Authors:
ZhongKai Zhou* and Xiaochong Ren
Key Laboratory of Food Nutrition and Safety, Ministry of Education, Tianjin University of Science and Technology, Tianjin 300457, China
Received: 12 March, 2015; Accepted: 14 April, 2015; Published: 17 April, 2015
Prof. Zhongkai Zhou (PhD), School of Food Engineering and Biotechnology, Tianjin University of Science and Technology, Tianjin, 300457, China, Tel: +862260601408; Fax +862260601375; Email:
Zhou Z, Ren X (2015) Consumption of Aloe vera Mucilage Attenuates Plasma Oxidative Stress and Dyslipidemia in Type 2 Diabetic Rats. Glob J Biotechnol Biomater Sci 1(1): 001-003.
© 2015 Zhou Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
A. vera mucilage; Dyslipidemia; Lipid metabolism
This study found that Aloe vera mucilage administration significantly improved biological parameters including lipids composition and oxidative stress in type 2 diabetic rats. Because the mRNA levels of lipogenic genes were significantly down-regulated (P<0.01) by the intervention of A. vera mucilage, this improvement of these biological parameters may be caused, at least in part, by the regulation of hepatic genes that are involved in lipids metabolism, thus resulting in an increase of cholesterol clearance and β-oxidation as well as a reduction of hepatic lipogenesis. The results in this study might provide a further beneficial effect of A. vera mucilage in the possible management of dyslipidemia-related diseases.
Introduction
Diabetes is one of the most costly and burdensome chronic diseases, which gains increasingly public health concern. More importantly, hyperglycemia is also accompanied by hyperlipidemia. Nowadays the agents used for diabetes treatment are mainly synthetic drugs and insulin. However, these drugs usually come with considerable side effects, such as hypoglycemia, drug-resistance, dropsy and weight gain. Thus, it is not surprising that is a great interest in novel approaches to Diabetes Mellitus (DM) management. In particular, there has been an increasing demand for natural products, and A. vera is one of them currently showing the potentials in this field.
A. vera leaves are consisted of chlorenchyma cells and thinner walled cells forming the parenchyma (filets). The parenchyma cells contain a transparent mucilaginous jelly which is referred to as Aloe vera gel. The gel has been widely accepted since the 4th century B.C. as a traditional medicine. The majority of the mucilaginous is acemannan, an acetylated glucomannan. The polysaccharide has been shown the potential to be an immunostimulant for enhancing the immunologic functions [11. Chow JT, Williamson DA, Yates KM, Goux WJ (2005) Chemical characterization of the immunomodulating polysaccharide of Aloe vera L. Carbohydr Res 340: 1131-1142. ].
Following the further research, more physiological properties of Aloe vera mucilage are being discovered. For example, Yagi, et al. [22. Yagi A, Hegazy S, Kabbash A, Abd-El Waha E (2009) Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharm J 17: 209-215.] found that the polysaccharides in A. vera L demonstrated the possible hypoglycemic effect. However, considering the multi-links among hyperglycemia, hyperlipidemia and hypertension in DM, there is litter known the effect of A. vera mucilage on dyslipidemia induced by DM. Thus, this paper aims to study the influence of A. vera mucilage administration on blood lipids composition in type 2 diabetic rats. Moreover, the regulations of gene expressions related to the lipids metabolism are also investigated following the intervention.
Materials and Methods
Materials
A. vera leaves were grown and collected from Yunnan, the South-East region of China. The dried Mucilage was prepared as followings: the A. vera leaves were washed using distilled water to remove dirt on the surface. The skin was carefully removed from the parenchyma by a scalpel-shaped knife. After homogenized in a blender, the mass was filtered to remove large particles using nylon cloth, and then it was spray dried (Mobile MinorTM, GEA, China) with inlet air temperature 175°C, outlet air temperature 95°C, and feed rate 1.5 L/h. The moisture content of the dried powder was 9.7%. The dried powder was used for examining its effect on anti-hyperlipidemia for the diabetic rats. Streptozotocin (STZ) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals were of reagent grade and used as received.
Animals and diets
Healthy male Sprague-Dawley rats (SD, non-diabetic) of ~ 190±10 g weight, purchased from the animal house, Chinese Military Medical Science Academy. 24 rats were divided into three groups randomly: normal control, model control, and A. vera mucilage intervention group. Normal control: healthy rats without intervention; Model control: STZ induced diabetic rats without intervention; Intervention group: STZ induced diabetic rats with A. vera mucilage supplement. SD rats were housed in plastic cages (4 rats/cage) with free access to food and water, under controlled environments. After one week's adaptive feeding with the basic diet, the rats were fasted for 12 h, followed by intravenous injection of STZ 45 mg/ kg b.w. except the rats in the normal control group. After 72 h of STZ injection, the blood glucose level (BGL) was measured and the rats with BGL level higher than 16.7 mmol/L were considered diabetic and were included for the experiment. All the rats were fed with the basal diet containing 7% fat, 13% protein, and a highly digestible starch for 6 weeks. In contrast, dried A. vera mucilage was administered for the interventional group with 1.2 g once daily for 6 consecutive weeks before they were sacrificed for analysis.
Biological analysis
At the end of this experiment (i.e. 6 weeks), blood samples were collected from the femoral artery before animals were sacrificed by cervical dislocation. The samples collected were stored at -80°C prior to the chemical analyses. Blood lipids composition, such as high-density lipoprotein-cholesterol (HDL-c), total cholesterol (TCH), triglyceride (TG) and nonesterified fatty acids (NEFAs) were measured according to the instructions of their corresponding kits (Dong'ou diagnosis Products Co Ltd, Zhejiang, China). Plasma malondialdehyde (MDA), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and reactive oxygen species (ROS) were measured by enzyme-linked immunosorbent assay (ELISA) (Sigma-Aldrich, USA).
After 6-weeks feeding, the rats were dissected immediately with sterile scissors. The liver was sampled, weighed and immediately frozen in liquid nitrogen, and stored at -80 before homogenizing for total RNA extraction. All animal trial procedures were approved by Ethical Committee for the Experimental Use of Animals in Center for Drug Safety Evaluation, Tianjin University of Science & Technology.
Gene expression of lipid and glucose metabolisms
The total RNA was extracted using the Trizol reagent (Takara). Total RNA isolated from liver was treated with RNase-free DNase to remove any contaminating genomic DNA. For RT-PCR operation, first strand cDNA was synthesized using the PrimeScript RT reagent kit with gDNA Eraser (Takara) according to the manufacturer's instructions. PCR of the detection genes was carried out (20 μL volume) containing 2 μM of each primer, 40 ng of cDNA, and 10 μL of SYBR Primix ExTag. Thermal cycling conditions included an initial denaturation step at 95°C for 5 min, and then 40 cycles of 95°C for 30 s, 58-60°C for 30 s and 72°C for 30 s. Fluorescence was measured at the end of each cycle. The 18S rRNA gene was used as an internal control to normalize target genes' expression. Three replicates of each reaction were carried out, and the relative transcript quantity was calculated according to the method of 2-ΔΔCT.
Results and Discussion
Effect of administration of A. vera mucilage on blood lipids composition
As shown in Table 1, the level of oxidative stress was significantly increased due to the induction of diabetic disease (P<0.001). Because high glucose concentration could induce the formation of reactive oxygen species (ROS), which would enhance the production of malondialdehyde (MDA) [33. Del Rio D, Stewart AJ, Pellegrini N (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 15: 316-328.], a byproduct of non-enzymatic lipid peroxidation, thus, MDA and ROS are commonly used biomarkers for evaluating the scale of oxidative damage. In this study, the data indicated that the plasma MDA of diabetic rats in the model control group (i.e. without intervention) increased by 51.4% (P<0.001), and accompanied by a significant increase in ROS, compared to the rats in the normal group (Table 1). This further confirmed that diabetic status usually exhibits higher oxidative stress due to persistent and chronic hyperglycemia, which thereby reduces the total antioxidant activities and thus promotes the generation of ROS [44. Kamalakannan N, Prince PSM (2006) Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol l98: 97-103.].
It was interesting to find that the intervention of A. vera mucilage could significantly attenuate plasma MDA level by 25.6% compared to the model control (P<0.05) (Table 1). In addition, the intervention also led to a reduction of ROS, indicating the greater improvement of oxidative stress status was achieved by the consumption of A. vera mucilage in the diabetic rats. Consistently, GSH-Px and SOD was found to be increased by 31.0% and 28.6%, respectively, following A. vera mucilage treatment compared to the model control group.
Moreover, diabetes mellitus caused dyslipidemia, reflected by a significantly higher in blood triglyceride (TG) and total cholesterol (TCH) (P<0.001) (Table 1). In contrast, the intervention of A. vera mucilage achieved a great reduction of 63.7% for TG (P<0.01) and 24.4% (P<0.05) for TCH, respectively, as compared to the rats in the model control group (i.e. non-treatment group). More importantly, this study also found that the intervention of A. vera mucilage could nearly restore the HDL-c concentration to a normal level in the diabetic rats (Table 1).
Effect of A. vera mucilage administration on mRNA levels of lipids metabolism
To elucidate the molecular mechanisms underlying the hypolipidemic effect of the intervention of A. vera mucilage, the changes in the expression levels of hepatic genes involved in lipids metabolism were analyzed and displayed in Figure 1. Considering that SREBP-1c preferentially enhances the transcription of genes required for fatty acid synthesis but not cholesterol synthesis, the mRNA levels of lipogenic genes such as sterol regulatory element binding protein-1 (SREBP-1, associated with the regulation of fatty acids and triglycerides synthesis and metabolism), and sterol regulatory element binding protein-2 (SREBP-2, response for cholesterol synthesis) were all determined. The significant decrease in the expression of both SREBP target genes indicated that the depression of cholesterol and fatty acid synthesis and thus a marked reduction of hepatic cholesterol and triglycerides were achieved following the A. vera mucilage intervention (P<0.01). Consistently, the intervention also significantly decreased the expression levels of other related lipogenic genes such as acetyl CoA carboxylase (ACC1), fatty acid synthase (FAS), fatty acid desaturase 1 (Fads1) and diacylglycerol acyltransferase 1 (Dgat1). Moreover, Insig-1 and Insig-2 play important roles both in glucose homeostasis and the regulation of intracellular cholesterol and fat metabolism [55. Goldstein JL, DeBose-Boyd RA, Brown MS (2006) Protein sensors for membrane sterols. Cell 124: 35-46.].
-
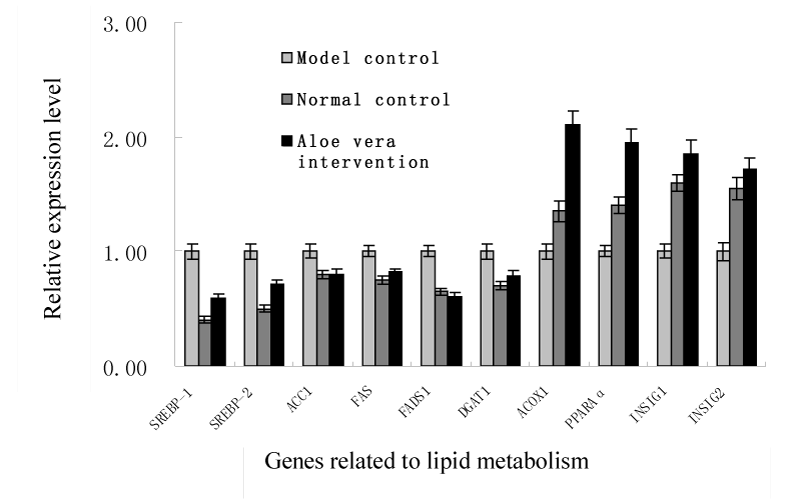
Figure 1:
Effect of Aloe vera mucilage intervention on the gene expression of lipids metabolism.
This study found that the expression of the two insulin induced genes was significantly promoted, which might be highly related to the improved lipids composition caused by Aloe vera mucilage intervention. Previous reports also suggested that hepatic over-expression of Insig-1 (or Insig-2) could inhibit the activation of SREBP-1c in the liver [66. Herbert A, Gerry NP, McQueen MB, Heid IM, Pfeufer A, et al. (2006) A common genetic variant is associated with adult and childhood obesity. Science 312: 279-283.]. In particular, the expression levels of Acox1 and PPARα genes were greatly up-regulated in the diabetic rats after the A. vera mucilage intervention (Figure 1), which might suggest that the reduction of TG was mainly due to the promotion of fatty acid β-oxidation.
Conclusion
This is the first time to investigate that the consumption of A. vera mucilage improves plasma oxidative stress and dyslipidemia in type 2 diabetic rats. The study of the molecular mechanisms underlying the hypolipidemic effect of the intervention of A. vera mucilage indicates that improvement of blood lipids composition might be associated with the inhibition of lipid accumulation in the liver via the down-regulation of genes involved in lipogenesis and the up-regulation of genes involved in lipid β-oxidation.
Acknowledgements
This work was supported by the NSFC (No. 31471701), China-European collaboration program (SQ2013ZOA100001), and 2015 Tianjin Research Program of Application Foundation and Advanced Technology.
- Chow JT, Williamson DA, Yates KM, Goux WJ (2005) Chemical characterization of the immunomodulating polysaccharide of Aloe vera L. Carbohydr Res 340: 1131-1142.
- Yagi A, Hegazy S, Kabbash A, Abd-El Waha E (2009) Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharm J 17: 209-215.
- Del Rio D, Stewart AJ, Pellegrini N (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 15: 316-328.
- Kamalakannan N, Prince PSM (2006) Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol l98: 97-103.
- Goldstein JL, DeBose-Boyd RA, Brown MS (2006) Protein sensors for membrane sterols. Cell 124: 35-46.
- Herbert A, Gerry NP, McQueen MB, Heid IM, Pfeufer A, et al. (2006) A common genetic variant is associated with adult and childhood obesity. Science 312: 279-283.



 Global Journal of Biotechnology and Biomaterial Science
Global Journal of Biotechnology and Biomaterial Science




Table 1:
Effect of A. vera mucilage administration on oxidative stress in the diabetic rats.
Results are expressed as means ± SD. Values in each column followed by different superscript are significantly different at *P</em><0.05, **P</em><0.01 and ***P</em><0.001, respectively, compared to the model control.