for a fish to maintain its shape is 3.0. Population which varies exponentially from 3.0 shows allometric growth [44]. The value of b in length-weight relationship is a bio-indicator in determining the quantity of feed consumed and growth pattern in fish and its variation depending on ecological conditions within the culture system [45]. The high b value reported in Farm A (3.070) agrees with [10,46]. This, therefore, indicates positive allometric growth. However, it was greater than [47-49]. Past studies on freshwater fishes including Tilapiines and Squirrelfish (Sargocentron rubrum) recorded negative allometric growth [34,38,45,50,52-54]. Despite reporting negative allometric, b values were within 2 and 4 which is in agreement with O. nilotcus findings by [55-56]. The negative allometric recorded in the present study could be the reason behind the slender bodies exhibited by O. jipe. In aquaculture, water quality, nutrition, availability of food, stage of development, ecological variations, and health status of the fish are key determinants for growth of fish. The aforementioned might be responsible for the negative allometric growth recorded in the present study [37,57-60]. The adjusted coefficient of determination (R2) ranged from 0.95 to 0.13 for Farm A (Figure 1) and B (Figure 2) respectively. The overall R2 recorded was 2.92. This is in line with the result reported for O. niloticus by [33,46,50], thus demonstrating a positive correlation between total length and weight (Figures 3,4).
Conclusions and recommendations
O. jipe has the potential for aquaculture based on the current study’s positive response. However, there is a need for all male culture trials, and breeding programs to ensure the selection of traits that will enhance the species culture preference by the local community over the current dominant O. niloticus. There is increased interest by the local community in the species’ culture and thus opportunity for adoption and conservation. To enhance the fish’s growth rate, FCR, and K-Factor, there is a need for further research on the species’ nutritional and general management requirements as well as employment of Genetic by Environment (G x E) strategies similar to those undertaken for O. niloticus.
The study further recommends the promotion of O. jipe community-based hatchery development for a sustainable supply of quality seed as the demand for the species grows. The hatchery will also serve as a mass breeding facility for the restocking of the Jipe ecosystem. To avert ecological risks such as that experienced through O. niloticus culture, a policy limiting the culture of O. jipe within its catchment area and its culture biosafety measures need reinforcement.
- Dunz AR, Schliewen UK. Molecular phylogeny and revised classification of the haplotilapiine cichlid fishes formerly referred to as "Tilapia". Mol Phylogenet Evol. 2013 Jul;68(1):64-80. doi: 10.1016/j.ympev.2013.03.015. Epub 2013 Mar 29. PMID: 23542002.
- Yue GH, Lin HR, Li JL. Tilapia is the Fish for Next-Generation Aquaculture. Int J Marine Sci Ocean Technol. 2016; 3(1):11-13.
- Eccles DH. FAO species identification sheet for fisheries purposes. A field guide to freshwater fishes of Tanzania. Prepared and published with the support of the United Nations Development Programme. FAO, Rome. 2019; 145.
- Lowe-McConnell RH. New species of Tilapia (Pisces Cichlidae) from Lake Jipe and the Pangani River, East Africa. 1995;129-162.
- Genner MJ, Turner GF, Ngatunga BP. A guide to the tilapia fisheries of Tanzania. 2018; 36.
- Mwachiro EC, Makilla D, Bett KD, Ndeje GK. A Comparative study of the cage and earthen pond culture of Oreochromis jipe, in Lake Jipe, Taita Taveta District, Kenya. Global Advanced Research Journal of Agricultural Science. 2012;1(7): 163-181.
- Ndetei R. The role of wetlands in lake ecological functions and sustainable livelihoods in Lake Environment: A case study on cross border Lake Jipe - Kenya/Tanzania. Kenya Wildlife. 2011:2:162-168. http://hdl.handle.net/1834/1492.
- Froese R, Pauly D. Oreochromis jipe (Lowe, 1955). Fish Base. 2019:181-186. https://www.fishbase.de/summary/Oreochromis-jipe.html.
- Odada E, Olago G, Daniel O. Proceedings of the 11th World Lakes Conference. 2006; 2:162-168.
- Njiru M, Ojuok JE, Okeyo-Owuor JB, Muchiri M, Ntiba MJ, Cowx IG. Some biological aspects and life history strategies of Nile tilapia Oreochromis niloticus (L.) in Lake Victoria, Kenya. African Journal of Ecology. 2006; 44:30-37.
- Ogutu-Ohwayo R. The decline of the native fishes of lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus and the Nile tilapia, Oreochromis niloticus. Environmental Biology of Fishes. 1990; 27(2): 81-96.
- Twong'o TK, Sikoyo GM, Wakhungu JW. Shared aquatic ecosystems of East Africa: Status and trends. University of Nairobi Research Archive. 2002. http://erepository.uonbi.ac.ke/handle/11295/51125
- Ministry of Fisheries and Livestock Development. Taita Taveta district Fisheries Report. The status of fisheries in Taita Taveta District, Kenya. 2001.
- FAO. The State of World Fisheries and Aquaculture. Towards Blue Transformation. Rome, FAO. 2022. https://doi.org/10.4060/cc0461en.
- FAO. Technical Guidelines for Responsible Fisheries. Aquaculture Development, No.5. Rome, Italy. 40. 1997. https://www.fao.org/3/W4493E/w4493e01.htm#bm1.
- Mohammed EY, Uraguchi ZB. Impacts of climate change on fisheries: implications for food security in Sub-Saharan Africa. In: Hanjra, M.A. (eds.) Global Food Security, Nova Science Publishers, Inc.: 2013; 114-135.
- FAO. Social and economic performance of tilapia farming in Africa. FAO Fisheries and Aquaculture Circular No. 1130. 2017. Rome, Italy.
- Adeyemi SO. Food and feeding habits of some commercially important fish species in Gbedikere Lake, Bassa, Kogi State, Nigeria. International Journal of Lake and River. 2009; 2:31-36.
- Orina P, Ikaya L, Abel R, Akwany L, Chepkirui M. Effects of different substrates on growth and survival of Labeo victorianus fry towards its conservation along Mara Basin”. Open Journal of Ecology. 2023;13(1):37-48. http://dx.doi.org/10.4236/oje.2023.131004.
- Omweno, JO, Orina PS, Getabu A, Outa NO. Growth and aquaculture potential of Tilapia jipe, Oreochromis jipe and Nile tilapia, Oreochromis niloticus. International Journal of Fisheries and Aquatic Studies. 2020; 8(3): 395-399.
- Orina PS, Charo-Karisa H, Munguti JM, Boera P, Abwao J, Kyule D. A comparative study of Labeo victorianus (Bouelenger, 1901) and Oreochromis niloticus (Linnaeus, 1758) grown in polyculture systems. Lakes & Reservoirs Research & Management. 2018: 23(1): 56-62.
- Ogada EO, Orina PS, Ogendi GO. Effect of stocking density and diet on the performance of Jipe tilapia (Oreochromis jipe) cultured in hapas at Sagana, Kenya. International Journal of Advanced Research. 2018; 6(10): 296-308. DOI: 10.21474/IJAR01/7811.
- Kembenya ME, Marcial HS, Outa NO, Sakakura Y, Hagiwara A. Captive breeding of threatened African carp (Labeo victorianus) of Lake Victoria. Journal of World Aquaculture Society. 2017; 48: 6-8.
- Mengistu BS, Mulder AH, Benzie AHJ, Komen H. A. Systematic literature review of the major factors causing yield gap by affecting growth, feed conversion ratio and survival in Nile tilapia (Oreochromis niloticus). Reviews in Aquaculture. 2020; 12(2):524-541.
- Boyd CE. A low feed conversion ratio is the primary indicator of efficient aquaculture. Responsibility. 2021. https://www.globalseafood.org/advocate/a-low-feed-conversion-ratio-is-the-primary-indicator-of-efficient-aquaculture/
- Ridha MT. Evaluation of mono sex culture of GIFT and non-improved strains of Nile tilapia Oreochromis niloticus in recirculating tanks. International Aquatic Research (Islamic Azad University, Toneka-bon Branch). 2011; 3(3): 189-195.
- Momi MA, Islam MS, Biswas S. Condition Factor. A health indicator for cultivable fish. 2021; 71-103.
- Lagler KF. Freshwater fishery biology. In: Brown, W.M.C. (Ed.), Comp, Dubuque, Iowa. 1956; 421.
- Yosuva M, Jeyapragash D, Manigandan V, Machendiranathan M, Saravanakumar A. Length-weight relationship and relative condition factor of Yellowfin tuna (Thunnus albacares) from Parangipettai coast, southeast coast of India. Zoology Ecology. 2018; 28(2):94-99.
- Jisr N, Younes G, Sakhn C, El-Dakdouki MH. Length–weight relationships relative condition factor of fish inhabiting the marine area of the Eastern Mediterranean city, Tripoli-Lebanon. The Egyptian Journal of Aquatic Research. 2018; 44(4):299-305.
- Abowei JN, Davies AO. Some pollutant parameters of Clarotes laticep (Rupell, 1829) from the freshwater reaches of the lower River, Niger Delta, Nigeria. American Journal of Scientific Research. 2009; 2:15-19.
- Morton A, Routledge RD. Fulton’s condition factor: is it a valid measure of sea lice impact on juvenile salmon? North American Journal of Fisheries Management. 2006; 26:56-62.
- Shija RS. Length-Weight Relationship and Fulton’s Condition Factor of the Nile Tilapia (Oreochromis niloticus L., 1758) in Lake Chamo, Ethiopia. J Agric Environ Sci. 2020; 5: 2616-3713.
- Farrag MS, AbouelFadl KY, Alabssawy NA, Toutou MM, El-Haweet AK. Fishery biology of Lessepsian immigrant squirrelfishes Sargocentron rubrum (Forsskal, 1775), Eastern Mediterranean Sea, Egypt. Egyptian Journal of Aquatic Research. 2018; 44: 307-313.
- Degsera A, Minwyelet M, Yosef T. Age and growth of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) from Lake Tana, Ethiopia. Afr J Aquat Sci. 2020; 45:509-519.
- Ali RAS, Elawad AN, Khalifa MM, El-Mor M.Length–weight relationship and condition factor of Liza Ramada from Eastern coast of Libya. International Journal of Fishery and Aquaculture Research. 2016; 2:1-9.
- Alhassan EH, Akongyuure DN, Asumang F. Determination of morphometric relationship and condition factors of four Cichlids from Golinga reservoir in the northern region of Ghana. Journal Biol Sci. 2015; 15:201-206.
- Dan- Kishiya AS. Length-weight relationship and condition factor of five fish species from a tropical water supply reservoir in Abuja, Nigeria. American Journal of Research Communication. 2013; 1:175-187.
- Simon KD, Mazlan AG. Length-weight and length-length relationships of archer and puffer fish species. Open Fish Sci J. 2008; 1: 19-22.
- Kimmerer W, Avent SR, Bollens SM, Feyrer F, Grimaldo FL, Moyle PB. Variability in length-weight relationships used to estimate biomass of estuarine fish from survey data. Trans. Am. Fish Soc. 2005; 134:481-495.
- Pauly D, Gayalino FC. Editors. FAO-ICLARM stock assessment tools (FiSAT): reference manual. FAO computerized information series. Fisheries. 1997; 8:262.
- Hile R. Age and growth of the Cisco, Leucichtys, and K. suercur in the Lakes of North Eastern highland and S. Bull. United State Bureau of Fishes. 1993; 48:211-314.
- Martin WR. The mechanics of the environmental control of body form is fish. University of Toronto study of Biology. Publication Ontario Fisheries Research Laboratory. 1949; 58(70):1-91.
- Allen KR. A method of fitting growth curves of the von Bertalanffy type to observed data. J Fish Res Board Can. 1996; 23:163-79.
- Ubong GN, Okon NA, Etitigwun JU. Assessment of Length-Weight Relationship of Nile Tilapia Oreochromis niloticus (Linnaeus 1758) from Qua Iboe River Estuary, Southeastern, Nigeria. Asian Journal of Biology. 2023; 17:21-33. ISSN: 2456-7124.
- Wagaw S, Mengistou S, Getahun A. Aspects of the growth and reproductive biology of Oreochromis niloticus (Linnaeus, 1758) in a tropical Soda Lake, Lake Shala, Ethiopia. Fish Aquat Sci. 2022; 25(7):380-389 DOI: https://doi.org/10.47853/FAS.2022.e34
- Kebede MT, Getahun A, Lemma B. Reproductive biology of commercially important fish species in Lake Langeno, Ethiopia. Asian Fish Sci. 2018; 31:319-39.
- Hipro LA. Reproductive biology of Oreochromis niloticus in Lake Beseka, Ethiopia. J Cell Anim Biol. 2013; 7:116-20.
- Assefa WW, Getahun A. Length-weight relationship, condition factor and some reproductive aspects of Nile tilapia Oreochromis niloticus in Lake Hayq, Ethiopia. Int J Zool Res. 2014; 4:47-60.
- Barman SK, Da BR, Kunda M, Mazumder SK, Uddin M J, Barman PP. Length-weight relationships and condition factors of mono and mixed-sex Nile tilapia (Oreochromis niloticus) in open water cage culture system. Journal of Fisheries. 2023. DOI: https://doi.org/10.17017/j.fish.431
- Onada O, Ogunola O. Improving Food Security in an Eco-Friendly Manner through Integrated Aquaculture. Open Access Library Journal. 2016; 3:1-6. doi 10.4236/oalib.1102476.
- Nehemia A, Justin DM, Rumisha C. Length-Weight relationship and condition factor of tilapia species grown in marine and freshwater ponds. Agriculture and Biology Journal of North America. 2012; 2151-7525. doi:10.5251/abjna.2012.3.3.117.124.
- Ude EF, Ugwu LC, Mgbenka BO, Nwani CD. Evaluation of length-weight relationship of fish species of Ebonyi River, Nigeria. Nigerian Journal of Fisheries. 2011; 8(1):136-144.
- Imam TS, Bala U, Balarabe ML, Oyeyi TI. Length-weight relationship and condition factor of four fish species from Wasai Reservoir in Kano, Nigeria. African Studies. 2010; 6:125-130.
- Otieno ON, Kitaka N, Njiru JM (2014) Length-weight relationship, condition factor, Length at first maturity and Sex ratio of Nile tilapia, Oreochromis niloticus in Lake Naivasha, Kenya. Int J Fish Aquat Stud. 2014; 2(2):67-72.
- Waithaka E, Mugo J, Obegi B, Keyombe JL. Socio-economic of the reintroduced Oreochromis niloticus in Lake Naivasha (Kenya). Int J Fish Aquat. 2015; 2(5): 142-146.
- Bhuyain AR, Barman SK, Hossain MM, Khan MH, Mim KK, Mazumder SK. Seasonal dynamics of heavy metal concentrations in water and fish from Hakaluki Haor of Bangladesh. Conservation. 2022; 2(3):473-484.
- Kunda M, Pandit D, Harun-Al-Rashid A. Optimization of stocking density for mono-sex Nile tilapia (Oreochromis niloticus) production in riverine cage culture in Bangladesh. Heliyon. 2021 Nov 9;7(11):e08334. doi: 10.1016/j.heliyon.2021.e08334. PMID: 34841100; PMCID: PMC8606329.
- Aktar MJ, Islam MJ, Barman SK, Kunda M. Assessment of fish biodiversity in the Teesta River of Bangladesh. Journal of Sylhet Agricultural University. 2020; 7(2):95-114.
- Froese R. Cube law, condition factor, and weight-length relationships: history, meta-analysis, and recommendations. Journal of Applied Ichthyology. 2006; 22:241-53.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
