Annals of Marine Science
A ‘Field of Mouths’: Damselfishes in the Intertidal of Heron Island Cay, Great Barrier Reef
John Lucas*, Leya Koh, Maximilian Rath, Jasper Synowski, Richard Vierick and Salomé Willer
Cite this as
Lucas J, Koh L, Rath M, Synowski J, Vierick R, et al. (2017) A ‘Field of Mouths’: Damselfishes in the Intertidal of Heron Island Cay, Great Barrier Reef. Ann Mar Sci 1(1): 001-004. DOI: 10.17352/ams.000001The study was undertaken in the intertidal zone on the lee side of Heron Island cay (southern Great Barrier Reef) where there is a high density of branching corals. We investigated the influence of coral colony size on the diversity of damselfish species (Pomacentridae) associated with the branching corals. Forty coral colonies were marked and the associated pomacentrids photographed and identified. Eleven or twelve species were identified and there were up to eight species associated with a coral colony. The relationships between diversity of fish species and coral colony area, and coral colony perimeter were very significant. Plankton samples in the vicinity of the fringing reef are sparse during the day when the fish are feeding compared to the rich samples at night when they aren’t feeding, suggesting a substantial impact on the zooplankton content of the water flowing over the fishes’ habitats. In contrast to the ‘wall of mouths’ on the windward crest of a patch reef, it is suggested that these pomacentrid inhabitants of the shallow waters fringing Heron Island cay constitute a ‘field of mouths’. During the day, they trap and retain the allochthinous organic material that flows across the shallow coral reef and act as a source of nutrients for this coral reef ecosystem.
Introduction
Many damselfishes (Pomacentridae) are planktivores that feed on zooplankters and other suspended organic particles in coral reef environments. Their plankton-picking may have a substantial impact on the water flowing through their territory during daylight: they are visual feeders [1]. Hamner et al. (1988) [2], described where schools of pomacentrids in the water column at the windward edge of a patch reef, Davies Reef, in the central region of the Great Barrier Reef, had a major impact on the zooplankton and organic paraticle content of the water flowing onto the reef. Hamner et al. (1988) [2], estimated that approximately 0.5 kg wet weight of plankton was removed per day from the ocean water flowing over each meter width of reef crest. There were ten species of hovering pomacentrids that ‘visually inspected and stripped zooplankton’ from the water during daylight. This led Hamner et al. (1988) [2], to describe these hovering planktivorous fish as as a ‘wall of mouths’.
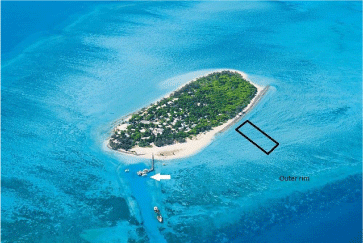
At Heron Island, southern Great Barrier Reef, the reefs immediately fringing this cay are not characterized by schools of pomacentrids in the water column at the weather edge. There are numerous coral colonies in the intertidal zone: their low heights are determined by the duration of desiccating exposures at low tides as a major factor. (Coral colony is used here for a structure consisting of hundreds or thousands of coral polyps that all originate from the settlement and metamorphosis of one coral planula) The tidal range is about 3 m and there are two tides each 24 hr. On the protected side of the cay where this study was conducted, the substrate remains covered when the tide drops below the height of the intertidal reef. A raised outer rim of the reef serves as a barrier that reduces the rate of water run-off and creates a shallow lagoon for the duration of the low tide (Figure 1). Fishes associated with coral colonies, such as the damselfish, can remain in their habitat.
There is a variety of coral colony sizes that mainly reflect their different ages. Pomacentrids were observed to be associated with the branching colonies over the broad expanse of intertidal reefs and this initial study set out to determine the nature of the pomacentrid fish’s community in a representative area of intertidal reef and especially to consider the influence of coral colony size on these species.
In previous studies, Komyakova et al. (2013) [3], examined the influence of a number of different abiotic and biotic factors on both fish species richness and total fish abundance on Lizard Island in the Great Barrier Reef. This study found that the best predictor for fish species richness was coral species richness. Other predictors were hard coral cover and topographic complexity. Another study conducted in northeast Brazilian reefs not only looked at natural factors influencing fish abundance and species richness but also at anthropogenic ones, such as tourism and fishing activity Pereira, et al. (2014) [4]. This study found that in these Atlantic reefs, fish abundance and species richness are strongly influenced by algae abundance. Coral abundance and coral diversity were found to be less important. In an earlier study, comparing different sites in the Indian Ocean, Chabanet et al. (1997) [5], found that fish abundance and fish species richness were correlated with various factors: architectural complexity of corals, coral diversity, coral species richness, coral size, coral abundance, living coral cover and encrusting coral cover. Other studies found relations between fish species richness and depth [6], coral quality and abundance [7], exposure to waves [8], and substratum complexity [8,9].
This study of pomacentrid species abundance was conducted in the intertidal zone of Heron Island, where parameters such as the exposure to waves, depth, coral species richness and anthropogenic factors were minor variables. It is a contribution to the existing research in this field because, although various possible predictors have been found, it appears that only one study [5], considered coral size as a predictor of fish species richness. That study was conducted at Reunion Island in the Indian Ocean and considered a variety of unrelated fish species and a variety of coral structures. This study considers related fish species in branching corals.
Materials and Methods
This study was made in late September, 2016. Forty branching coral colonies within 100 m of the shore, mainly Acropora species, were measured and tagged at low tide (Figure 1). The colonies were selected to give a range of sizes, and they were selected for reasonably circular shape and for not having major irregularities in their perimeter. Their diameters were measured twice at right angles to take account of imperfect circularity. The colonies were then tagged with numbered blue ribbons.
During high-tide each coral colony was photographed underwater a number of times about two minutes after it was approached. This enabled the fishes to accustom to the observers and move out from concealment within the coral branches. However, it was impossible to be sure that all individuals of all species were not concealed and were included in the photographs. Thus, no attempt was made to count the number of individuals in each photographic field. Fish were considered to be associated with a coral if they were at a distance where they could quickly hide in that coral.
The photographs were subsequently examined on a large computer monitor, with further magnification if needed, to identify species. The identifications were obtained from illustrations from at least two sources, including on-line images. There were some species that were relatively common and could be readily identified once their broad features were recognized. Some other less-common species were more difficult to identify.
The area of each coral colony was calculated by the formula
a x b x π (where a and b were the two measured dimensions)
the perimeter was calculated by the approximate formula
π x√[2 x (a/2)2 + (b/2)2]
Results
Eleven or twelve damselfish species were identified (Table 1). All species have previously been recorded from the Great Barrier Reef. There were some substantial differences amongst the fish species in the number of coral colonies inhabited. They ranged from the ubiquitous lemon damselfish(Pomacentrus moluccensis), which occurred in 81% of the corals (Figure 2), to some where a single individual was seen in one coral, e.g. the three-spot damselfish (Pomacentrus tripunctatus). P. moluccensis was not only ubiquitous, but from general observations it tended to be quite abundant in many of the corals where it occurred. This was a typical pattern for the more common species such as the Philippine damselfish (Pomacentrus phillippinus), the humbug damselfish (Dascyllus auruanus) (Figure 2) and the golden damselfish (Amblyglyphidodon aureus), which tended to occur in groups rather than as single individuals.
The corals ranged in surface area from 0.043 m2 to 2.294 m2. Three of the smallest corals had no fish occupants, but others had two, perhaps related to shape of the colony.
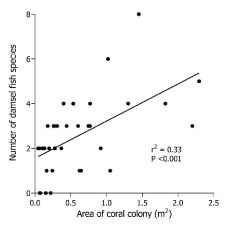
Figure 3 shows a plot of number of fish species against area of coral colony. In this regression model, coral area was found to be a highly significant (p< 0.001)(r2 = 0.33) predictor of the number of fish species associated with that coral colony.
Pomacentrids tend to find refuge in the perimeters of coral colonies and perimeters were calculated. They ranged from 0.74 – 5.4 m. In a regression model, the perimeter of the coral colony was found to be a highly significant (P<0.0001)(r2 = 0.434) predictor of the number of fish species associated with that coral colony, even more so than coral area.
Discussion
We found a complex population of pomacentrid fishes in the studied area. No data were obtained on densities of fishes, but, as already noted, general observation found that there were often multiple individuals of a species inhabiting the same coral colony. The question arises as to how up to eight species of fishes that are planktivorous and branching coral inhabitants can co-exist in close proximity?
The answer or at least part of the answer is in Hamner et al. (1988) [2], who analysed the gut contents of ten species of their ‘wall of mouths’ pomacentrids in a huge study. They classified these gut contents into 14 categories: algal fragments, copepods, eggs, amphipods, etc. Only one species, an Abudefduf species, was found to have all 14 categories of food items in the gut at one time, but this was for the accumulated data of the guts of 24 individuals at that sampling time, not for one fish. Algal fragments were ignored by a number of species, but the ubiquitous lemon damselfish of our study (Pomacentrus moluccensis) had a propensity for algal fragments, although it also took some copepods, larvaceans and forams.
Clearly there is no simple competition for each planktonic food item as it becomes available, but species have preferences. The larger coral colonies may allow more species to exploit their preferred plankters as they pass in the fishes’ vicinity.
There is another kind of sampling that apparently relates to this study and adds another dimension. It is inevitable that zooplantkters will be removed from the water passing over the intertidal reef during the day. The fishes must be feeding and it is a matter of their impact. Long-term samples taken at night with a 200 µm mesh plankton net adjacent to this reef area consistently contain numerous larvae of fishes, polychaetes, bivalves, and crabs, shrimps and other decapod crustaceans; swimming amphipods and isopods; and some copepods (for which the mesh size is too large)( Figure 1). Equivalent plankton samples taken in the same place during the day have consistently sparse contents. These observations are consistent with the water flowing over the fringing reef being subjected to heavy predation by visual planktivores during daylight hours.
Hamner et al. (1988) [2], make a strong case for the reef crest pomacentrids having a major role in trapping and retaining the allochthinous organic material (i.e. material formed elsewhere) that flows onto the coral reef during the day then passing it on as nutrients to the coral reef ecosystem. They saw this especially through the fishes’ feces.
Unlike the vertical ‘wall of mouths’ on the windward crest of a reef, this broadly-distributed population of pomacentrids in this shallow reef region is more like a ‘field of mouths’ which prey on the zooplankters and organic particles passing over the shallow reef during daylight hours. As well as feces, their bodies are available for subsequent predation or scavenging as a source of nutrients for the adjacent coral reef ecosystem.
We thank Andy Barnes for his program leadership and the staff of the University of Queensland’s Heron Island Research Station for their assistance.
- Johansen JL, Jones GP (2013) Sediment-induced turbidity impairs foraging performance and prey choice of planktivorous coral reef fishes. Ecol Appl 23: 1504–1517. Link: https://goo.gl/G6A8hI
- Hamner WM, Jones MS, Carleton JH, Hauri IR, Williams D (1988) Zooplankton, planktivorous fish, and water currents on a windward reef face: Great Barrier Reef, Australia. Bulletin of Marine Science 42: 459-479. Link: https://goo.gl/9zkhP7
- Chabanet P, Ralambondrainy H, Amanieu M, Faure G, Galzin R (1997) Relationships between coral reef substrata and fish. Coral Reefs 16: 93-102. Link: https://goo.gl/6PVl5o
- Pinheiro HT, Martins AS, Joyeux JC (2013) The importance of small-scale environment factors to community structure patterns of tropical rocky reef fish. Journal of the Marine Biological Association of the United Kingdom 93: 1175-1185. Link: https://goo.gl/57UZ8o
- Syms C, Jones P (2000) Disturbance, habitat structure, and the dynamics of a coral-reef fish community. Ecology 81: 2714-2729. Link: https://goo.gl/taeXha
- Friedlander AM, Brown EK, Jokiel PL, Smith WR, Rodgers KS (2003) Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 22: 291-305. Link: https://goo.gl/Rz1YEO
- Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32: 315-326. Link: https://goo.gl/mweM4O

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
