Annals of Marine Science
Lichen as biomonitor of atmospheric elemental composition from Potter Peninsula, 25 de Mayo (King George) Island, Antarctica
Mariana S Rivera1, Soledad Perez Catan2, Carla Di Fonzo3, Laura Dopchiz3, Maria A Arribere2, Martin Ansaldo3, Maria I Messuti4 and Debora F Bubach2*
2Laboratory for Neutron Activation, Bariloche Atomic Center, National Atomic Energy Commission, Río Negro, Argentina
3Ecophysiology and Ecotoxicology Laboratory, Argentine Antarctic Institute, Campus Migueletes, (1650) San Martín, Buenos Aires, Argentina
4Institute of Research on Biodiversity and the Environment, National University of Comahue, National Council of Scientific and Technical Research, Bariloche University Regional Center, Quintral 1250, (8400) S.C. of Bariloche, Río Negro, Argentina
Cite this as
Rivera MS, Catan SP, Fonzo CD, Dopchiz L, Arribere MA, et al. (2018) Lichen as biomonitor of atmospheric elemental composition from Potter Peninsula, 25 de Mayo (King George) Island, Antarctica. Ann Mar Sci 2(1): 009-015. DOI: 10.17352/ams.000009Lichens are powerful biomonitor of airborne pollution around point sources or long range transport because they are perennial allowing bioindication at long period. The element concentrations in foliose and fruticose lichen species from Potter Peninsula located in 25 de Mayo (King George) Island is reported. The coefficient of the variation for most of the elements was up to 50% except for as and Br, K and Se. The Principal Component Analysis showed differences among sampling sites according to human activities respect to the special protected areas. Aluminium, Cr, Hg, Pb and Se concentrations are linked local waste burning, global inputs, and the melt-water processes, while Br and Se were associated with marine biogenic cycle. This information could be a valuable tool for future atmospheric studies.
Introduction
Lichenized fungi (lichens) are well known as biomonitor organisms used to evaluate the air quality. The lack of a wax cuticle and stomata allows to nutrients and contaminants to be absorbed over the whole surface. The absorbed elements can be taken from the dry and wet deposition and by short and long-range transport [1-3]. Likewise, they can extract elements from the melt-water such as Al, Co, Cr, Pb, Mn, Ni and REE especially for Antarctic ecosystems [4].
The lichens reported data are mainly about global pollutants such as Pb, Cd, Zn, Cu, Cr and Hg [5-7]. The use of new materials of technologic application (nanoparticles), plus the increment of industrial and population development, and the effects of global warming could improve the transport, deposition and availability of pollutants. On the other hand, the increase of global temperature is heterogeneous and it occurs more rapidly in Antarctic and Arctic Continents [5]. Actually, it will need to extend the knowledge about the background levels of more elements as control tools due to those are being released to the environment [8]. For this reason, to expand the knowledge about the elements present in Polar Regions represents a safeguard for the future.
The Antarctic Protocol provided strict environmental management and protection guidelines, and established the obligation to clean-up abandoned work sites. Also, establishes principles for planning and conducting of all Antarctic activities. However, the local impacts due to the increasing of human presence seem inevitable [1]. In the 2008 Antarctic Treaty Consultative Meeting, some of these areas have been designated as Antarctic Specially Protected Areas (ASPA; https://www.ats.aq/e/ep.htm) with the purpose to preserve these environments due to their high sensitivity to disturbances. Element determination, concentration levels and potential toxicity, in key species are very important in order to understand and to elucidate the impact on the Antarctic terrestrial ecosystem.
Species of the genus Usnea has a wide distribution around the world including Antarctica and they have frequently used as bioindicator of presence of elements and compounds [1]. The element contents in lichens from Antarctica are widely reported in the literature, in particular several works reported the elemental composition in lichens from 25 de Mayo (King George) Island, South Shetland Islands, Antarctica [2,9,10]. However, the information about more elements and their concentrations in Antarctic lichens is still insufficient [11].
During three Antarctic summer campaigns, lichens sampling in Potter Peninsula located in 25 de Mayo (King George) Island were made with the aim to evaluate the effects of human presence in the area and the coastline ecosystems. In the present work the concentration of 27 elements in lichens of the third last campaign are reported in order to identify the elements associated with the anthropic source or biogeochemical process. Those results were compared with our previous data.
Materials and Methods
Study area
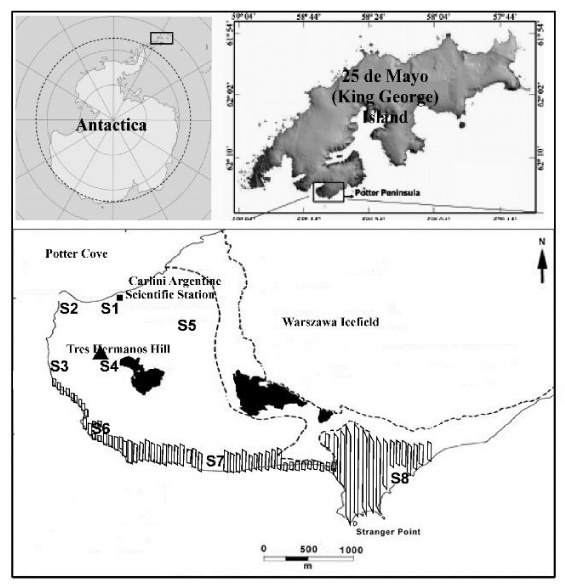
The Potter Peninsula is placed at the southernmost end of 25 de Mayo (King George) Island, South Shetland Islands, Antarctica, extending its area from 58°35’ to 58°41’ W and from 62°13.9’ to 62°15.7’ S (Figure 1), where the Carlini Argentine Scientific Station is located. The ice-free area encompasses approximately 6 km2, bordered by the Warszawa Icefield to the NE, the Bransfield Strait to the SE, the Maxwell Bay to the SW and Potter Cove to the NW. On the coastline, an area specially protected (width ca. 500 m) is defined as ASPA-132 according to the current regulation [12]. The morphology of Potter Peninsula is characterized by a glacial landscape with steep cliffs in parts of the along the coast and in the interior, the countryside flat hilly stand out a protruding andesitic columns named Tres Hermanos hill (196 m). The geology is dominated by Tertiary effusive basalt-andesite and related pyroclastic rocks. The climate conditions are characteristic of marine Antarctic weather (average annual temperature of 2.8°C); snowfall and scarce rainfall in summer; and strong winds predominant from the southwest sector [13]. The development of Antarctic terrestrial biotic communities is mainly limited by the freshwater availability, low temperatures and limited photoperiod [1]. Therefore, few groups of organisms can colonize or survive in this area; most of them are lichens and bryophytes that grow on the ice-free coastal areas (rocks and soil) during summer [14]. The ASPA-132, protected area is characterized as a wilderness representative area with high biological diversity and richness, which includes penguin colonies, elephant seals and sea lions, and skuas and petrels in the elevated zone, as well as dominant lichens in rocky formations of the highest sites and close to the beach (Figure 1).
Sampling of lichens and soil
Saxicolous and terricolous lichens were collected during austral summer since 2011 to 2014, at eight different sites of Potter Peninsula (Figure 1). The sampled material was based on the availability of access and the possibility to evaluate anthropic impact [10]. According to the different human activities, three sites were considered: Carlini Station (S1), heliport (S2) and water dam (S4); intermediate points to the east (S3) close to the Tres Hermanos hill, and (S5) at the southeast of Carlini Station close to Warszawa Icefield. Finally, other three zones (S6, S7 and S8) located within the ASPA-132, are the farthest at 2.5 to 3.7 km away from to the human settlement (Figure 1).
Lichens were collected by random walk method and surface soils were taken at 2 to 3 cm deep nearby of the lichens. For the collection, plastic spoons, latex gloves and titanium and Teflon devices were used. All the collected material was kept in sterile polyethylene bags frozen at -20°C until processed.
Prior to the elemental analysis, a fraction of lichen specimens were separated for identification. The destined thalli for analysis were cleaned under stereo dissecting microscope at room temperature (22°C). Afterwards, thalli were washed two times with ASTM water grade 1 (American Society for Testing and Materials) and dried in a laminar flow hood. Each sample was made up at least by 10 dried thalli that were ground and homogenate. In SUPRASIL AN® quartz ampoule 130 to 180 mg of each set were weighted and sealed for irradiation while 50 to 100 mg samples of the soil were placed in plastic contain.
Lichen identification
The study was based on the fresh material deposited in Centro Atómico Bariloche collected by the authors, and on desiccated selected material from BCRU herbarium to compare. Specimens were examined and identified using a stereo dissecting microscope (Olympus SZ30) and a light microscope (Leitz Laborlux 11). Anatomical features were studied on hand-cut sections mounted in water and in lactophenol cotton blue; 10% potassium hydroxide (10% KOH) or 1% Lugol´s Iodine solution directly and after a KOH pre-treatment. All measurements were made in water [15,16]. Secondary lichens compounds were identified by High Performance Thin-Layer Chromatography (HPTLC) [17].
Elemental analysis
The elemental concentrations were determined by Instrumental Neutron Activation Analysis (INAA). The samples were irradiated in the RA-6 research nuclear reactor, Centro Atómico Bariloche, Argentina (φth≅1.5×1013 n.cm-2.s-1; φepi≅6×1011 n.cm-2.s-1).
Three gamma ray spectra, with different decay times, were collected using an intrinsic HPGe detector 30% relative efficiency and a 4096 channel analyser, whereas the spectra were analysed by using the GAMANAL routine included in the GANAAS package, distributed by International Atomic Energy Agency (IAEA). The elemental concentrations were determined using the absolute parametric method. Analytical errors were computed as the propagation of the uncertainties associated with the nuclear parameters, and the efficiency of the gamma ray detection system. The IAEA 336 Lichen Reference Material was analysed together with the samples, for analytical quality control (QC) showing good agreement with the recommended certified values. The QC analysis is reported in the Supplementary Data, Table 1.
The measured elements were: antimony (Sb), arsenic (As), barium (Ba), bromine (Br), caesium (Cs), calcium (Ca), cobalt (Co), chromium (Cr), hafnium (Hf), iron (Fe), mercury (Hg), potassium (K), rubidium (Rb), selenium (Se), scandium (Sc), silver (Ag), sodium (Na), strontium (Sr), tantalum (Ta) thorium (Th), uranium (U), zinc (Zn), and the Rare Earth Elements (REE) like lanthanum (La), samarium (Sm) and terbium (Tb).
Aluminium (Al) and lead (Pb) were analysed in lichens samples by acid digestion with 6mL of 70% HNO3, 0.2mL of 60% HF and 1mL of 30% H2O2 and the soils with a 67% solution of HNO3: H2O2 (10:1, v/v), and 1mL HF. The completed digestions were made in a microwave digestion system (Milestone Ethos D) (EPA 3052) and the measurements were made by Atomic Absorption Spectrometry with Graphite Furnaces (GF-AAS) (Perkin Elmer PinAcle 900 Atomic Absorption Spectrometer). Precision of analysis was estimated by the coefficient of variation of four replicates and was found to be within 10% for all elements. The limit of detection to the equipment was 5 µg/L and the LQ was 0.0125 µg/g.
Data analysis
The statistical analysis was performed with XLSTAT program (Copyright 1995-2009, Addinsoft). When the concentration of an element was below the detection limit, one third of this value was used for statistical analysis and descriptive purposes. Data were analysed using a multivariate statistical method. Principal Component Analysis (PCA) using the elements contents as quantitative variables was applied. The considered significance level in statistical tests was α ≤0.05.
Agglomerative Hierarchical Clustering (AHC) based on Spearman correlation coefficient over the element concentrations in the lichens was used in order to evaluate comparatively the elemental composition in each sites (S1-S8) with respect to the lichen habitus (e.g. foliose vs. fruticose). The dendrogram was based on Similitude Index with average link method.
A lithophile element (e.g. REE) was used as geochemical tracers (GT) for the discrimination of the elements associated with geological particulate material (PM) from the soil entrapped in the thalli. They are used to quantify the contribution of detrital compounds associated with the PM to the overall composition of the thalli for differentiating the possible sources of contribution of elements. This was made by using correlation matrix and linear regressions based on the Pearson test.
Likewise, we calculated other environmental indexes such as the Enrichment Factor (EF) and the Load Pollution Index (PLI). The EF allows the comparison of the sample with the composition of the substratum [18]. This factor was estimated for all lichen samples and each element (X) discriminated by collection site, according to:
The concentration of element (X) and samarium (SM) with subscript “liq” designates the lichen sample and “sub” for the soil content in sample of the same sites [19]. The EF(x) values greater than 1 indicates that the element in the lichen is enriched compared to the substrate. In this work, an element was considered enriched when EF(x) values were greater than or equal to [5].
Pollution Load Index (PLI) was used to assess the environmental quality of the area, and it represent one specific site. The formula used by [20], takes into account the load of all elements given by:
CFx is the ratio between the concentration of X element in the lichen and the concentration of the same element in the control site. Control was defined as site with base element concentration level for the study area. Pollution Load Index values less than 1 indicated that the elements in the area are close to the background concentrations and PLI > 1 indicate different deterioration degrees to the environmental quality [21].
Results
Species evaluation
Specimens collected in Potter Peninsula are shown on Table 1 according to the taxonomic location, habitus and collection sites of each species. These were identified as: Himantormia lugubris (Hue) I. M. Lamb; Physconia muscigena (Ach.) Poelt; Rhizoplaca aspidophora (Vain.) Redón; Sphaerophorus globosus (Huds.) Vain; Usnea antarctica Du Rietz and Usnea aurantiacoatra (Jacq.) Bory.
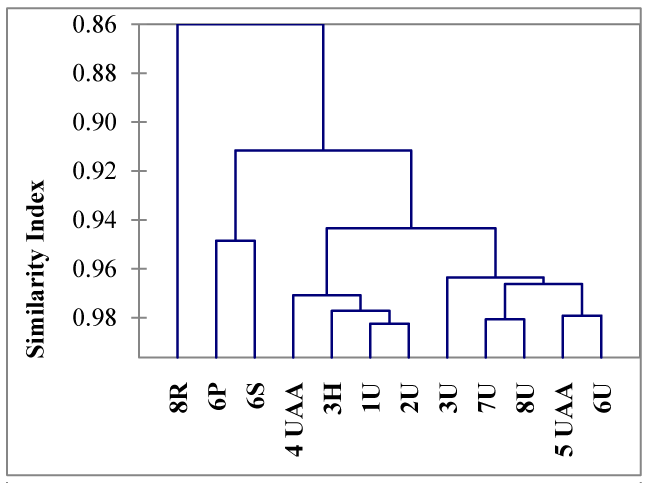
The dendrogram (Figure 2) shows the results of applying AHC strategy to data. In this figure the R. aspidophora sample (8R), of foliose habitus, presents the lower similarity of the assemblies and two main clusters. The cluster 1 include: U. antarctica samples (1U, 2U, 3U, 6U, 7U, 8U), U. aurantiacoatra (4UAA, 5UAA) and H. lugubris (3H), all of them of fruticose habitus. Cluster 2 comprises P. muscigena (6P) and S. globosus (6S) both from the same site but different habit, foliose and fruticose respectively. The major similitude aggrupation observed were related with the habitus more than the sampling site.
Spatial evaluation
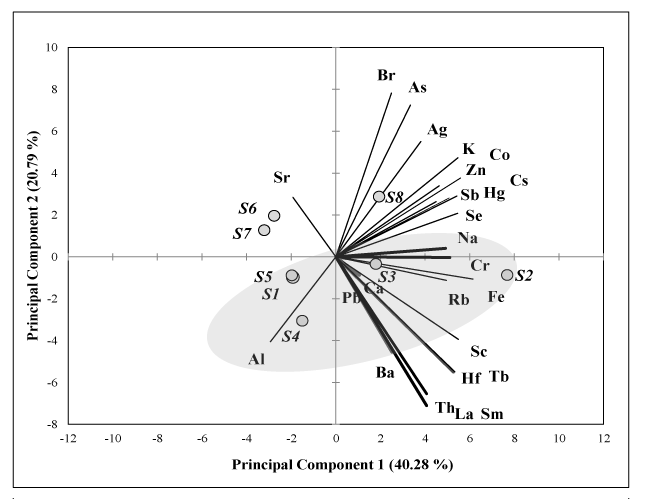
Principal Component Analysis (PCA): The elemental concentrations of the Usnea species thalli are shown in Supplementary Data, Table 2, and the PCA results in Figure 3. The observations are scattered by the elements according to the sampling sites. The total explained variation in the biplot was 61.07% by component 1 and 2. The observations S1, S4 and S5 were grouped by a greater contribution of Al and S2 was scatter by the effect of Cr, Fe and Rb, while the observation less explained was S3. On the other hand, S8 was separated from the rest of observations by the influence of elements like As, Br and Ag and finally, S6 and S7 were grouped by Sr contribution.
Enrichment factor (EF): Antimony, Sc, Hf, La, Ta, Tb and Th correlated significantly with the GT (Sm) which belongs to the lanthanides group. This relationship was not significant for Al, As, Ba, Br, Ca, Cs, Zn, Co, Cr, Sr, Fe, Hg, Ag, Pb, K, Rb, Se, Na and U (p >0.05), most of them belong to the transition and alkaline metals groups.
The EF(x) values of non-lithophile elements by sampling sites are presented on Table 2, in general, the S6, S7 and S8 were the sites with the highest EF values for Br, Ca and Hg. However, Al and Se were enriched in all sites. In adition, Br presented high EF in S2 and S3, and it was observed in S1 by Pb and Hg.
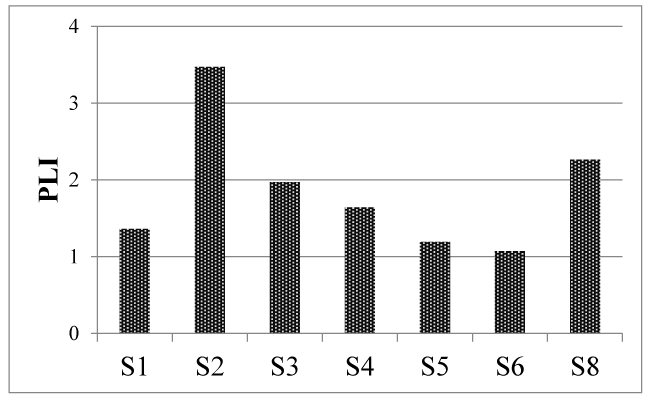
Polution Load Index (PLI): The site used as control for PLI calculus was S7. The choice was due to the fact that this site presented the lowest concentrations for the most elements (Supplementary Data, Table 2). Figure 4 shows the PLI values, being S2 the more deteriorated site with PLI to 3.47, followed by S8 (PLI: 2.26) that also presented a high level of deterioration; the lowest PLI (1.07) was for S6 site.
Discussion
Lichens accumulate elements, some of them environmental pollutants, for a long period, in excess of 100 years due to their longevity [22]. The relation between the growth form and the accumulation capacity has been reported as a factor that affects the element accumulation patterns in lichens [23,24]. The results in Supplementary Data, Table 2 showed for the mostly elements, e.g.: Sb, As, Ba, Cs, Co, Hf, Fe, La, Rb, Sc and Sm, major concentrations in foliose than fruticose form, while for Br, Ca, Hg and Ag were similar in both habitus taking account the average, standard deviations and median of the concentrations. Our results are agree with some authors whose suggested that foliose form has the highest exposed surface to the atmosphere and this related to higher ability to incorporate elements [25].
The dendrogram Figure 2 showed high similarity index (0.964) for homogenous grouping to the family Parmeliaceae with respect to the other clades belong to the family Physciaceae. This may be a coincidence. The criteria on the lichen family taxa somewhat ambiguous and depends on the authors. Genus Usnea and Himantormia are classified within the Parmelaceae family which is very diverse and including two foliose and fruticose habitus (Thgorsten et al. 2012). In other hand, genus Usnea is also classified in the Usneasea family excluding the genus Himantormia [26].
The dendrogram nest the lichens of for their habitus in spite of the fruticose S. globosus is an exception, which is grouped together with the foliose P. muscigena; unlike the other fruticose species, S. globosus, was found in association with bryophytes (mostly mosses). The growing condition in high humid microenvironments could make to the elements more available for the lichens [27]. Environmental changes have considerable influences on physico-chemical process such us pH, redox potential or oxygen diffusion rates [27]. The nutrients and elements can be leached and extracted from the colonized rock, roughened by molten water that flows downward and can seep into the cushions of lichens and mosses. Those processes could explain the high concentration of some elements in S. globosus and the ensemble showed in Figure 2 with fruticose lichens. In addition [28], found in mosses samples from dry and barren Antarctic terrestrial ecosystems, that raw concentrations of elements often reflect the biogeochemical nature of soils and rocks rather than atmospheric input, generally Al, Fe, Cr, and other lithophile elements.
The PCA of the Usnea sp., showed clearly a distinction of sampling sites, the sites near to Carlini Station (S1 to S5) had a major contribution of elements, which could related to the human activities like was shown in previous studies at the 25 de Mayo (King George) Island [1,2]. The reported results in this work together with our data from the two previous sampling campaigns showed the same trends about the element effects on antrophic and ASPA area [10]. A great variation in the element concentrations was observed in all champagnes, the coefficient of variation lower than 50% was found for as and Br, K and Se (Supplementary Data, Table 2).
The ACP-graphic in Figure 3 includes Al and Pb contents producing the spread of sampling sites in slightly different way to presented by [10], where S2 is in other different quadrant that S1, S4 and S5.
The burning of waste as plastics, paper, batteries, and fuel oils, is most important activity that releases ashes with elements to the environment and gases as Hg directly into the atmosphere. Aluminium, Ba and Ca are some of the contained elements in the ash matrix while other as Cu, Pb, Sb, Se and Zn are deposited on surface. In the other hand, the element concentrations likes Mn, Fe and Cu in the effluents coming directly from the glacier underside where extremely higher than those derived ice free areas at the bottom of the Tres Hermanos hill, emphasize the relevance of leaching process followed by thaw waters [29]. These are in agreement with [30], whose inform several heavy metals concentrations as Pb, Cd, Fe and Cr in surface sediments from Potter Cove. This authors suggest that most of the metals found in Potter Cove constitute a redistribution of autochthonous materials within the ecosystem, and its can be considered to be present at natural background levels in surface sediments. Those informed processes in the literature, have not doubt that they contributed to dispersing elements in the study areas.
The site S4, is a place close to Tres Hermanos hill, was used like garbage dump until 1998 when the Madrid Protocol (1991) came into operation and remediation works were carried out [31]. The S2 samples correspond to the heliport area and they had a major lithophile element contributions as Ba, Hf, Fe, La, Sm, Sc and Th, which may be associated with the resuspensions of the surrounding sediments; Pb and, an important Cr contribution observed in S2 could also came from the emplacement of the fuel cisterns. This site and S4, moreover could be influenced by the water from Tres Hermanos hill, while S1 and S5 are affected by the melt water come from the Icefield [29,30]. The sites far away from the human settlement (S6, S7 and S8) were separated from those close to Carlini Station (S1 to S5) by the greatest contribution of Br, biological elements such as Se, Zn, K and pollutants as Hg, Ag and as. These sampling sites are included in the ASPA-132 where there are several marine mammal settlements and seabird colonies of penguins, skuas and seagulls. The bioconcentration of pollutants elements and detoxification process in mammals and sea birds from Arctic and Antarctic area have been known base on excrement, guano and feathers analysis among others [32-36]. These authors proposed process sea-land bio-transport of chemical pollutants as As, Hg, Se and Ag.
The PCA results were in agreement to the EF (Table 2) where the higher values were in S6 and S7 for Al, Br and Se following by Hg and Pb. Bromine and Se were enriched in all sites and campaigns which can be able to biogenic marine cycles, while the other elements could be indicate anthropogenic impact [3,10,18,37]. Nevertheless, [4], found increased level of elements such as Al, Cr, Pb and other REEs, in water and glacier meltwater from 25 de Mayo (King George) Island. This represents a significant impact on the biogeochemistry of coastal seawater in Antarctica and it could explain the high Al and Se EF values in all studied sites and the Pb EF in S7. Moreover, biomethylation is known as an important chemical process in the ocean, which can lead to volatile compounds of heavy metals, likes Cd, Hg, Pb, Ti and halogens as Br. Some results reported by [38], demonstrate that the bacteria and microalgae from the polar ocean are potential sources for the production of methylated heavy metals. These take place principally in the pack-ice section, whereas in the polar ocean under the closed ice sheet and the shore of the Antarctic Peninsula, methylated heavy metals diminish. Despite of the stability of the compounds in seawater is different, there are no doubts that they play an important role in the biogeochemical cycle of this regions like suggested by high EF values in pristine sites (S6 to S8).
The PLI values could be considered as contradictory to the EF. This is because S2 was the most deteriorate site, although in general, the EF values for this site were low. The PLI is a standardized method for the comparison of different geographical areas which take account all the elements, and indicates the background level when PLI is equal to 1 [39]. When it value increases, this indicates different pollution degree in the environment, whereas the EF values is particular for each element and considered soil. These explain the differences interpretations about both indexes in each area, in particular, PLI in S1and S2, and EF in S1, due to the high concentrations in the soil, for example, Cr and Pb by accidental fuel oil spills (Supplementary Data, Table 3).
The elemental contents in S8 showed a homogeneous contribution including As, Br, Ag and biological elements (Supplementary Data, Table 2). In this site, the important biological activities related with animal colonies and biogeochemical cycle, were the reasons, which justified the designation as second deteriorated area by the high PLI.
Conclusion
Based on three sampling campaigns, the area close to the Carlini Station (S1 to S5) is enrichment of different elements respect to the sites far away from it, which includes in the ASPA-132 (S6 to S8). The most important remarks are:
The presence of the pollutants elements related to anthropogenic sources as the local waste burning (Al, Sb, Cr, Pb, Hg, and Se), soil resuspensions (e.g.: lithofile elements) and, fuel spills (Pb and Cr) also, the global inputs (Cr, Pb and Hg).
The natural pollutants and the process like soil leaching and melt water from glacier (Al, Cr and Pb) and biogeochemical process (Br, Se, Hg, Zn, Fe, Tl) are identified as an influence in the elements contents of the Antarctic lichens. Furthermore, the results of our work support the idea that the marine biogenic cycle and sea-land bio-transport are the main processes that would affect the coastlines environments.
The authors acknowledge to the RA-6 reactor staff for the irradiation of the samples and to the Chemical Laboratory, INVAP SE staff for the support and assistance in the sample analysis. This work was partially funded by PICTO 0091 (FONCyT/DNA) project. MIM is grateful to Consejo Nacional de Investigaciones Científicas y Técnicas and Universidad Nacional del Comahue.
Carla Di Fonzo, Laura Dopchiz, Martín Ansaldo. Logistics, sampling, sampling preparation and discussions
Mariana S. Rivera, Soledad Perez Catán2, María A. Arribere, María I. Messuti and Débora F. Bubach sampling preparation and analysis, species identifications and discussions.
- Bargagli R (2008) Environmental contamination in Antarctic ecosystems. Sciences of the Total Environment 400: 212 ̶ 226. Link: https://tinyurl.com/ybdvjsyc
- Tapia Espinoza RE (2011) Evaluación de la calidad del aire en la Península Fildes, Isla Rey Jorge, Antártica, biomonitoreo de líquenes como herramienta de gestión. Tesis para optar al Grado de Magíster en Gestión y Planificación Ambiental. Universidad de Chile. Link: https://tinyurl.com/ydb3lnjp
- Bubach D, Pérez Catán S, Arribére M, Guevara SR (2012) Bioindication of volatile elements emission by the Puyehue–Cordón Caulle (North Patagonia) volcanic event in 2011. Chemosphere 88: 584 ̶ 590. Link: https://tinyurl.com/yd5lfopc
- Kim I, Kim G, Choy EJ (2015) The significant inputs of trace elements and rare earth elements from melting glaciers in Antarctic coastal waters. Polar Research 34: 24289. Link: https://tinyurl.com/y6vnez8f
- Bargagli R (2005) Antarctic ecosystems. Environmental contamination, climate change, and human impact. Berlin: Springer. Link: https://tinyurl.com/y8gc3hn4
- Yogui GT, Sericano JI (2008) Polybrominated diphenyl ether flame retardants in lichens and mosses from King George Island, maritime Antarctica. Chemosphere 73: 1589 ̶ 1593. Link: https://tinyurl.com/ybgclcoj
- Zvěřina O, Láska K, Cervenka R, Kuta J, Coufalík P, et al. (2014) Analysis of mercury and other heavy metals accumulated in lichen Usnea antarctica from James Ross Island, Antarctica. Environmental Monitoring and Assessment 186: 9089 ̶ 9100. Link: https://tinyurl.com/ybo6rdke
- Steig EJ, Schneider DP, Rutherford SD, Mann ME, Comiso JC, et al. (2009) Warming of the Antarctic ice-sheet surface since the 1957 International Geophysical Year. Nature 457: 459 ̶ 463. Link: https://tinyurl.com/y8lt3mfh
- Wojtuń B, Kolon K, Samecka-Cymerman A, Jasion M, Kempers AJ (2013) A survey of metal concentrations in higher plants, mosses, and lichens collected on King George Island in 1988. Polar Biology 36: 913 ̶ 918. Link: https://tinyurl.com/yax4gtrt
- Bubach D, Perez Catán S, Di Fonzo C, Dopchiz L, Arribére M, et al. (2016) Elemental composition of Usnea sp lichen from Potter Peninsula, 25 de Mayo (King George) Island, Antarctica. Environmental Pollution 210: 238 ̶ 245. Link: https://tinyurl.com/ydhh5mfq
- Carthy Mcd (2002) Lichenometry. In NIMIS, P.L., SCHEIDEGGER, C. & WOLSELEY, P.A., eds., Monitoring with lichens ̶ Monitoring lichens. NATO Science Series (Series IV: Earth and Environmental Sciences). Dordrecht: Springer 379 ̶ 383. Link: https://tinyurl.com/y7eehsu5
- (2007) UNEP (United Nations Environment Programme), Global Environment Outlook (GEO 4) Environment for Development. United Nations Environment Programs. Link: https://tinyurl.com/ybfnhuor
- Rodríguez GO (2016) Patrimonio natural. Península Potter, Antártida Argentina. Link: https://tinyurl.com/y9lxyxh3
- Alberdi M, Bravo La, Gutiérrez A, Gidekel M, Corcuera Lj (2002) Ecophysiology of Antarctic vascular plants. Physiologia Plantarum 115: 479 ̶ 486. Link: https://tinyurl.com/yag8cg2j
- Ovstedal DO, Lewis Smith RI (2001) Lichens of Antarctica and South Georgia: a guide to their identification and ecology. Cambridge: Cambridge University Press. Link: https://tinyurl.com/y7allf54
- Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, et al. (2009) The lichens of Great Britain and Ireland. British Lichen Society. British London: Lichen Society. Link: https://tinyurl.com/ydx7vgb5
- Orange A, James PW, White FJ (2001) Microchemical methods for the identification of lichens. London: British Lichen Society. Link: https://tinyurl.com/ycockf7p
- Klos A, Rajfur M, Šrámek E, Wactanwek M (2011) Use of lichen and moss in assessment of forest contamination with heavy metals in praded and glacensis euroregions (Poland and Czech Republic). Water, Air & Soil Pollution 222: 367 ̶ 376. Link: https://tinyurl.com/yblt6v2f
- Bergamaschi L, Rizzio E, Giaveri G, Profumo A, Loppi S, et al. (2002) Determination of baseline element composition of lichens using samples from high elevations. Chemosphere 55: 933 ̶ 939. Link: https://tinyurl.com/y7oj7nw3
- Norouzi S, Khademi H, Cano AF, Acosta JA (2016) Biomagnetic monitoring of heavy metals contamination in deposited atmospheric dust, a case study from Isfahan, Iran. Journal of Environmental Management 173: 55 ̶ 64. Link: https://tinyurl.com/y8xqqmv2
- Boamponsem Lk, Adam Ji, Dampare Sb, Nyarko Bjb, Essumang Dk (2010) Assessment of atmospheric heavy metal deposition in the Tarkwa gold mining area of Ghana using epiphytic lichens. Nuclear Instruments and Methods in Physics Research, Sec. B 268: 1492 ̶ 1501. Link: https://tinyurl.com/y959qsoe
- Godoy JM, Schuch LA, Nordemann DJR, Reis VRG, Ramalho M, et al. (1998) 137Cs, 226,228Ra, 210Pb and 40K concentrations in Antarctic soil, sediment and selected moss and lichen samples J Environmetal Radioactivity 41: 33 ̶ 45. Link: https://tinyurl.com/ydhe2zd6
- Clair SB, Clair LL, Weber DJ, Nolan F ( 2002) Element accumulation patterns in foliose and fruticose lichens from rock and bark substrates in Arizona. Bryologist 105: 415 ̶ 421. Link: https://tinyurl.com/y9k75z9f
- Rola K, Osyczka P, Kafel A (2016) Different heavy metal accumulation strategies of epilithic lichens colonising artificial post-smelting wastes. Archives of Environment Contamination and Toxicology 70: 418 ̶ 428. Link: https://tinyurl.com/y9wdmu9x
- Monaci F, Fantozzi F, Figueroa R, Parra O, Bargagli R (2012) Baseline element composition of foliose and fruticose lichens along the steep climatic gradient of SW Patagonia (Aisén Region, Chile). Journal of Environmental Monitoring 14: 2309-2316. Link: https://tinyurl.com/y85gaj5k
- Thell A, Crespo A, Divakar Pk, Kärnefelt I, Leavitt SD, et al. (2012) A review of the lichen family Parmeliaceae – history, phylogeny and current taxonomy. Nordic Journal of Botany 30:641–664. Link: https://tinyurl.com/ybl2r54d
- Bölter M (1992) Environmental conditions and microbiological properties from soils and lichens from Antarctica (Casey Station, Wilkes Land). Polar Biology 11: 591 ̶ 599. Link: https://tinyurl.com/yb36jqrn
- Lodenius M (2014) Biomonitoring of air borne metal pollution. WIT Transactions on Ecology and the Environment 183: 75 ̶ 85. Link: https://tinyurl.com/y8q7f6ba
- Abele D, Adrian A, Dorothee D, Gonzalez O, Kriews M, et al. (2006) Potter Cove and metal concentrations in Laternula elliptica shells. The Antarctic ecosystem of Potter Cove, King-George Island (Isla 25 de Mayo). Synopsis of research performed 1999-2006 at the Dallmann Laboratory and Jubany Station. Link: https://tinyurl.com/y7pn6ocz
- Andrade S, Poblet A, Scagliola M, Vodopivez C, Curtosi A, et al. (2001) Distribution of Heavy Metals in Surface Sediments from an Antarctic Marine Ecosystem. Environmental Monitoring Assessment 66: 147–158. Link: https://tinyurl.com/y89qadkt
- Jerez S, Motas M, Benzal J, Diaz J, Barbosa A (2013) Monitoring trace elements in Antarctic penguin chicks from South Shetland Islands, Antarctica. Marine Pollution Bulletin 69: 67 ̶ 75. Link: https://tinyurl.com/y99kv2tf
- Nie Y, Liu X, Sun L, Emslie SD (2012) Effect of penguin and seal excrement on mercury distribution in sediments from the Ross Sea region, East Antarctica. Sciences of Total Environment 433: 132-140. Link: https://tinyurl.com/y8u43kb7
- Liu X, Nie Y, Sun L, Emslie SD (2013) Eco-environmental implications of elemental and carbon isotope distributions in ornithogenic sediments from the Ross Sea region, Antarctica. Geochimica et Cosmochimica Acta 117: 99-114. Link: https://tinyurl.com/y97dwtaf
- Huang T, Yang L, Chu Z, Sun L, Yin X (2016) Geochemical record of high emperor penguin populations during the Little Ice Age at Amanda Bay, Antarctica. Science of the Total Environment 565: 1185 ̶ 1191. Link: https://tinyurl.com/y8tgxtmb
- Catán SP, Bubach D, Di Fonzo C, Dopchiz L, Arribére M, et al. (2017) Pygoscelis antarctica feathers as bioindicator of trace element risk in marine environments from Barton Peninsula, 25 de Mayo (King George) Island, Antarctica. Environmental Sciences and Pollution Research 24: 10759-10767. Link: https://tinyurl.com/y7a95afr
- Santamans AC, Boluda R, Picazo A, Gil C, Ramos-Miras J, et al. (2017) Soil features in rookeries of Antarctic penguins reveal sea to land biotransport of chemical pollutants. Link: https://tinyurl.com/y8ph4kct
- Pacheco AMG, Freitas MD (2009) Trace-element enrichment in epiphytic lichens and tree bark at Pico Island, Azores, Portugal. Journal of the Air and Waste Management Association 59: 411 ̶ 418. Link: https://tinyurl.com/y9bltdvy
- Heumann KG (2001) Biomethylation in the Southern Ocean and its contribution to the geochemical cycle of trace elements in Antarctica, In CAROLI, S., CESCON, P. & WALTON, D.W.H., eds. Environmental contamination in Antarctica. A challenge to analytical chemistry. Amsterdam: Elsevier 181-217. Link: https://tinyurl.com/ybhfwe2o
- Angulo E (1996) The Tomllinson Pollution Load Index applied to heavy metals “Mussel-Watch” data: A useful index to assess coastal pollution. Sciences of the Total Environment 187: 19 ̶ 56. Link: https://tinyurl.com/ya3l4sf2

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
